- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Construction of Activity-based Anorexia Mouse Models
(*contributed equally to this work) Published: Vol 13, Iss 15, Aug 5, 2023 DOI: 10.21769/BioProtoc.4730 Views: 1757
Reviewed by: Pilar Villacampa AlcubierreAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Assessing Rough-and-tumble Play Behavior in Juvenile Rats
Jonathan W. VanRyzin [...] Margaret M. McCarthy
Jan 5, 2020 5524 Views

Protocol for Measuring Free (Low-stress) Exploration in Rats
Wojciech Pisula and Klaudia Modlinska
Jan 20, 2020 4452 Views

Operant Vapor Self-administration in Mice
Renata C. N. Marchette [...] Khaled Moussawi
May 20, 2021 4553 Views
Abstract
Anorexia nervosa (AN) is a psychiatric disorder mainly characterized by extreme hypophagia, severe body weight loss, hyperactivity, and hypothermia. Currently, AN has the highest mortality rate among psychiatric illnesses. Despite decades of research, there is no effective cure for AN nor is there a clear understanding of its etiology. Since a complex interaction between genetic, environmental, social, and cultural factors underlines this disorder, the development of a suitable animal model has been difficult so far. Here, we present our protocol that couples a loss-of-function mouse model to the activity-based anorexia model (ABA), which involves self-imposed starvation in response to exposure to food restriction and exercise. We provide insights into a neural circuit that drives survival in AN and, in contrast to previous protocols, propose a model that mimics the conditions that mainly promote AN in humans, such as increased incidence during adolescence, onset preceded by negative energy balance, and increased compulsive exercise. This protocol will be useful for future studies that aim to identify neuronal populations or brain circuits that promote the onset or long-term maintenance of this devastating eating disorder.
Keywords: Anorexia nervosa (AN)Background
Anorexia nervosa (AN) is a severe psychiatric disorder characterized by extreme hypophagia, severe body weight loss, distorted self-image, and maladaptive food choices (Mitchell and Peterson, 2020). The onset of AN usually occurs in adolescence or young adulthood (Favaro et al., 2009) and it mainly affects females (roughly 92%) (Udo and Grilo, 2018). It has the highest mortality rate among psychiatric disorders (Treasure et al., 2015), 12 times higher than the death rate associated with all causes of death for females 15–24 years old (Smink et al., 2013; Fichter and Quadflieg, 2016). Currently, there are no effective pharmacological cures for AN (Crow, 2019). To inform new therapies and to identify individuals at risk for the disorder, a deeper understanding of the neurobiology underlying AN is urgently needed. The etiology of AN is complex since it involves interactions between genetic, environmental, social, and cultural factors. Such complexity has made it difficult to develop an appropriate animal model so far (Siegfried et al., 2003). Previous studies have used approaches involving genetic, environmental, and/or dietary manipulations to study AN in animal models. Genetically modified mouse models have been used to ablate or activate specific neuronal populations, with immediate reduction of food intake (Luquet et al., 2005; Calvez et al., 2011). While most of these studies might provide insights into neural circuits causing anorexia (simply seen as the loss of appetite for food), their translatability to humans is minimal since the nature and timing of the stressors are different from those that increase the risk of developing AN in humans (François et al., 2022).
The best characterized animal model of AN is the activity-based anorexia model (ABA) (Klenotich and Dulawa, 2012). This is a bio-behavioral phenomenon described in rodents that models the key symptoms of AN (self-induced starvation and compulsive running, to list a few of them) and is based on the principle that mice will prefer to run instead of eating even when food is available, if previously exposed to food restriction. The ABA, however, cannot capture the sociocultural factors that might promote AN.
Here, we propose a method to couple a genetically modified mouse model to a specific ABA protocol designed for adolescent female and male mice. We chose to investigate the involvement of the hypothalamic agouti-related peptide and neuropeptide Y (AgRP/NPY)-expressing neurons. These neurons are physiologically active during fasting, but also modulate complex non-feeding behaviors (Dietrich et al., 2015). To impair AgRP neuron function, we studied AgRP neuron-specific diphtheria toxin receptor-expressing mice (AgRP-DTR), which allow ablation of AgRP neurons early postnatally by administering diphtheria toxin at P5 (Luquet et al., 2005). By combining ABA with a transgenic mouse model and further choosing a critical time window (adolescence), it is feasible to get insights into genetic × environmental factors (such as early life stress) that contribute to the onset or propagation of AN. This protocol will be useful for future studies that aim to identify the contribution of specific neuronal populations to AN symptomatology. Combining the two approaches in adolescent mice will also allow the unraveling of mechanisms that contribute to resilience or vulnerability to AN.
Materials and reagents
Mouse cages (Tecniplast, catalog number: GM500)
Wire bar lids (Tecniplast, catalog number: GM500)
Bedding, Teklab corncob bedding (Envigo, catalog number: 7092)
Standard rodent diet, Teklab global 18% protein rodent diet (Envigo, catalog number: 2018S)
Paper towels
4-week-old female and male mice, Agouti-related protein mice (AgRP-DTR mice)
70% ethanol (VWR Chemicals, catalog number: 20842.298, to be dissolved in water to final concentration)
NaCl powder (Sigma, catalog number: 7647-14-5)
Diphtheria toxin (List Biological, catalog number: 150)
Equipment
In-cage running wheel (Starr Life Science Corporation, USA)
Infrared sensor for running wheel (Starr Life Science Corporation, USA)
Standard Windows PC (operating system Windows 7 or later)
DataPort1224 (DP1224) (Starr Life Science, USA)
Powered USB hub (Conrad Electronic AG, Digitus DA-70229 10 Port USB 2.0-Hub/catalog number:1027267-5k) (to provide sufficient USB ports and equipment spacing flexibility, one USB port support for each DP1224s)
Analytical balance (1 mg) (MettlerToledo, Balance XPE206DR, catalog number: 30132913)
Compact scale (0.1 g) (Haslab, HCB 302 Highland portable precision, catalog number: 105654302)
Red headlamp (Energizer, online vendor)
Software
VitalView Data Acquisition System software version 5.1 (Starr Life Science, USA https://www.starrlifesciences.com/product/activity-software/)
GraphPad Prism version 9 (GraphPad software, San Diego, CA, https://www.graphpad.com)
Note: Do not run other software on your Windows PC while VitalView is acquiring data.
Procedure
AgRP ablation
Weigh the mice at P5.
Dissolve the diphtheria toxin powder in water or saline (0.90% w/v of NaCl) to a final concentration of 5 μg/mL (stock solution).
Inject subcutaneously a dose of 5 μg/kg in heterozygous and homozygous mice for the selected mutation (expression of the diphtheria toxin receptor in AgRP neurons) (Figure 1). The preferable site for injection is over the shoulders, into the loose skin over the neck. A new needle should be used for each animal to reduce the risk of site infection. Allow full absorption of the substance before putting back the mouse into the cage.
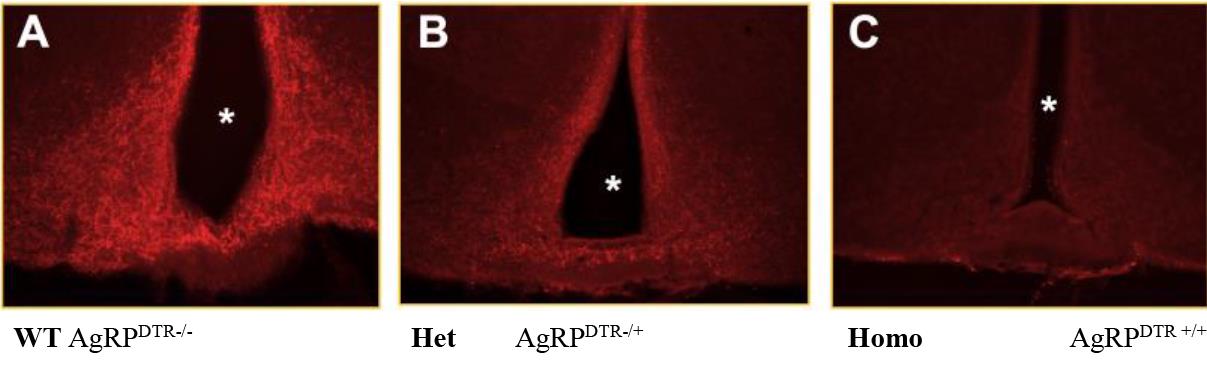
Figure 1. Ablation of AgRP neurons by diphtheria toxin injections on P5 in the arcuate nucleus. Both control and AgRPDTR/+ mice were injected as pups with DT (50 μg/kg) on P5. Representative immunostaining of ARC neurons of control (WT) and AgRPDTR/+ mice are shown. The asterisks indicate third ventricle. Scale bar, 50 μm. AgRP: Agouti related peptide. DTR: diphtheria toxin receptor. ARC: arcuate.Weigh the mice and their food intake daily up to day 21 to assess standard growth.
Activity-based anorexia model
Place cages in a temperature-controlled room dedicated only to ABA testing. Set the light timers to the 12:12 h light/dark cycle.
Cage setup: clean the cage and the running wheel with 70% ethanol and afterward dry with a paper towel. Add the bedding (up to 4 cm) and the in-cage running wheel. Verify that the wheel can freely turn without touching the bedding.
Insert the infrared sensor on the side of the running wheel and connect it to the DP1224.
Boot the computer and start the running wheel software by double-clicking on the icon.
Label every cage and the corresponding sensor in alphabetic or numerical order. Set up the configuration of the data acquisition (a detailed data acquisition software manual is available online from Starr Life website).
Single-house 36-day-old mice in a cage containing bedding, a water bottle, and ad lib food.
Allocate the mice in two groups:
Running group have ad lib access to food and water and access to the running wheel.
Sedentary group have ad lib access to food and water without access to the running wheel.
Acclimation: allow animals to acclimate for four days.
For the following four days, measure weight and food intake every morning.
Use a dedicated container for the food, to avoid food contamination with feces and urine. Minimize the time that each cage is moved, weighing first the food and afterward the mouse from the same cage. When removing the mouse from the cage, manually stop the running wheel to prevent recording biased running wheels counts.
Food restriction (72 h/three days).
Approximately at noon on the fourth day of acclimation, remove the food from both groups (sedentary and running).
Before the onset of the dark phase, pre-weigh 50 g of pellet. Store food at room temperature in cups labeled with the mouse ID and cover with aluminum foil. Use a fresh pellet for every day of food restriction to avoid contamination.
At 7:00 pm, when the lights are usually turned off, place the pre-weighed pellets in the overhead food bin of each cage for 2 h.
At 9:00 pm, carefully remove the leftover pellets, place it in the labeled containers, and cover with aluminum foil. Clean the food bin with a dry paper. Wear a red headlamp while you are in the room during the dark phase.
In the morning after food restriction, weigh the mice and the food intake from the leftover pellet. Ensure that there is no food left in the bedding of the cage.
To determine weight loss, calculate the % baseline body weight dividing the current weight by the body weight recorded on the last day of acclimation. Any mouse that loses more than 25% of its body weight is at risk of death and must be removed from the experiment. Mice removed from the experiment are placed in a new cage without the running wheel and with ad lib food and water.
Recovery: approximately at noon of the third day of food restriction, remove all remaining mice from the experiment and place them in a new cage without the running wheel and with ad lib food and water. Allow the animals to recover for at least one week before undergoing additional behavioral testing.
Under the File menu in the VitalView program window, click “stop acquisition.”
Data analysis
Body weight data
Perform a survival analysis (e.g., Kaplan-Meier) of both running and sedentary groups across the three days of food restriction. Percentage survival (on the y-axis) indicates the percentage of animals within each group that were not removed from the experiment. Mice removed earlier from the experiment because they lost more than 25% of their initial body weight show higher vulnerability to ABA. The survival analysis allows evaluation of any intervention (drug and/or diet) that might improve adaptation to ABA (Figure 2, right).
Both during acclimation and food restriction, calculate the body weight separately for females and males and express it as grams (Figure 2, left).
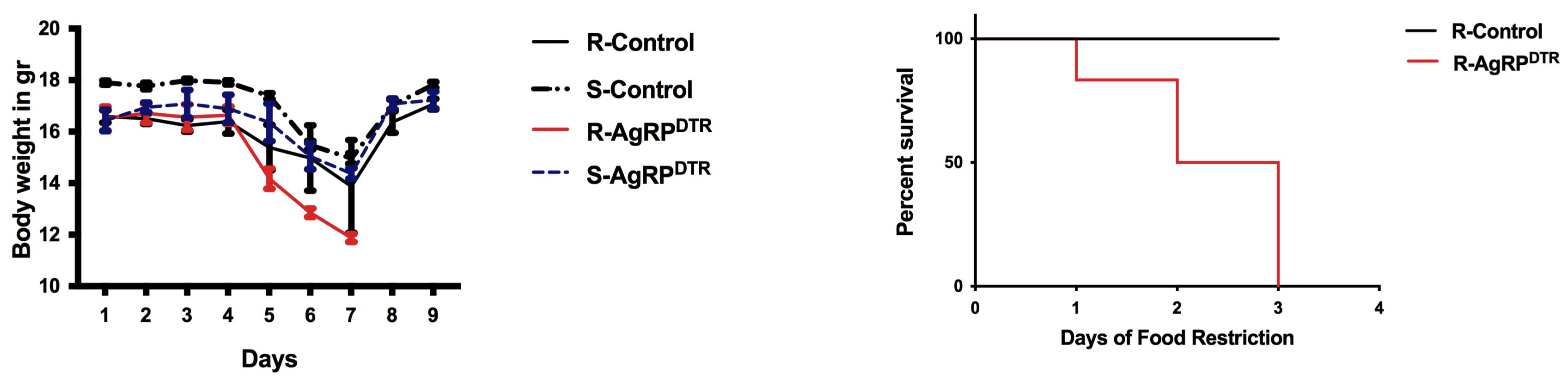
Figure 2. Body weight and survival curve data. Error bars indicate SEM; running(R)-control, n = 16; sedentary (S)-control, n = 12; R-AgRPDTR, n = 16; S-AgRPDTR, n = 14.
Food intake data
Calculate the food intake as kcal/day. For standard diet (7% simple sugars, 3% fat, 50% polysaccharide, 15% protein) energy = 3.5 kcal/g. During acclimation, food intake is indicated as kcal/day, while during food restriction is indicated as kcal/2 h (Figure 3).
Make separate calculations for males and females.
After calculating the average, compare running and sedentary groups using a two-way ANOVA, followed by multiple comparisons test.
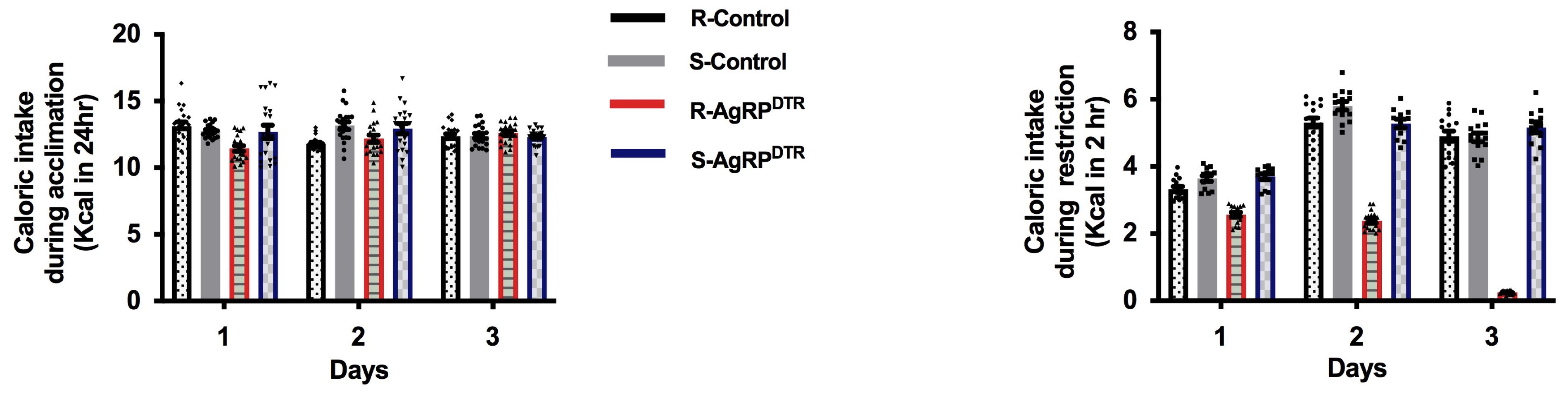
Figure 3. Food intake. Food intake shown as kcal in 24 h during acclimation (left) or kcal in 2 h during food restriction (right). Error bars indicate SEM; running(R)-control, n = 16; sedentary (S)-control, n = 12; R-AgRPDTR, n = 16; S-AgRPDTR, n = 14.
Running wheel data
Use the VitalView Data Acquisition System software to extract the running wheel data. The program converts the data to an Excel file by selecting the Export option in the File menu. The running wheel count is reported by day. Calculate the average total running across the acclimation and the food restriction. Use a two-way ANOVA test for group comparisons followed by multiple comparisons test.
Additionally, and separately, calculate the average light cycle (12 h) and dark cycle (12 h) running for each group and use two-way ANOVA test for group comparisons followed by multiple comparisons test. In our as well as in different experimental paradigms, an abrupt increase of running during the light phase is associated with poor adaptation to the ABA and often precedes discharge from the experiment for most mice. Running that precedes presentation of food is indicated as food anticipatory activity.
Optional: Calculate the running wheel count over the 2-h period of food restriction and put them in correlation with the food intake. One of the features of ABA is that mice prefer to run instead of eating even if food is available.
Make separate calculations for males and females (Figure 4).
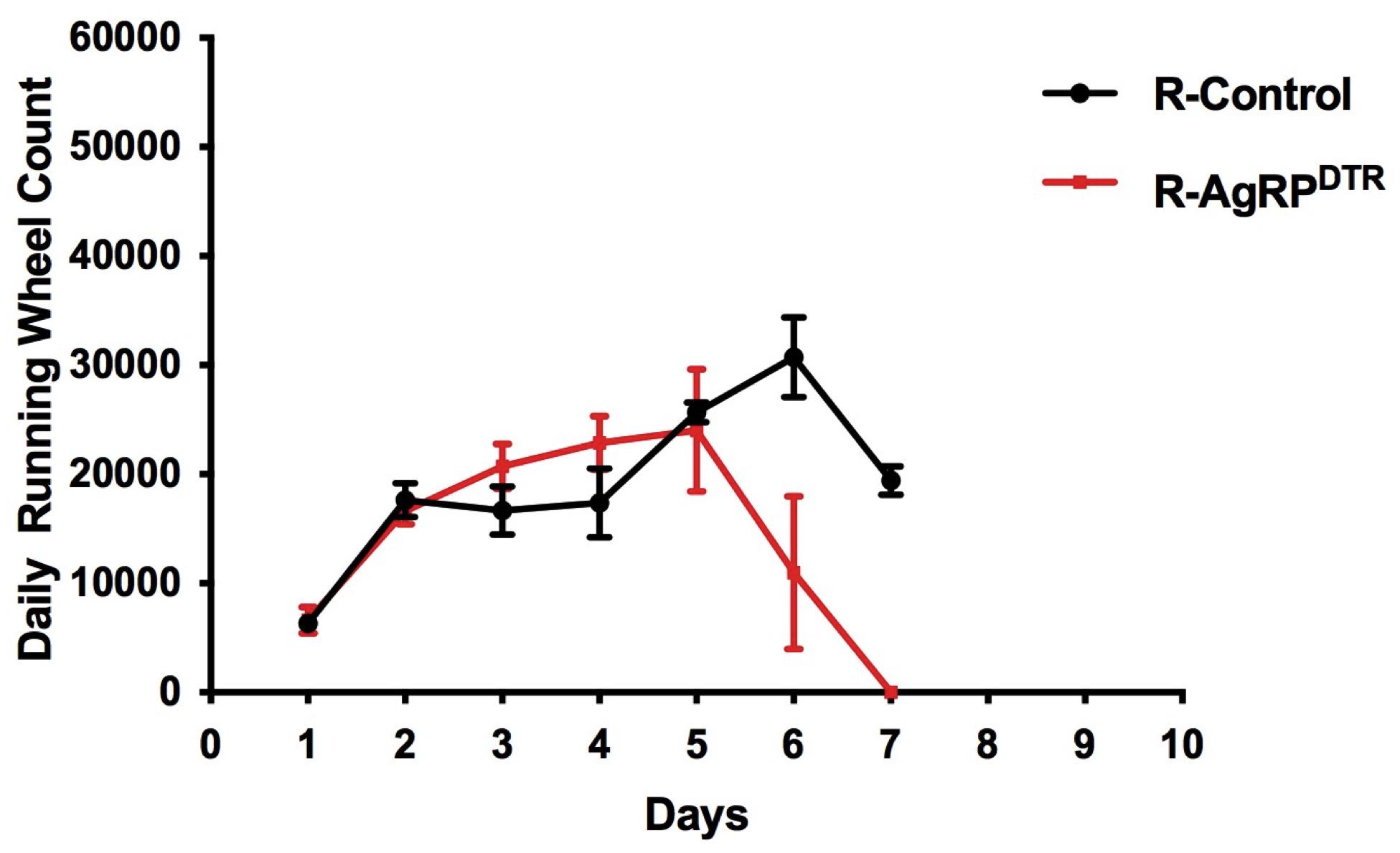
Figure 4. Running wheel data. Total running wheel count averaged across acclimation (days 1–4) and food restriction (days 5–7). Error bars indicate SEM; running(R)-control, n = 16; sedentary (S)-control, n = 12; R-AgRPDTR, n = 16; S-AgRPDTR, n = 14. The units for the cumulative (24 h) wheel count are revolutions per minute (RPM).
Notes
Notes on the Procedure
Carefully assess the weight of the mice before injection with the diphtheria toxin. In our experience, mice that weigh less than 2.5 g at P5 are more prone to die after the injection.
The temperature in the room dedicated to ABA should be strictly controlled and range from 22 to 25 °C. Room temperature has been shown to affect vulnerability to ABA (Gutiérrez et al., 2002).
In this study, we used littermate mice in the experiments. This choice ensures that both the genetic background and environment are comparable through several experiments.
Handling of animals should be kept to a minimum; if possible, one operator should handle the mice throughout the experiment. Food intake and weight should be measured at the same time each day. Scents and perfumes should be avoided. All the above precautions minimize animal stress exposure by reducing unpredictability.
Adolescent mice are more vulnerable to weight loss and death induced by starvation compared to adult mice or rats. Therefore, the current protocol applies some strategies to improve their survival during ABA. These include providing food for 2 h instead of 1 h during food restriction and limiting food restriction to 72 h. Using a different age group from that specified in this protocol may require changes in food restriction duration.
Give food-restricted mice a large pellet to reduce the possibility that food will fall into the cage and mice continue to eat beyond the allowed time window.
To prevent mouse dehydration, food can be provided wet during food restriction.
To prevent data loss, power the computer through a backup power supply. Even a short interruption of power will force the computer to restart, and data acquisition will stop.
Notes on Data analysis
It is always advisable to plot the data for female and male mice separately, since their food intake and body weight differ at the baseline and after food restriction.
All data shown in Figures have been previously published (Miletta et al., 2020).
Notes on validation
Technically, it is challenging to get a consistent level of AgRP ablation through several mice generations. However, being consistent about the time of the diphtheria toxin injection can minimize it.
Acknowledgments
This work was partly funded by the Swiss National Science Foundation (Early Postdoc.Mobility P2BEP3_172252 to M.C.M.), National Institutes of Health grants AG052005 and DK111178, and a Klarman Family Foundation grant (to T.L.H.). T.L.H. was also supported by grant NKFI-126998 from the Hungarian National Research, Development and Innovation Office. The described model was adapted from previous studies in which activity-based anorexia model was tested with different conditions (Klenotich and Dulawa, 2012). The mouse strain was first described by Luquet et al. (2005) but not in correlation with the ABA.
Competing interests
The authors have no financial or no-financial competing interests to report.
Ethics considerations
This animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Yale University (2022-07942).
References
- Calvez, J., Fromentin, G., Nadkarni, N., Darcel, N., Even, P., Tome, D., Ballet, N. and Chaumontet, C. (2011). Inhibition of food intake induced by acute stress in rats is due to satiation effects. Physiol Behav 104(5): 675-683.
- Crow, S. J. (2019). Pharmacologic Treatment of Eating Disorders. Psychiatr Clin North Am 42(2): 253-262.
- Dietrich, M. O., Zimmer, M. R., Bober, J. and Horvath, T. L. (2015). Hypothalamic Agrp neurons drive stereotypic behaviors beyond feeding. Cell 160(6): 1222-1232.
- Favaro, A., Caregaro, L., Tenconi, E., Bosello, R. and Santonastaso, P. (2009). Time trends in age at onset of anorexia nervosa and bulimia nervosa. J Clin Psychiatry 70(12): 1715-1721.
- Fichter, M. M. and Quadflieg, N. (2016). Mortality in eating disorders - results of a large prospective clinical longitudinal study. Int J Eat Disord 49(4): 391-401.
- François, M., Fernandez-Gayol, O. and Zeltser, L. M. (2022). A Framework for Developing Translationally Relevant Animal Models of Stress-Induced Changes in Eating Behavior. Biol Psychiatry 91(10): 888-897.
- Gutiérrez, E., Vazquez, R. and Boakes, R. A. (2002). Activity-based anorexia: ambient temperature has been a neglected factor. Psychon Bull Rev 9(2): 239-249.
- Klenotich, S. J. and Dulawa, S. C. (2012). The activity-based anorexia mouse model. Methods Mol Biol 829: 377-393.
- Luquet, S., Perez, F. A., Hnasko, T. S. and Palmiter, R. D. (2005). NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310(5748): 683-685.
- Miletta, M. C., Iyilikci, O., Shanabrough, M., Sestan-Pesa, M., Cammisa, A., Zeiss, C. J., Dietrich, M. O. and Horvath, T. L. (2020). AgRP neurons control compulsive exercise and survival in an activity-based anorexia model. Nat Metab 2(11): 1204-1211.
- Mitchell, J. E. and Peterson, C. B. (2020). Anorexia Nervosa. N Engl J Med 382(14): 1343-1351.
- Siegfried, Z., Berry, E. M., Hao, S. and Avraham, Y. (2003). Animal models in the investigation of anorexia. Physiol Behav 79(1): 39-45.
- Smink, F. R., van Hoeken, D. and Hoek, H. W. (2013). Epidemiology, course, and outcome of eating disorders. Curr Opin Psychiatry 26(6): 543-548.
- Treasure, J., Zipfel, S., Micali, N., Wade, T., Stice, E., Claudino, A., Schmidt, U., Frank, G. K., Bulik, C. M. and Wentz, E. (2015). Anorexia nervosa. Nat Rev Dis Primers 1: 15074.
- Udo, T. and Grilo, C. M. (2018). Prevalence and Correlates of DSM-5-Defined Eating Disorders in a Nationally Representative Sample of U.S. Adults. Biol Psychiatry 84(5): 345-354.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Miletta, M. C. and Horvath, T. L. (2023). Construction of Activity-based Anorexia Mouse Models. Bio-protocol 13(15): e4730. DOI: 10.21769/BioProtoc.4730.
Category
Neuroscience > Behavioral neuroscience > Animal model > Mouse
Medicine > Anorexia nervosa > Mouse model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link