- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana
Published: Vol 13, Iss 15, Aug 5, 2023 DOI: 10.21769/BioProtoc.4756 Views: 2284
Reviewed by: Aswad KhadilkarSam-Geun KongAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Streamlining Protein Fractional Synthesis Rates Using SP3 Beads and Stable Isotope Mass Spectrometry: A Case Study on the Plant Ribosome
Dione Gentry-Torfer [...] Federico Martinez-Seidel
May 5, 2024 2869 Views

An Activity-Based Proteomics with Two-Dimensional Polyacrylamide Gel Electrophoresis (2D-PAGE) for Identifying Target Proteases in Arabidopsis Apoplastic Fluid
Sayaka Matsui and Yoshikatsu Matsubayashi
Mar 5, 2025 1989 Views

Advancing 2-DE Techniques: High-Efficiency Protein Extraction From Lupine Roots
Sebastian Burchardt [...] Emilia Wilmowicz
Oct 5, 2025 1772 Views
Abstract
The chloroplast lumen contains at least 80 proteins whose function and regulation are not yet fully understood. Isolating the chloroplast lumen enables the characterization of the lumenal proteins. The lumen can be isolated in several ways through thylakoid disruption using a Yeda press or sonication, or through thylakoid solubilization using a detergent. Here, we present a simple procedure to isolate thylakoid lumen by sonication using leaves of the plant Arabidopsis thaliana. The step-by-step procedure is as follows: thylakoids are isolated from chloroplasts, loosely associated thylakoid surface proteins from the stroma are removed, and the lumen fraction is collected in the supernatant following sonication and centrifugation. Compared to other procedures, this method is easy to implement and saves time, plant material, and cost. Lumenal proteins are obtained in high quantity and purity; however, some stromal membrane–associated proteins are released to the lumen fraction, so this method could be further adapted if needed by decreasing sonication power and/or time.
Keywords: Thylakoid lumenBackground
The chloroplast is the organelle that conducts photosynthesis in plants and algae. A major compartment of the chloroplast is the thylakoid lumen, which is enclosed by the thylakoid membrane. The Arabidopsis lumen proteome consists of at least 80 proteins based on mass spectrometry analyses (Peltier et al., 2002; Schubert et al., 2002) and up to 127 proteins according to lumenal targeting peptide prediction (Almagro Armenteros et al., 2019). All lumenal proteins characterized so far are nuclear-encoded and post-translationally transported into chloroplasts [see for reviews on lumenal proteins targeting (Albiniak et al., 2012) and function (Kieselbach and Schröder, 2003; Järvi et al., 2013)]. Lumenal proteins support and modulate photosynthetic activity directly or indirectly (examples of proteins are given in parenthesis), with known function in electron transport (plastocyanin PC, photosystem I subunit PsaN, and photosystem II subunit PsbO), in protein processing (C-terminal processing protease ctpA), assembly (immunophilins), and degradation (Deg proteases), in redox regulation (membrane protein with lumen thioredoxin domain HCF164) and in photoprotection (violaxanthin de-epoxidase and lipocalin in the plastid LCNP). However, the function of several lumenal proteins remains to be elucidated (some of which are Psb-like and pentapeptide repeat-containing proteins) and their regulation is not fully understood. The activity, stability, and distribution in the lumen compartment as soluble or membrane-associated proteins, or protein–protein interactions can be regulated by post-translational modifications such as phosphorylation or N-terminal acetylation (Gollan et al., 2021), or through redox modification or disulfide bond modulation, e.g., by lumen thiol oxidoreductase 1 of the lumenal domain of the kinase STN7 (Wu et al., 2021; for reviews, see Buchanan and Luan, 2005; Kang and Wang, 2016).
The major challenge when performing studies on the lumen proteome is the balance between purity and the quantity of protein needed for further analysis. Indeed, lumenal proteins are lowly abundant compared to thylakoid membrane protein light harvesting complex LHCII and stromal Rubisco [which represent more than 50% of total chloroplast protein content (Hall et al., 2011)]. Fractionation of the chloroplast and isolation of lumenal proteins thus enable their detection and accurate quantification by working within the dynamic range of detection, for example in immunoblot assays (the dynamic range is the lowest to highest concentration of a given protein that can be reliably detected). Then, the accumulation of a protein of interest can be investigated in different growth conditions or stress treatments and in mutants; the lumen proteome has been analyzed from 6–8-week-old plants grown under standard growth conditions (i.e., 120 μmol photons m-2·s-1, 21 °C, 8:16 h light/dark) (Peltier et al., 2002; Schubert et al., 2002) and also comparing the end of the dark vs. light period (Granlund et al., 2009) or after cold acclimation (Goulas et al., 2006). In addition, localization and distribution of the proteins, as well as assessment of protein complexes and interaction, can be inferred. Recently, Gollan et al. (2021) refined further localization studies to distinguish free lumenal proteins from membrane-associated ones, using Yeda press to isolate soluble lumenal proteins and subsequent washes with urea and salt to release inner membrane-associated proteins.
To isolate lumen proteins from plants, first the chloroplasts are obtained by homogenization of leaves followed by centrifugation; then, chloroplasts are lysed by osmotic shock, and thylakoid membranes are collected by centrifugation and washed to remove stromal proteins and peripheral membrane proteins. Finally, thylakoid membranes are ruptured to release lumenal proteins with a Yeda press (Kieselbach et al., 1998) or a sonicator (Peltier et al., 2000; Levesque-Tremblay et al., 2009); alternatively, thylakoid membranes are solubilized to release lumenal proteins using a detergent such as Triton X-114 followed by phase partitioning at 30–37 °C (Bricker et al., 2001), 0.04% Triton X-100 (McKinnon et al., 2020), or 0.05% n-Dodecyl β-D-maltoside (Chang et al., 2021).
Here, we report a simple procedure by which a lumenal fraction can be isolated in pure form from the thylakoids in Arabidopsis by sonication, which we adapted from previously described methods (Peltier et al., 2000; Levesque-Tremblay et al., 2009). Lumen isolation by sonication is of interest for several reasons: 1) the Yeda press is no longer commercially available (Hall et al., 2011), so unless already existing in the laboratory, access to one is limiting; 2) operating a sonicator is easier and faster than using the Yeda press for more than two samples (due to faster washing time of instruments parts); and 3) use of detergent can be costly and can disrupt native interactions. Also, the yield with sonication is comparable to using the Yeda press (15–30 μg of lumenal proteins per gram of leaf) and a decreased isolation time (from six to three hours for two samples) is valuable to limit proteolysis and preserve more native states of proteins and complexes during the lumen isolation. All methods present the disadvantage of stromal membrane–associated protein contaminants in the lumenal fraction that are not removed during the washes of thylakoid membranes (Bricker et al., 2001) (for example, see Figure 2, ATPb); the presence of these contaminants could be decreased by using a shorter sonication time and/or decreased power (Peltier et al., 2000). Overall, the sonication method is easy to implement, saves time, plant material, and cost, and is suitable for most studies—unless a large quantity of proteins is required, in which case the Yeda press method should be favored.
Materials and reagents
1.5 mL microcentrifuge tube (Sigma-Aldrich, catalog number: HS4323)
15 mL centrifuge tube (Thermo Fisher Scientific, catalog number: 339650)
Amicon ultra-0.5 centrifugal filter unit (EMD Millipore, catalog number: UFC500324)
10.4 mL polycarbonate bottle with cap assembly (Beckman Coulter, catalog number: 355603)
50 mL open-top thick-wall polycarbonate tube (Beckman Coulter, catalog number: 363647)
10 μL pipette tip (Thermo Fisher Scientific, catalog number: 3521-HR)
200 μL pipette tip (Thermo Fisher Scientific, catalog number: 3551-HR)
1,000 μL pipette tip (Thermo Fisher Scientific, catalog number: 3101-HR)
Disposable glass Pasteur pipettes 230 mm (VWR, catalog number: 612-1702)
1,000 mL plastic bag (e.g., Tingstad, catalog number: 398301-1)
Glass funnel (e.g., Sagitta, catalog number: 87807)
250 mL flask (e.g., Sagitta, catalog number: 86425)
25 mL beaker (e.g., VWR, catalog number: 213-0192)
Cuvette for chlorophyll quantification [e.g., Hellma, catalog number: HL104-002-10-40 (quartz, preferred) or Sarstedt, catalog number: 67.742 (plastic; ensure to measure right away so acetone does not degrade plastic and affect spectrophotometer reading)]
Paintbrush 6 mm (Ahlsell, catalog number: 384364)
Miracloth 22–25 μm pore size (Calbiochem, catalog number: 475855)
Arabidopsis thaliana plants (wild type and soq1-1 mutant, ecotype: Columbia-0)
Milli-Q water
2-Mercaptoethanol (Thermo Fisher Scientific, catalog number: 21985023)
Glycerol (Sigma-Aldrich, catalog number: G5516)
Bromophenol blue (Sigma-Aldrich, catalog number: B0126)
Ethanol (Sigma-Aldrich, catalog number: EX0290)
Acetic acid (Sigma-Aldrich, catalog number: 695092)
Coomassie blue R-250 (Sigma-Aldrich, catalog number: 1.12553)
Tris(hydroxymethyl)aminomethane (Tris base) (Sigma-Aldrich, catalog number: 648310-M)
Glycine (Sigma-Aldrich, catalog number: G8898)
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771)
Tween 20 (Sigma-Aldrich, catalog number: P9416)
Non-fat dried milk (e.g., Semper)
Tricine (Sigma-Aldrich, catalog number: T0377)
D-sorbitol (Sigma-Aldrich, catalog number: S1876)
Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: E9884)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: 529552)
Sodium L-ascorbate (Sigma-Aldrich, catalog number: A4034)
L-cysteine (Sigma-Aldrich, catalog number: C7352)
Sodium fluoride (Sigma-Aldrich, catalog number: 215309)
MgCl2 (Sigma-Aldrich, catalog number: M2670)
Concentrated HCl (37%) (Sigma-Aldrich, catalog number: 320331)
Disodium hydrogen phosphate (Na2HPO4) (Sigma-Aldrich, catalog number: 567550)
Sodium dihydrogen phosphate (NaH2PO4) (Sigma-Aldrich, catalog number: 1.06370)
Benzamidine (Sigma-Aldrich, catalog number: 12072)
ϵ-Aminocaproic acid (Sigma-Aldrich, catalog number: A2504)
Phenylmethanesulfonyl fluoride solution (PMSF) (Sigma-Aldrich, catalog number: 93482)
cOmpleteTM, EDTA-free protease inhibitor cocktail (Roche, catalog number: 4693132001)
Quick StartTM Bradford Protein Assay kit (Bio-Rad, catalog number: 5000201)
PageRuler prestained protein ladder (Thermo Fisher Scientific, catalog number: 26616)
Immobilon-P PVDF Membrane (Millipore Sigma, catalog number: IPVH00005)
ECL bright kit for immunodetection (Agrisera, catalog number: AS16 ECL-N)
Anti-PsaD, 1:1,000 dilution (Agrisera, catalog number: AS09 461)
Anti-Lhcb4, 1:7,500 dilution (Agrisera, catalog number: AS04 045)
Anti-RbcL, 1:7,500 dilution (Agrisera, catalog number: AS03 037)
Anti-PC, 1:2,000 dilution (Agrisera, catalog number: AS06 141)
Anti-ATPb, 1:5,000 dilution (Agrisera, catalog number: AS05 085)
Horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody, 1:10,000 dilution (Sigma-Aldrich, catalog number: A6154)
1 M Tris-HCl stock solution (6.8 and 7.5) (see Recipes)
100 mM Sodium phosphate stock solutions (pH 7.8) (see Recipes)
Extraction buffer (see Recipes)
Resuspension buffer (see Recipes)
Lysis buffer (see Recipes)
Washing buffer (see Recipes)
4× sample loading buffer for SDS-PAGE (see Recipes)
Running buffer for SDS-PAGE (see Recipes)
Transfer buffer for immunodetection (see Recipes)
TBST buffer (see Recipes)
Blocking buffer (see Recipes)
Coomassie blue stain (see Recipes)
Coomassie blue destain (see Recipes)
Equipment
10 μL pipette (Thermo Fisher Scientific, catalog number: 4641040N)
20 μL pipette (Thermo Fisher Scientific, catalog number: 4641060N)
200 μL pipette (Thermo Fisher Scientific, catalog number: 4641080N)
1,000 μL pipette (Thermo Fisher Scientific, catalog number: 4641100N)
Plant growth cabinet (CLF plant Climatics, model: E-41L2)
Custom-designed LED panel, built by JBeamBio with cool white LEDs BXRA-56C1100-B-00 (Farnell)
Laboratory balance (e.g., Fisher Scientific, catalog number: 14-557-421)
Blender (e.g., Coline, 300 mL mixer cup with 2-bladed knife)
High-speed centrifuge (Beckman Coulter, model: Avanti J-20XP) with JA-25.50 fixed angle aluminum rotor (Beckman Coulter, catalog number: 363055)
Ultracentrifuge (Beckman Coulter, model: LE-70) with type 70.1 Ti fixed-angle titanium rotor (Beckman Coulter, catalog number: 342184)
Benchtop centrifuge (Beckman Coulter, catalog number: B06322)
UV-visible spectrophotometer (Hitachi, catalog number: U-5100)
Sonicator (Sonics, model: VCX130) with 2 mm microtip (Sonics, catalog number: 630-0417)
Dry block heater (MRC, catalog number: DBSC-001)
Immunodetection imaging system (Azure, model: c600)
Procedure
Plant material and growth conditions
Wild-type Arabidopsis thaliana and soq1-1 mutant were grown in a growth chamber with 120 μmol photons m-2·s-1 light intensity and 60% humidity at 20 °C during the day for 8 h and 18 °C during the night for 16 h. For cold and high light conditions (Cold HL), plants are illuminated in the cold room (4 °C) for 6 h at 1,600 μmol photons m-2·s-1 light intensity.
Thylakoid lumen isolation by sonication
Prepare extraction buffer [see Recipe 1 (Fristedt et al., 2009)], resuspension buffer (see Recipe 2), lysis buffer (see Recipe 3), and washing buffer (see Recipe 4). Keep at 4 °C.
Harvest the Arabidopsis rosettes from intended conditions (e.g., growth light and cold and high light conditions) into plastic bags. After removing the air, seal the bags and put them in the dark in the cold room. We usually use four rosettes from plants grown under short-day (8:16 h day/night) conditions for seven weeks (~10 g of fresh weight).
Blend the plant samples in 90 mL of extraction buffer three times for 5 s using a blender.
Filtrate the homogenate through a glass funnel with four layers of Miracloth to a 250 mL flask on ice.
Transfer the filtrated homogenate to 50 mL open-top thick-wall polycarbonate tubes (two tubes) and centrifuge at 1,000× g for 5 min.
Resuspend the chloroplast pellet in 35–40 mL of resuspension buffer using a soft paintbrush and centrifuge at 1,000× g for 5 min.
Note: We initially use a small volume (~3 mL) to resuspend the chloroplast using a paintbrush, flash freeze one or two aliquots (100 μL) in liquid nitrogen, and store them at -70 °C until immunoblot analysis. Then, we add resuspension buffer to 35–40 mL.
Resuspend the chloroplast in lysis buffer at a final chlorophyll concentration of 0.2 mg/mL and centrifuge at 6,000× g for 5 min.
Note: We usually keep the chloroplast in lysis buffer at 4 °C for 10 min to break the chloroplast envelopes by osmotic shock before centrifugation.
Concentrate approximately 2 mL of supernatant using Amicon ultra-0.5 centrifugal filters. Flash freeze the stromal aliquots (50 μL) in liquid nitrogen and store them at -70 °C until immunoblot analysis.
Wash the thylakoid pellet with 35–40 mL of washing buffer and centrifuge at 6,000× g for 5 min.
Note: We usually use a small volume (~3 mL) to resuspend the pellet using a paintbrush and then add washing buffer to 35–40 mL.
Resuspend the thylakoids in washing buffer, usually ~4 mL, so that the chlorophyll concentration is approximately 0.5 mg/mL. The method to measure the chlorophyll concentration is from Porra et al. (1989). Flash freeze the thylakoid aliquots (100 μL) at -70 °C until immunoblot analysis.
Transfer the thylakoid mixture to a 25 mL pre-cooled beaker and sonicate 10 times for 30 s ON (power: 130 watt) and 10 s OFF at 4 °C.
Note: We usually put half of the beaker into ice to keep the samples cold during the sonication operation.
Transfer the samples from the beaker to a 10.4 mL polycarbonate bottle, balance the samples with the washing buffer carefully, and ultracentrifuge at 200,000× g for 2 h at 4 °C to separate the thylakoid membranes in the pellet and the soluble lumenal proteins in the supernatant.
Transfer the supernatant (~3 mL) carefully to a new 15 mL centrifuge tube using a disposable glass Pasteur pipette and concentrate the soluble lumen sample (final volume is ~150 μL) at 14,000× g for 10 min at 4 °C using Amicon ultra-0.5 centrifugal filters. Flash freeze the lumen aliquots (25 μL) and store them at -70 °C until immunoblot analysis.
Resuspend the thylakoid membrane after isolation of lumen proteins in washing buffer (~4 mL) at a chlorophyll concentration of 0.5 mg/mL. Flash freeze the thylakoid membranes aliquots (100 μL) and store them at -70 °C until immunoblot analysis.
Sample preparation for SDS-PAGE
Take out samples (thylakoids, thylakoid membrane after isolation of lumen proteins, and lumen fraction) from -70 °C on ice and thaw rapidly at 37 °C on a heat block (less than a minute).
Wash the thylakoid and thylakoid membrane samples with 1 mL of 120 mM Tris-HCl (pH 6.8) and centrifuge at 20,000× g for 5 min at 4 °C.
Resuspend the pellets gently by adding 200 μL of 120 mM Tris-HCl (pH 6.8).
Measure the samples concentration using the Bradford assay (Kruger, 1994)
SDS-PAGE and immunoblot
Prepare the polyacrylamide gels for SDS-PAGE.
Note: The optimal concentration of separating gel depends on the protein of interest. We usually use a 15% separating gel for lumenal plastocyanin protein (PC; PC1 runs closer to 14 kDa while PC2 runs closer to 19 kDa) and 12% separating gel for thylakoid membrane light-harvesting complex b4 protein (Lhcb4, 29 kDa), core subunit A of photosystem I (PsaA, 55–60 kDa), stromal ribulose bisphosphate carboxylase oxygenase large subunit (RbcL, 53 kDa), beta subunit of ATP synthase (ATPb, 54 kDa), and suppressor of quenching 1 (SOQ1, 108 kDa).
Load the samples (5 μg of protein and 5 µL PageRuler prestained protein ladder) and run the polyacrylamide gels.
Note: We run the gels at a lower voltage (80 V) for 20 min and then increase to a higher voltage (120 V) until the dye front has reached the bottom of the gel.
After running the gel, transfer the proteins to a PVDF membrane in transfer buffer (see Recipe 7) by wet transfer at 200 mA for 90 min at 4 °C.
After transferring, incubate the PVDF membrane with blocking buffer (see Recipe 9) for 1 h at room temperature (20–25 °C).
Incubate the PVDF membrane with the primary antibody diluted with blocking buffer for 1 h at room temperature (20–25 °C).
Note: Here, we used anti-PsaD, anti-Lhcb4, anti-RbcL, anti-PC, rabbit-specific antibodies against a C-terminal peptide of SOQ1 (TVTPRAPDAGGLQLQGTR) (1:200 dilution, produced and purified by peptide affinity by ThermoFisher), and anti-ATPb.
Wash the PVDF membrane with TBST buffer (see Recipe 8) (10 min × three times)
Note: We used the horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody.
Incubate the PVDF membrane with the secondary antibody diluted with blocking buffer for 1 h at room temperature (20–25 °C).
Wash the PVDF membrane with TBST buffer (10 min × three times).
Incubate the PVDF membrane with the ECL bright kit for immunodetection.
Data analysis
We used sonication to break the thylakoids and collected thylakoid lumen by ultracentrifugation. The purity of the different fractions was assessed by immunoblot analysis using various antibodies against stromal (RbcL), thylakoid membrane (PsaA, Lhcb4), and thylakoid lumen (PC) proteins (Figure 1). The results in Figure 1 confirmed the purity of thylakoids, thylakoid membrane after isolation of lumen proteins (named membrane), and lumen. There are some lumen contaminants in the stroma fractions, possibly due to some thylakoids being broken when we lysed chloroplasts by osmotic shock. Of note, the lumen fraction is not completely pure. We observed a small amount of the stromal subunit of ATP synthase (ATPb) in the lumen, suggesting that there are some thylakoid membrane contaminants in the isolated lumen fraction (Figure 2).
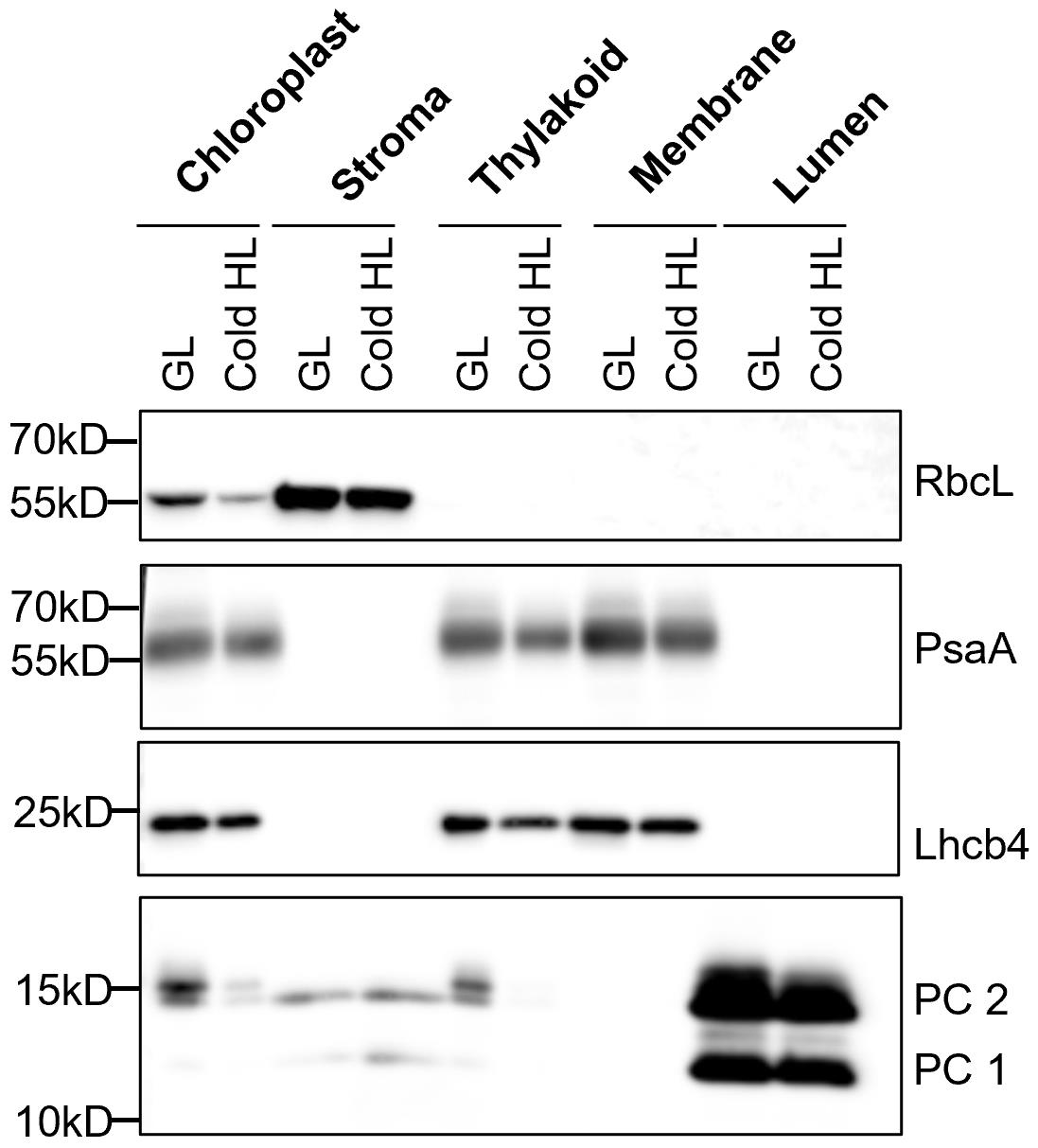
Figure 1. Immunoblot analysis of marker proteins in chloroplast sub-fractions. The protein samples were prepared from 7-week-old Arabidopsis plants (Col-0) under growth light (GL, 120 μmol photons m-2·s-1) and 6 h cold and high light (Cold HL, 4 °C, 1,600 μmol photons m-2·s-1) conditions. Samples were loaded at the same quantity of total protein (5 μg). Four chloroplast proteins (RbcL, PsaA, Lhcb4, and PC) are shown. From Yu et al. (2022).By applying this method, we can investigate the localization and accumulation of chloroplast proteins under different conditions (e.g., Cold HL) (Yu et al., 2022). Using either the Yeda press or sonication methods, we found that SOQ1, a thylakoid membrane-anchored protein involved in negative regulation of photoprotection qH (Brooks et al., 2013; Malnoë et al., 2018), accumulated as full length and also as three distinct truncated lumenal forms [comprising three domains thioredoxin-like (T) NHL (N) C-terminal domain (C) named TNC, two domains NC, or the C-terminal domain only (CTD)] in growth light and Cold HL conditions (Figure 2). Because of the large volumes of buffers used during the preparation with the Yeda press, no protease inhibitors were added except EDTA for metalloproteinases inhibition; together with the long isolation time, a concern was that the truncated SOQ1 forms observed in the lumen fraction were an artifact of the preparation. In comparison, the small volumes of buffers used in the sonication method allow to add inhibitors of different proteases (e.g., Roche cOmpleteTM protease inhibitor cocktail tablet, one tablet for 50 mL buffer). In the presence of protease inhibitors and a shorter isolation time, we confirmed the existence of SOQ1 truncated forms in the lumen fraction. The full-length SOQ1 present in the lumen fraction is likely due to contamination from the thylakoid membrane. These results were published in Yu et al. (2022).
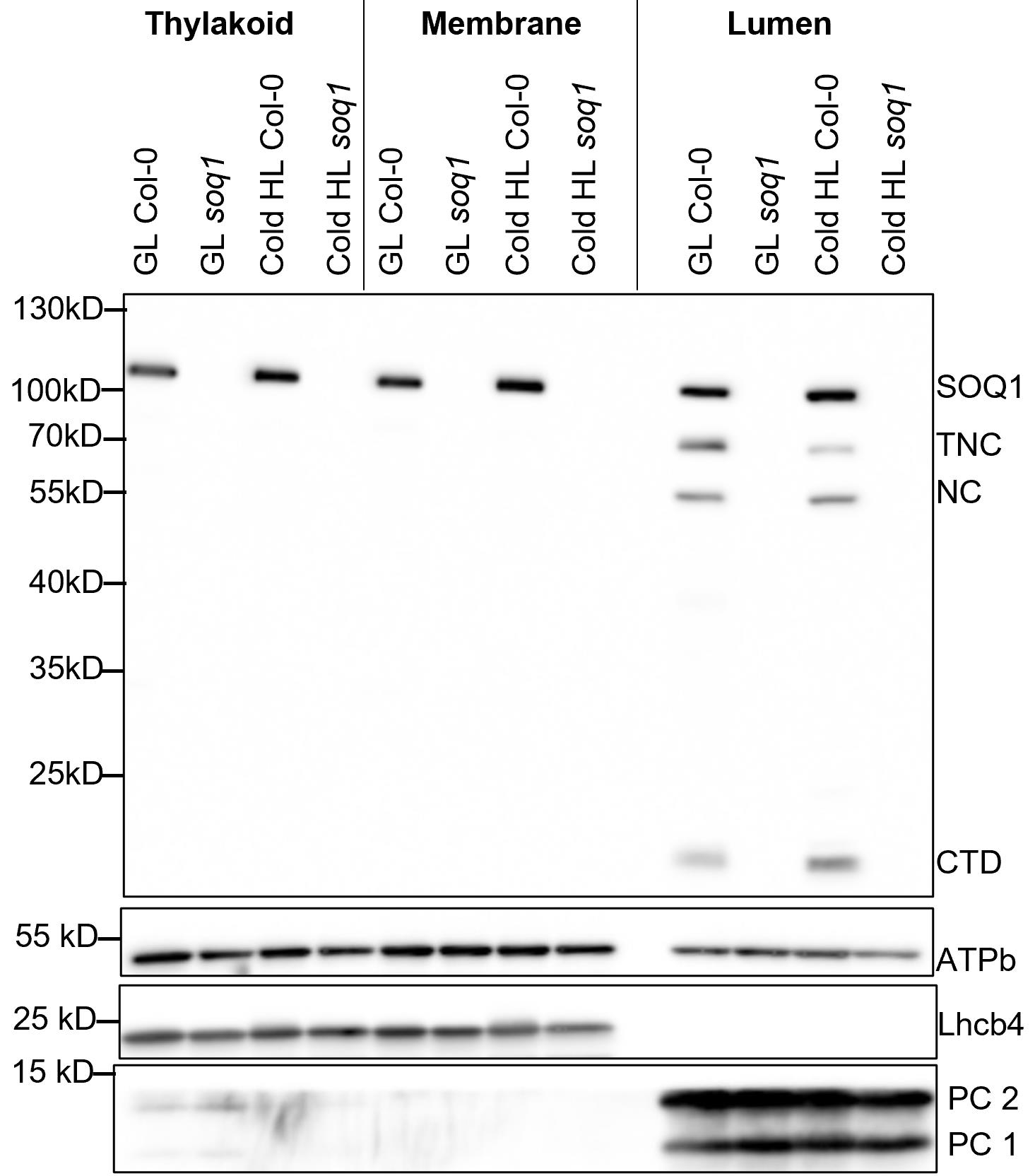
Figure 2. The accumulation of SOQ1 in chloroplast sub-fractions. The protein samples were prepared from 7-week-old wild type (Col-0) and soq1 mutant under growth light (GL, 120 μmol photons m-2·s-1) and 6 h cold and high light (Cold HL, 4 °C, 1,600 μmol photons m-2·s-1) conditions. Samples were loaded at the same quantity of total protein (5 μg). Four chloroplast proteins (SOQ1, Lhcb4, PC, and ATPb) are shown. Three distinct truncated forms (TNC, NC, and CTD) of SOQ1 are accumulated in the lumen in GL and Cold HL conditions. TNC: thioredoxin-like, NHL and C-terminal domains (calculated molecular weight is 73 kDa); NC: NHL and C-terminal domains (calculated molecular weight is 53 kDa); CTD: C-terminal domain (calculated molecular weight is 17 kDa). From Yu et al. (2022).
Notes
All steps for thylakoid lumen isolation are performed in the cold room (4 °C). The centrifuge equipment with the rotor is pre-cooled to 4 °C. The paintbrushes should be washed after each isolation step. All buffers for thylakoid lumen isolation are prepared one day before and stored in the cold room. BSA, sodium ascorbate, and L-cysteine should be added in the extraction buffer right before use [see also notes from Hall et al. (2011)]. The protease inhibitors can be prepared with the stock concentration of 100 mM benzamidine (stored at -20 °C), 500 mM ϵ-aminocaproic acid, and 100 mM PMSF stored at 4 °C for several months [see also notes from Bouchnak et al. (2018)]. The protease inhibitors with final concentration of 1 mM benzamidine, 5 mM ϵ-aminocaproic acid, and 0.2 mM PMSF and/or 1× Roche cOmpleteTM protease inhibitor cocktail tablet must be added in all buffers right before use.
Recipes
(*) Added right before use
Extraction buffer
50 mM tricine-NaOH (pH 7.8)
330 mM sorbitol
1 mM EDTA
10 mM KCl
*0.15% (w/v) bovine serum albumin (traps fatty acids)
*4 mM sodium ascorbate (limits protein oxidation)
*7 mM L-cysteine (limits protein oxidation)
*Protease inhibitors
Resuspension buffer
50 mM sodium phosphate (pH 7.8)
330 mM sorbitol
10 mM sodium fluoride (NaF) (inhibits phosphatase)
*Protease inhibitors
Lysis buffer
10 mM sodium phosphate (pH 7.8)
5 mM MgCl2
10 mM NaF
*Protease inhibitors
Washing buffer
50 mM sodium phosphate (pH 7.8)
100 mM sorbitol
10 mM NaF
5 mM MgCl2
*Protease inhibitors
4× sample loading buffer for SDS-PAGE
8% (w/v) SDS
20% (w/v) 2-mercaptoethanol
40% (v/v) glycerol
0.008% (w/v) bromophenol blue
25 mM Tris-HCl (pH 6.8)
Running buffer for SDS-PAGE
25 mM Tris base
192 mM glycine
0.1% (w/v) SDS
Transfer buffer for immunodetection
25 mM Tris base
192 mM glycine
20% (v/v) ethanol
0.0375% (w/v) SDS
Tris-buffered saline with 0.1% Tween 20 (TBST) buffer
20 mM Tris-HCl (pH 7.5)
150 mM NaCl
0.1% (v/v) Tween 20
Blocking buffer
5% (w/v) non-fat dried milk in TBST
Coomassie blue stain
0.1% (w/v) Coomassie blue R-250
40% (v/v) ethanol
10% (v/v) acetic acid
50% (v/v) Milli Q water
Coomassie blue destain
30% (v/v) ethanol
10% (v/v) acetic acid
60% (v/v) Milli Q water
1 M Tris-HCl stock solution (6.8 and 7.5, 500 mL)
Mix 60.57 g of Tris base with 400 mL of Milli Q water
Adjust the pH to 6.8 or 7.5 by adding concentrated HCl
Add Milli Q water until final volume is 500 mL
Note: The stock solution is used to prepare 25 mM and 120 mM Tris-HCl (pH 6.8) or 20 mM Tris-HCl (pH 7.5)
100 mM Sodium phosphate stock solution (pH 7.8)
10.4 mM NaH2PO4
89.6 mM Na2HPO4
Note: The stock solution is used to prepare 10 mM and 50 mM sodium phosphate (pH 7.8).
Acknowledgments
This protocol was adapted from Peltier et al. (2000) and Levesque-Tremblay et al. (2009). We thank W.P. Schroder for help with lumen preparation using the Yeda press method and critical reading of this protocol. This work was supported by a starting grant to A.M. from the Swedish Research Council Vetenskapsrådet (2018-04150), European Commission Marie Skłodowska-Curie Actions Individual Fellowship Reintegration Panel (845687) to A.M., by a consortium grant from the Swedish Foundation for Strategic Research (ARC19-0051) and by grants to UPSC from the Knut and Alice Wallenberg Foundation (2016.0341 and 2016.0352), and the Swedish Governmental Agency for Innovation Systems (2016-00504).
Competing interests
The authors declare that there is no conflict of interest.
Ethics considerations
The material used in this protocol is Arabidopsis thaliana.
References
- Albiniak, A. M., Baglieri, J. and Robinson, C. (2012). Targeting of lumenal proteins across the thylakoid membrane. J. Exp. Bot 63(4): 1689–1698.
- Almagro Armenteros, J. J., Salvatore, M., Emanuelsson, O., Winther, O., von Heijne, G., Elofsson, A. and Nielsen, H. (2019). Detecting sequence signals in targeting peptides using deep learning. Life Sci. Alliance 2(5): e201900429.
- Bouchnak, I., Moyet, L., Salvi, D., Kuntz, M. and Rolland, N. (2018). Preparation of Chloroplast Sub-compartments from Arabidopsis for the Analysis of Protein Localization by Immunoblotting or Proteomics. J. Vis. Exp. 140: e58581
- Bricker, T. M., Prevost, M., Vu, V., Laborde, S., Womack, J. and Frankel, L. K. (2001). Isolation of lumenal proteins from spinach thylakoid membranes by Triton X-114 phase partitioning. Biochim. Biophys. Acta-Bioenerg. 1503(3): 350–356.
- Brooks, M. D., Sylak-Glassman, E. J., Fleming, G. R. and Niyogi, K. K. (2013). A thioredoxin-like/β-propeller protein maintains the efficiency of light harvesting in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 110: 2733–2740.
- Buchanan, B. B. and Luan, S. (2005). Redox regulation in the chloroplast thylakoid lumen: a new frontier in photosynthesis research. J. Exp. Bot. 56: 1439–1447.
- Chang, W., Li, C., Cui, Z., Li, W., Song, H., Chang, H., Fu, W., Wang, C., Huang, T., Luo, Y., et al. (2021). Diverged Early From CtpB and CtpC, CtpA Has Evolved to Process D1 Precursor in Oxygenic Photosynthetic Organisms. Front. Plant Sci. 12: e676036.
- Fristedt, R., Willig, A., Granath, P., Crèvecoeur, M., Rochaix, J. D. and Vener, A. V. (2009). Phosphorylation of Photosystem II Controls Functional Macroscopic Folding of Photosynthetic Membranes in Arabidopsis. Plant Cell 21(12): 3950–3964.
- Gollan, P. J., Trotta, A., Bajwa, A. A., Mancini, I. and Aro, E. M. (2021). Characterization of the Free and Membrane-Associated Fractions of the Thylakoid Lumen Proteome in Arabidopsis thaliana. Int. J. Mol. Sci 22(15): 8126.
- Goulas, E., Schubert, M., Kieselbach, T., Kleczkowski, L. A., Gardeström, P., Schröder, W. and Hurry, V. (2006). The chloroplast lumen and stromal proteomes of Arabidopsis thaliana show differential sensitivity to short- and long-term exposure to low temperature. Plant J. 47(5): 720–734.
- Granlund, I., Hall, M., Kieselbach, T. and Schröder, W. P. (2009). Light Induced Changes in Protein Expression and Uniform Regulation of Transcription in the Thylakoid Lumen of Arabidopsis thaliana. PLoS One 4(5): e5649.
- Hall, M., Mishra, Y. and Schröder, W. P. (2011). Preparation of stroma, thylakoid membrane, and lumen fractions from Arabidopsis thaliana chloroplasts for proteomic analysis. Methods Mol. Biol. 775: 207–222.
- Järvi, S., Gollan, P. J. and Aro, E. M. (2013). Understanding the roles of the thylakoid lumen in photosynthesis regulation. Front. Plant Sci. 4: e00434.
- Kang, Z. H. and Wang, G. X. (2016). Redox regulation in the thylakoid lumen. J. Plant Physiol 192: 28–37.
- Kieselbach, T., Hagman, Ã., Andersson, B. and Schröder, W. P. (1998). The Thylakoid Lumen of Chloroplasts. J. Biol. Chem 273(12): 6710–6716.
- Kieselbach, T. and Schröder, W. P. (2003). The proteome of the chloroplast lumen of higher plants. Photosynth. Res 78(3): 249–264.
- Kruger, N. J. (1994). The Bradford method for protein quantitation. Methods Mol. Biol. 32: 9–15.
- Levesque-Tremblay, G., Havaux, M., and Ouellet, F. (2009). The chloroplastic lipocalin AtCHL prevents lipid peroxidation and protects Arabidopsis against oxidative stress. Plant J. 60: 691–702.
- Malnoë, A., Schultink, A., Shahrasbi, S., Rumeau, D., Havaux, M. and Niyogi, K. K. (2018). The Plastid Lipocalin LCNP Is Required for Sustained Photoprotective Energy Dissipation in Arabidopsis. Plant Cell 30(1): 196–208.
- McKinnon, L. J., Fukushima, J., Endow, J. K., Inoue, K. and Theg, S. M. (2020). Membrane Chaperoning of a Thylakoid Protease Whose Structural Stability Is Modified by the Protonmotive Force. Plant Cell 32(5): 1589–1609.
- Peltier, J. B., Emanuelsson, O., Kalume, D. E., Ytterberg, J., Friso, G., Rudella, A., Liberles, D. A., Söderberg, L., Roepstorff, P., von Heijne, G., et al. (2002). Central Functions of the Lumenal and Peripheral Thylakoid Proteome of Arabidopsis Determined by Experimentation and Genome-Wide Prediction. Plant Cell 14(1): 211–236.
- Peltier, J. B., Friso, G., Kalume, D. E., Roepstorff, P., Nilsson, F., Adamska, I. and van Wijka, K. J. (2000). Proteomics of the Chloroplast: Systematic Identification and Targeting Analysis of Lumenal and Peripheral Thylakoid Proteins. Plant Cell 12(3): 319–341.
- Porra, R. J., Thompson, W. A. and Kriedemann, P. E. (1989). Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta-Bioenerg. 975(3): 384–394.
- Schubert, M., Petersson, U. A., Haas, B. J., Funk, C., Schröder, W. P. and Kieselbach, T. (2003). Proteome map of the chloroplast lumen of Arabidopsis thaliana. J. Biol. Chem. 278(15): 13590.
- Wu, J., Rong, L., Lin, W., Kong, L., Wei, D., Zhang, L., Rochaix, J. D. and Xu, X. (2021). Functional redox links between lumen thiol oxidoreductase1 and serine/threonine-protein kinase STN7. Plant Physiol 186(2): 964–976.
- Yu, G., Hao, J., Pan, X., Shi, L., Zhang, Y., Wang, J., Fan, H., Xiao, Y., Yang, F., Lou, J., et al. (2022). Structure of Arabidopsis SOQ1 lumenal region unveils C-terminal domain essential for negative regulation of photoprotective qH. Nat. Plants. 8(7): 840–855.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Hao, J. and Malnoë, A. (2023). A Simple Sonication Method to Isolate the Chloroplast Lumen in Arabidopsis thaliana. Bio-protocol 13(15): e4756. DOI: 10.21769/BioProtoc.4756.
Category
Plant Science > Plant biochemistry > Protein > Isolation and purification
Plant Science > Plant physiology > Abiotic stress
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









