- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Synthesis of Bacteria-mimetic Gold Nanoparticles for Phagocytosis by Immune Cells
(*contributed equally to this work) Published: Vol 13, Iss 12, Jun 20, 2023 DOI: 10.21769/BioProtoc.4695 Views: 2389
Reviewed by: Alka MehraSameer NadafChhuttan L MeenaKarem A Court

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Ciliary and Flagellar Membrane Vesicle (Ectosome) Purification
William Dentler
Jun 20, 2014 10764 Views

Plasma Membrane Preparation from Lilium davidii and Oryza sativa Mature and Germinated Pollen
Ning Yang [...] Tai Wang
May 20, 2017 9172 Views

Stopped-flow Light Scattering Analysis of Red Blood Cell Glycerol Permeability
Patrizia Gena [...] Giuseppe Calamita
Aug 20, 2020 4815 Views
Abstract
Cell-based carrier exhibits inherent advantages as the next generation of drug delivery system, namely high biocompatibility and physiological function. Current cell-based carriers are constructed via direct payload internalization or conjugation between cell and payload. However, the cells involved in these strategies must be firstly extracted from the body and the cell-based carrier must be prepared in vitro. Herein, we synthesize bacteria-mimetic gold nanoparticles (GNPs) for the construction of cell-based carrier in mice. Both β-cyclodextrin (β-CD)-modified GNPs and adamantane (ADA)-modified GNPs are coated by E. coli outer membrane vesicles (OMVs). The E. coli OMVs induce the phagocytosis of GNPs by circulating immune cells, leading to intracellular degradation of OMVs and subsequent supramolecular self-assembly of GNPs driven by β-CD-ADA host–guest interactions. In vivo construction of cell-based carrier based on bacteria-mimetic GNPs avoids the immunogenicity induced by allogeneic cells and restriction by the number of separated cells. Due to the inflammatory tropism, endogenous immune cells carry the intracellular GNP aggregates to the tumor tissues in vivo.
Graphical overview
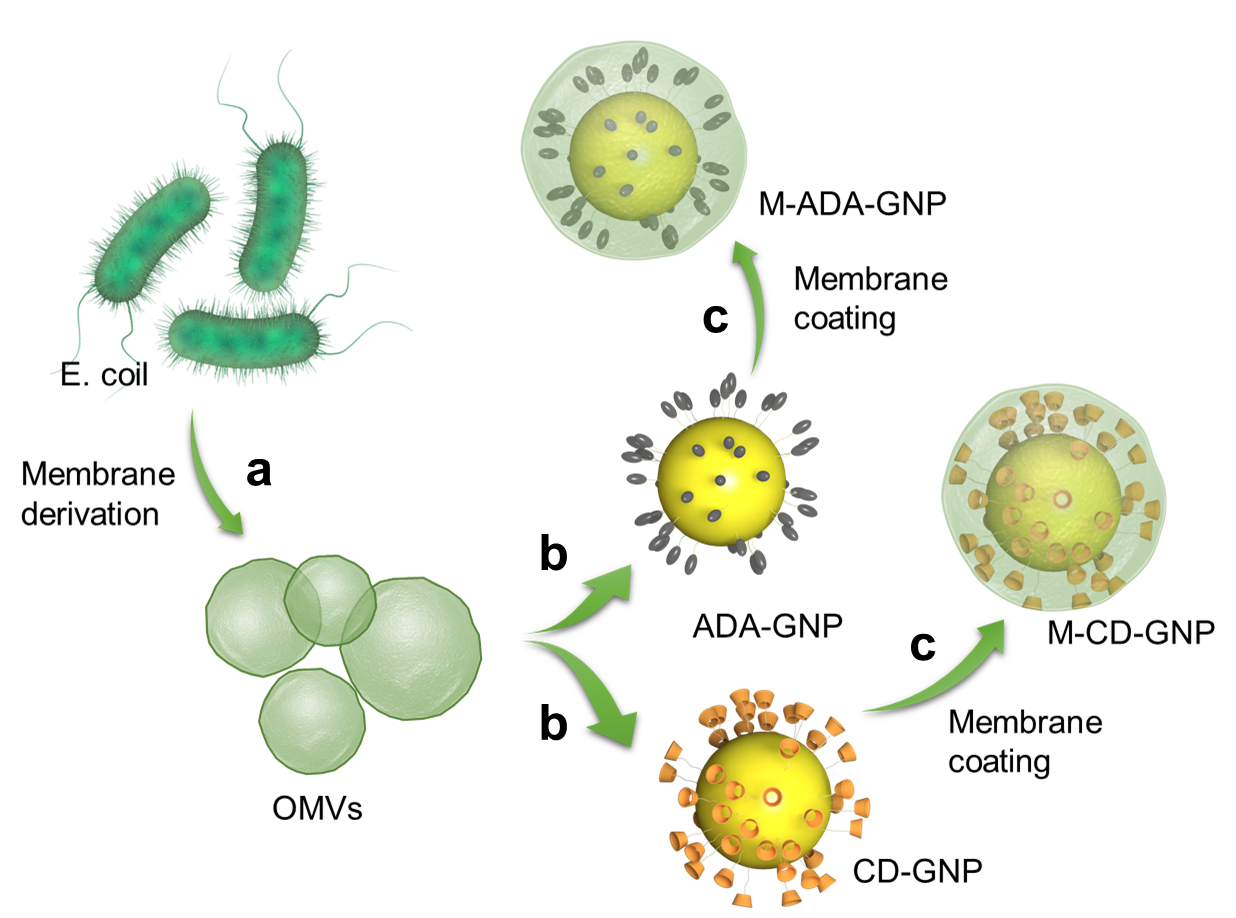
Collect the outer membrane vesicles (OMVs) of E. coli by gradient centrifugation (a) and coat on gold nanoparticles (GNP) surface (b) to prepare OMV-coated cyclodextrin (CD)-GNPs and OMV-coated adamantane (ADA)-GNPs (c) via ultrasonic method
Background
As a self component, cells exhibit inherent physiological properties in drug delivery, such as biocompatibility and homing effect (Yang et al., 2022). These help the drug payload to escape clearance by the mononuclear phagocyte system and perform the physiological function–based hitchhiking delivery. Recently, cell-based carriers were mainly constructed via direct drug internalization; this procedure was very simple, needing only in vitro cell culture with the payload (Li et al., 2021). In addition, another construction strategy was surface conjugation between cell and payload, which could be divided into covalent conjugation, receptor–ligand interaction, host–guest interaction, and physical interaction (Krueger et al., 2018; Li et al., 2018). The host cell must be artificially modified in vitro and then conjugated with payload. Thus, cell-based carriers, via either intracellular drug internalization or cell-surface attachment, were constructed in vitro before in vivo administration. This presented three challenges in clinical translation and application: 1) large-scale preparation is barely possible due to the limitation of separating sufficient number of endogenous cells; 2) in vitro drug loading processes may inevitably affect the physiological function of the carrier cells; and 3) transporting cells correspond to one single host, and would generate immune rejection when applied to other individuals.
In light of these issues, bacteria-mimetic gold nanoparticles (GNPs) were developed for in vivo construction of immune cell–based carriers and efficiently and stably hitchhiking delivery. In this design, both β-cyclodextrin (β-CD)-modified GNPs (CD-GNPs) and adamantane (ADA)-modified GNPs (ADA-GNPs) are coated by E. coli outer membrane vesicles (OMVs). Host–guest interactions involve two molecules or materials that can form complexes through unique structural relationships and noncovalent binding. Due to host–guest interactions between β-CD and ADA (Loescher and Walther, 2020; Wang et al., 2021), the host β-CD could bind with guest ADA to form a supramolecular complex. Thus, CD-GNPs bound with ADA-GNPs to produce GNP aggregates. It has been observed that the GNP aggregates show higher absorption ratio and therefore, higher temperature rise compared to single GNPs. The effect of photothermal therapy (PTT) was significantly improved in the form of GNP aggregates in comparison to GNPs. The E. coli OMVs coating induce the phagocytosis of GNPs by circulating immune cells, leading to intracellular degradation of OMVs and subsequent supramolecular self-assembly of GNPs. In addition to the increased PTT efficiency, GNP aggregation increased the nano-size of GNP into micro-size of GNP aggregate, which significantly inhibits the leakage of GNPs from immune cells (Zhang et al., 2021). This bacterial OMV-coating strategy for immune cell phagocytosis was not only applied to GNPs, but also extended to other therapeutic reagents that directly modulate immune cells or target disease tissues via immune cell hitchhiking delivery.
As different cells (platelets, stem cells, macrophages, T cells, or even tumor cells) have different physiological functions, the coating material could be specifically designed for phagocytosis according to the required type of cells as carriers for hitchhiking delivery.
Materials and reagents
Falcon tube (Falcon, catalog number: 352098)
Millipore with 10 kDa filter (Millipore, catalog number: UFC901024)
Nylon membrane filter with 0.45 pore size (Sigma-Aldrich, catalog number: Z290815-100EA)
HAuCl4·3H2O (Sigma-Aldrich, catalog number: 520918)
Sodium citrate (Aladdin, catalog number: S189183-100g)
Mono(6-mercapto-6-deoxy)-beta-cyclodextrin (Shandong Binzhou Zhiyuan Biotechnology Co., Ltd, catalog number: 81644-55-5)
1-adamantanethiol (Aladdin, catalog number: A169723-5g)
E. coli (ATCC, catalog number: 33694)
Polypropylene bacterial culture tube (Falcon, catalog number: 352057)
DMSO (Sigma-Aldrich, catalog number: D8418-100ML)
Lysogeny broth (LB) (Thermo Fisher Scientific, catalog number: 10855021)
LB medium (see Recipes)
Sodium citrate solution (5%) (see Recipes)
HAuCl4 solution (0.25 mM) (see Recipes)
Mono(6-mercapto-6-deoxy)-beta-cyclodextrin solution (2 mM) (see Recipes)
GNP solution (10 mM) (see Recipes)
1-adamantanethiol in DMSO (2 mM) (see Recipes)
Equipment
Centrifuge (Thermo Fisher Scientific, Heraeus Multifuge X3, model: 10325804)
Dynamic light scattering (Malvern, Nano-ZS)
Ultra-high-speed centrifuge (Beckman, Optima XPN-100 Ultracentrifuge)
Sonication bath (Branson, Bransonic ultrasonic cleaner, model: 5510E-DTH)
Transmission electron microscope, 120 kV (HITACHI, model: HT7800)
Microplate reader (Molecular Devices, SpectraMax, model: M5)
Vacuum oven (Thermo Fisher Scientific, Vacutherm, model: VT6205)
Software
Software associated with plate reader used (SoftMax Pro 7 Software)
Software associated with dynamic laser scanning used (Malvern Zetasizer Software v7.11)
Software associated with transmission electron microscope used (Gatan DigitalMicrograph 3.9)
Procedure
Preparation of GNPs via Turkevich method
Use HAuCl4in combination with sodium citrate as a reducing and capping agent for the nanoparticles.
Prepare HAuCl4 solution (0.25 mM; see Recipes) in a conical flask.
Put 100 mL of HAuCl4 solution (0.25 mM) into a 500 mL round-bottom flask with single neck and stir at 130 °C in an oil bath in a magnetic stirrer.
When HAuCl4 solution starts to boil, add 0.7 mL of sodium citrate solution (5%; see Recipes) into HauCl4 solution.
When the solution turns wine red, add 0.7 mL of sodium citrate solution (5%) again and repeat the addition process of sodium citrate solution twice.
Cool the reaction solution down to room temperature and transfer the entire reaction mixture to multiple 2 mL polypropylene microcentrifuge tubes. Centrifuge at 12,000× g for 30 min to collected precipitated GNPs.
Add 100 mL of ddH2O to disperse the precipitated GNPs and centrifuge at 12,000× g for 30 min again. Repeat the washing twice with deionized water and collect purified GNPs.
Characterize GNPs by transmission electron microscope (TEM) and dynamic light scattering (DLS). TEM imaging shows the morphology of Au nanoparticles and DLS analysis reflects the size distribution.
Place the precipitated GNPs in a 2 mL polypropylene microcentrifuge tube and dry GNPs in the vacuum oven at 25 °C for two days (Figure 1a).
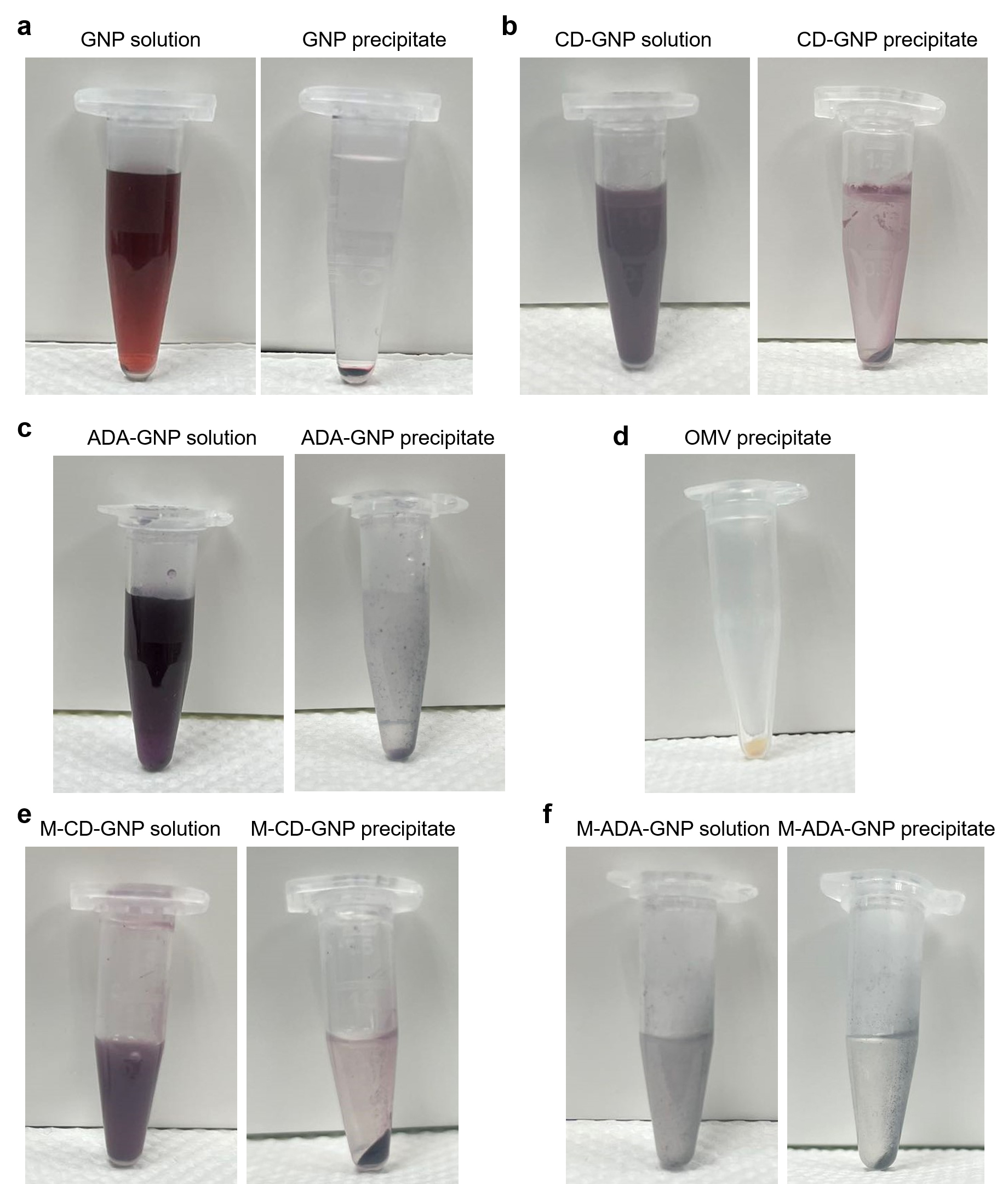
Figure 1. Photos of different gold nanoparticles (GNP) precipitates. a) GNP solution and GNP precipitate. b) CD-GNP solution and CD-GNP precipitate. c) ADA-GNP solution and ADA-GNP precipitate. d) Isolated outer membrane vesicles (OMV) precipitate. e) OMV-coated CD-GNP solution and OMV-coated CD-GNP precipitate. f) OMV-coated ADA-GNP solution and OMV-coated ADA-GNP precipitate.
Preparation of CD-GNPs
Put 1,000 mL of ddH2O in a conical flask and add 2.3 g of mono(6-mercapto-6-deoxy)-beta-cyclodextrin to prepare mono(6-mercapto-6-deoxy)-beta-cyclodextrin solution (2 mM; see Recipes).
Dry GNPs in a vacuum oven and dissolve 1.97 g of GNP power in 1,000 mL of ddH2O to prepare GNPs solution (10 mM; see Recipes).
Add 2 mL of mono(6-mercapto-6-deoxy)-beta-cyclodextrin solution (2 mM) to 2 mL of GNPs solution (10 mM) in a beaker and stir in a magnetic stirrer for 2 h at room temperature.
Centrifuge the resultant solution at 21,000× g for 8 min in a 2 mL polypropylene microcentrifuge tube and collect the precipitated CD-GNPs.
Dry CD-GNPs in a vacuum oven at 25 °C for two days (Figure 1b).
Preparation of ADA-GNPs
Dissolve 1-adamantanethiol in DMSO (2 mM, 2 mL; see Recipes).
Add GNP solution (10 mM, 2 mL) into 1-adamantanethiol solution in a beaker and stir for 2 h.
Centrifuge the resultant solution at 21,000× g for 8 min in a polypropylene microcentrifuge tube and collect the precipitated ADA-GNPs.
Dry ADA-GNPs in a vacuum oven at 25 °C for two days (Figure 1c).
Isolation of bacterial OMVs
Inoculate E. coli in a polypropylene bacterial culture tube containing 50 mL of LB medium (see Recipes) for three days, and then centrifuge resulting bacterial culture in a 50 mL Falcon tube at 2,000× g for 5 min. Disperse the precipitated bacteria in ddH2O to achieve a OD600 value of 1.0, measured in a microplate reader at wavelength of 600 nm.
Filter 50 mL of the supernatant through a 0.45 pore size nylon membrane filter.
Transfer the resulting filtrate into Millipore 10 kDa filter and centrifuge at 5,000× g for 5 min to obtain the OMVs-rich solution in a Falcon tube.
Centrifuge the OMVs-rich solution in a high-speed polypropylene copolymer (PPCO) centrifuge tube at ultra-high speed of 150,000× g for 3 h.
Collect the precipitated OMVs in a polypropylene microcentrifuge tube and store at -80 °C (Figure 1d).
Synthesis of OMV-coated CD-GNPs
Lyophilize OMVs to get OMV power.
Mix 1 mg of OMVs and 1 mg of CD-GNPs in 50 mL of ddH2O in Falcon tubes. Divide 50 mL of the resulting solution into two 50 mL Falcon tubes.
Sonicate the Falcon tubes for 10 s at intervals of 10 s in a sonication bath at a frequency of 40 kHz (100 W) for 2 min at room temperature.
Centrifuge the subsequent solution in the Falcon tube at 5,000× g for 30 min to precipitate OMV-coated CD-GNPs (Figure 1e).
Place the solution of OMV-coated CD-GNPs in copper mesh and dry for TEM imaging, to analyze the coating layer on GNP surface.
Place the solution of OMV-coated CD-GNPs in a quartz cuvette for DLS analysis, to measure the diameter change of GNP before and after OMV coating.
Synthesis of OMV-coated ADA-GNPs
Mix 1 mg of OMVs and 1 mg of ADA-GNPs in 50 mL of ddH2O solution. Divide 50 mL of the resulting solution into two 50 mL Falcon tubes.
Sonicate Falcon tubes for 10 s at intervals of 10 s in a sonication bath at a frequency of 40 kHz (100 W) for 2 min at room temperature.
Centrifuge the subsequent solution in the Falcon tube for 30 min at 5,000× g to precipitate OMV-coated ADA-GNPs (Figure 1f).
Place the solution of OMV-coated ADA-GNPs in copper mesh and dry for TEM imaging, to analyze the coating layer on GNP surface.
Place the solution of OMV-coated ADA-GNPs in quartz cuvette for DLS analysis, to measure the diameter change of GNP before and after OMV coating.
Data analysis
Normalize peak value of diameter curve to 1 (y-axis, corresponding to the frequency of occupied particle diameter).
TEM is a microscopy technique capable of imaging at a significantly higher resolution than light microscopes. This enables the instrument to capture fine details of nano-sized nanoparticles. GNP solution was added on ultra-thin carbon film and then analyzed by TEM. The appropriate view was chosen and imaged. Data file containing figure information was generated. Finally, this profile was loaded into Digital Micrograph and the TEM figure was obtained. The data analysis step is shown in Figure 2. Figure 3a and 3b show the morphology of GNP nanoparticles and GNP aggregates. DLS is a technique in physics that can be utilized to determine the size distribution profile of nano-sized particles in suspension or in solution. GNP solution was added into a glass cell and then analyzed by DLS. An Excel file with diameter distribution and corresponding intensity were generated. Finally, these data were loaded into GraphPad Prism with x-axis of diameter value and y-axis of intensity value, and the figure of size distribution was obtained. The photo of DLS instrument and data analysis step are shown in Figure 4. DLS shows a large size distribution of GNP aggregates in comparison to that of ADA-GNPs and CD-GNPs (Figure 3c).
Figure 2. Data generation steps by TEM analysis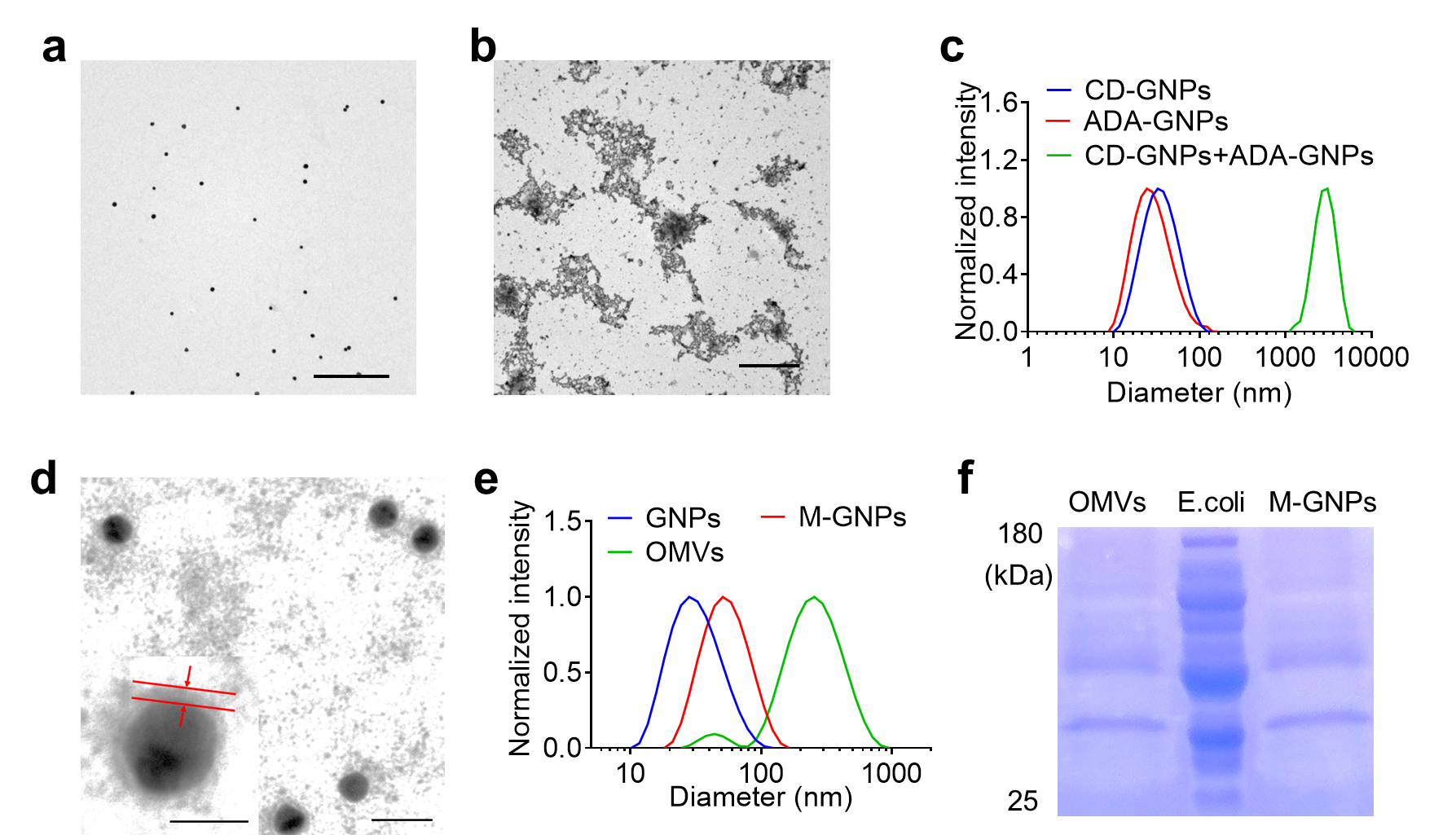
Figure 3. Characterization of outer membrane vesicles (OMV)-coated gold nanoparticles (GNPs). a) TEM image of GNPs. Scale bar: 500 nm. b) TEM image of GNP aggregates. Scale bar: 2 μm. c) Size distribution of the mixture of CD-GNPs and ADA-GNPs determined by DLS. d) TEM image of M-GNPs. Scale bar: 50 nm. Insert: amplified M-GNPs (the scale bar was 20 nm). e) Size distribution of GNPs, M-GNPs, and OMVs determined by DLS. f) The protein bands of M-GNPs, OMVs, and E. coli in the gel by Coomassie Blue staining.OMV-coated CD-GNPs/OMV-coated ADA GNPs were characterized by TEM and DLS. TEM was utilized to analyze the coating layer on GNP surface; a transparent membrane layer with ~6 nm thickness was observed on GNP surface (Figure 3d). DLS was utilized to measure the diameter change of GNP before and after OMV coating; an increased size from 30 to 45 nm after coating of GNP with OMV was observed (Figure 3e).
Fit the frequency-diameter using a X-Y model with GraphPad Prism 9.0 software (Figure 4c and 4e).
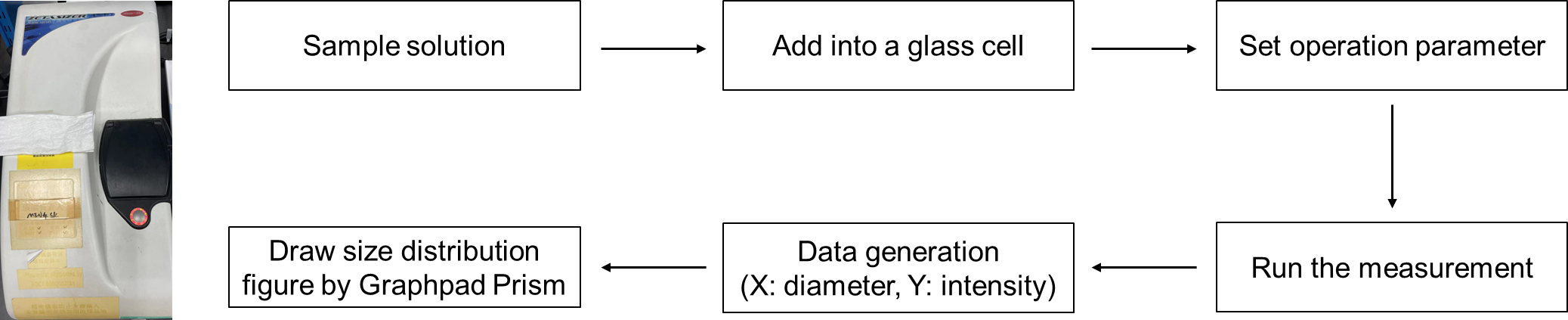
Figure 4. Photo of DLS instrument and data generation steps
Recipes
HAuCl4 solution (0.25 mM)
HAuCl4·3H2O, 85 mg
ddH2O, 1,000 mL
Mono(6-mercapto-6-deoxy)-beta-cyclodextrin solution (2 mM)
Mono(6-mercapto-6-deoxy)-beta-cyclodextrin, 2.3 g
ddH2O, 1,000 mL
GNP solution (10 mM)
GNP, 1.97 g
ddH2O, 1,000 mL
1-adamantanethiol in DMSO (2 mM)
1-adamantanethiol, 336 mg
DMSO, 1,000 mL
LB medium
Tryptone, 10 g/L
Yeast extract, 5 g/L
NaCl, 10 g/L
Sodium citrate solution (5%)
Sodium citrate, 50 g
ddH2O, 1,000 mL
Acknowledgments
This research was funded by Dr. Stanley Ho Medical Development Foundation (Grant No.: SHMDF-OIRFS/2021/002), Science and Technology Development Fund (FDCT) Macau SAR (Grant No.: 0065/2021/A2), and National Natural Science Foundation of China (22071275).
The current protocol is derived from the original paper (Gao et al., 2022).
Competing interests
R.W. and C.G. have one pending patent based on the bacteria-mimetic gold nanoparticles presented in this manuscript.
References
- Gao, C., Wang, Q., Li, J., Kwong, C. H. T., Wei, J., Xie, B., Lu, S., Lee, S. M. Y. and Wang, R. (2022). In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci Adv 8(19): eabn1805.
- Krueger, T. E. G., Thorek, D. L. J., Denmeade, S. R., Isaacs, J. T. and Brennen, W. N. (2018). Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl Med 7(9): 651-663.
- Li, T., Dong, H., Zhang, C. and Mo, R. (2018). Cell-based drug delivery systems for biomedical applications. Nano Res 11(10): 5240-5257.
- Li, Z., Wang, Y., Ding, Y., Repp, L., Kwon, G. S. and Hu, Q. (2021). Cell-Based Delivery Systems: Emerging Carriers for Immunotherapy. Adv Funct Mater 31(23): 2100088.
- Loescher S. and Walther A. (2020). Supracolloidal Self‐Assembly of Divalent Janus 3D DNA Origami via Programmable Multivalent Host/Guest Interactions. Angew Chem Int Engl 59 (14): 5515-5520.
- Wang, H., Wu, H., Yi, Y., Xue, K-F., Xu, J-F., Wang, H., Zhao, Y. and Zhang, X. (2021). Self-Motivated Supramolecular Combination Chemotherapy for Overcoming Drug Resistance Based on Acid-Activated Competition of Host–Guest Interactions. CCS Chemistry 3(8): 1413-1425.
- Yang, L., Yang, Y., Chen, Y., Xu, Y. and Peng, J. (2022). Cell-based drug delivery systems and their in vivo fate. Adv Drug Deliv Rev 187: 114394.
- Zhang, J., Yang, L., Huang, F., Zhao, C., Liu, J., Zhang, Y., and Liu, J. (2021). Multifunctional hybrid hydrogel enhanced antitumor therapy through multiple destroying DNA functions by a triple‐combination synergistic therapy. Adv Healthc Mater, 10(21): 2101190.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gao, C., Tang, M., Lee, S. M. Y. and Wang, R. (2023). Synthesis of Bacteria-mimetic Gold Nanoparticles for Phagocytosis by Immune Cells. Bio-protocol 13(12): e4695. DOI: 10.21769/BioProtoc.4695.
- Gao, C., Wang, Q., Li, J., Kwong, C. H. T., Wei, J., Xie, B., Lu, S., Lee, S. M. Y. and Wang, R. (2022). In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci Adv 8(19): eabn1805.
Category
Cell Biology > Organelle isolation > Membrane
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









