- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Direct Adeno-associated Viruses Injection of Murine Adipose Tissue
Published: Vol 13, Iss 10, May 20, 2023 DOI: 10.21769/BioProtoc.4674 Views: 1855
Reviewed by: Mohammed Mostafizur RahmanRachael E. HokensonAnonymous reviewer(s)
Abstract
The adipose tissue is a central metabolic organ that regulates whole-body energy homeostasis. The abnormal expansion of adipose tissue leads to the progression of obesity. The adipose tissue microenvironment is affected by pathological hypertrophy of adipocytes, highly correlated with systemic metabolic disorders. In vivo genetic modification is a great tool for understanding the role of genes involved in such processes. However, obtaining new conventional engineered mice is time consuming and costly. Here, we provide a simple and speedy method to efficiently transduce genes into adipose tissue by injecting the adeno-associated virus vector serotypes 8 (AAV8) into the fat pads of adult mice.
Keywords: Adipose tissueBackground
Obesity is a severe global health problem characterized by excess adipose tissue expansion and strongly associated with metabolic diseases such as diabetes, cardiovascular and hepatic lipid diseases, and some types of cancers (Haslam and James, 2005; Swinburn et al., 2011; Wabitsch et al., 2015). The growth of the adipose tissue can be attributed to the enlargement of the existing adipocytes (hypertrophy) or the formation of new adipocytes (hyperplasia) (Salans et al., 1973; Spalding et al., 2008; Jo et al., 2009). Unlike the protected role of adipocyte hyperplasia (Vishvanath and Gupta, 2019), hypertrophic adipocytes are more responsible for lipid homeostasis disorders and pathological consequences (Haczeyni et al., 2018). Moreover, besides its usage as a container for lipid storage, the adipose tissue also plays crucial roles in regulating metabolic and endocrine functions. Therefore, there is an urgent need to clarify the obesity pathogenic mechanism, develop safe anti-obesity therapeutic strategies, and further avoid the pandemic dimensions of obesity.
Genetic engineering of mice is a commonly used approach to clarify gene function in vivo. However, the production of new transgenic mice is time consuming and costly (Jimenez et al., 2013; Bates et al., 2020). Some undesired side effects can be observed in conventional genetically modified mice: the manipulated gene that works on the whole body may interfere with its primary function on a specific tissue. In vivo gene transfer to the target organ has become a faster, lower-cost, and more specific strategy. Using this strategy, the time points of gene delivery can be arranged based on experimental requirements. It can also eliminate the undesired effects on embryo development.
Adeno-associated virus (AAV) vectors, discovered in the 1960s, are considered one of the safest and most promising tools for in vivo delivery of gene therapies (Atchison et al., 1965; Wang et al., 2019). They are small (25–26 nm in diameter), non-enveloped viruses composed of an icosahedral capsid that contains a linear single-stranded DNA genome (approximately 4.7–4.9 Kb). In contrast to the adenovirus, retrovirus, and lentivirus, AAVs show a relatively safe profile with apathogenicity and low immunogenicity (Cao et al., 2011). They can transduce genes into dividing and non-dividing cells (Flotte et al., 1994), allowing long-term transgene expression in such tissues. Among the 13 AAV serotypes that have been identified (Pipe et al., 2019), at least three serotypes (AAV2/8/9) exhibit efficient gene transfer to adipose tissue of adult mice (O'Neill et al., 2014; Uhrig-Schmidt et al., 2014; Bates et al., 2020). Recently, we successfully applied this protocol on direct AAV8 injection into the subcutaneous adipose tissue. In this protocol, we demonstrate a detailed procedure for efficient gene delivery into adipocytes of adult mice.
Materials and Reagents
Animals: 8–12-week-old C57BL/6JNarl mice (National Laboratory Animal Center)
Rodent MD’sTM Rimadyl (Carprofen, 2 mg/tablet) (Bio-Serv, catalog number: SMD150-2)
AAV8 vectors carrying enhanced green fluorescent protein (GFP) as reporter gene driven by CMV promoter (obtained from National RNAi Core Facility at Academia Sinica, Taiwan)
PBS (Gibco, catalog number: 10010023)
75% ethanol
FORANE® isoflurane (Abbott, catalog number: B506)
Bacitracin-neomycin ointment (Shiteh, catalog number: 022990)
Equipment
Matrx® anesthesia machine (Midmark, catalog number: VIP3000) (Figure 1, ①)
Anesthetic gas recovery machine (Step, catalog number: R-600) (Figure 1, ②)
Anesthetized mouse facemask (RWD, catalog number: 68635) (Figure 1, ③)
Surgery tweezers (Shineteh, catalog number: ST-TW011) (Figure 1, ④)
Surgery scissor (Shineteh, catalog number: ST-S011PK) (Figure 1, ⑤)
50 µL syringe (Hamilton, catalog number: 80901); needles sold separately (model: 1705 LT) (Figure 1, ⑥)
Needle (30 G × ½) (BD Precision GlideTM, catalog number: 305106) (Figure 1, ⑦)
Needle holder (Shineteh, catalog number: ST-H212) (Figure 1, ⑧)
Sterilized Suture, 4-0, 12 mm 3/8 circle (UNIK, catalog number: SC124) (Figure 1, ⑨)
Biosafety Cabinet (ClassII)
Hair clipper (Orbaner, catalog number: MB-022)

Figure 1. Setup of experiment. ① Anesthesia machine, ② Anesthetic gas recovery machine, ③ Anesthetized mouse facemask, ④ Surgery tweezers, ⑤ Surgery scissor, ⑥ Syringe, ⑦ 30 G needle, ⑧ Needle holder, ⑨ Sterilized suture.
Procedure
Replace the diet with rodent MD’sTM Rimadyl 16 h before surgery to prevent postoperative pain.
Anesthetize mice in an anesthesia chamber filled with isoflurane at a flow rate of 5% in 100% oxygen.
Assess the level of anesthesia by pinching the mice’s hind toes or tail end (no withdrawal should be observed).
Transfer mice to a biosafety cabinet and put on an anesthetized mouse facemask. Adjust the rate of isoflurane to 1.5%–2.5% in 100% oxygen.
Shave a small area in the flanks and proximal hip joints with a hair clipper (Figure 2A).
Video 1 shows the procedures in steps 5–10.
Video 1. Procedures of direct adeno-associated virus (AAV) injection of murine adipose tissue. The video was produced at National Yang Ming Chiao Tung University (NYCU), where all procedures followed guidelines from the NYCU and were approved by the Institutional Animal Care and Use Committee (IACUC) of NYCU (IACUC approved number: 1090706, 1090706r, 1100332).Clean the shaved region with 75% ethanol.
Prepare an AAV8 solution in PBS. AAV8 will be administered at a total of 1 × 1011–2 × 1011 viral genomes (VG) AAV8 in a 30 μL volume for one fat pad per mouse. Fit a 30G needle securely with Hamilton syringe. Preload syringe with the AAV8 solution. Avoid air in the syringe.
Tent the skin with the tweezers and make a 0.5–1 cm incision in the skin using surgical scissors.
Expose the fat pad and carefully insert a 30G needle until the needle bevel enters the tissue. Inject the AAV8 slowly into 4–5 distinct spots (6–8 μL per spot) of the fat pad. Hold the needle in place for a little while after each injection to prevent backflow into the syringe (Figure 2B).
Hold the needle of the 4-0 braided silk with a needle holder and suture the incision (Figure 2C). (Alternative: close the incision with Michel's suture clips.)
Apply ointment to the incision region until it heals to prevent post-operative infection.
Transfer the mice to a clean cage on a heating pad. Monitor the mice closely until they regain consciousness.
Each experimental mouse must be single housed in a cage after surgery until the incision heals. Provide mice with Rimadyl within 48 h after the surgery to alleviate pain.
After one week from injection, the GFP signal is detectable.
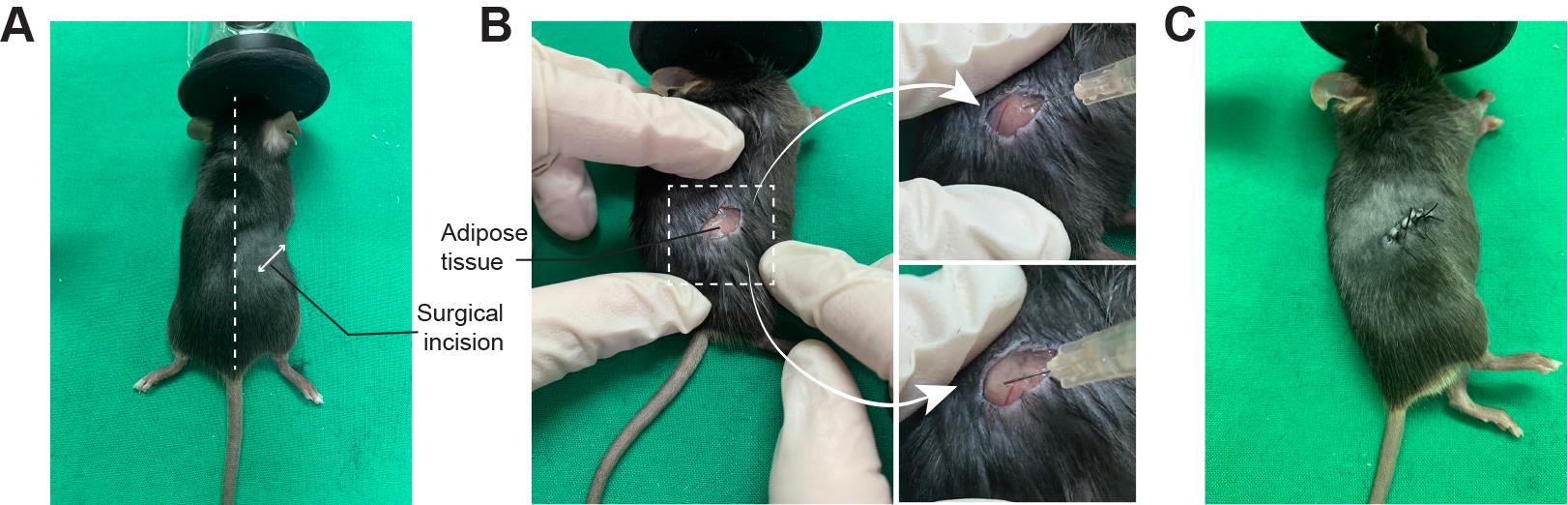
Figure 2. Direct adipose adeno-associated virus (AAV) injection procedure. A. Shave and clean the surgery area at the flank and proximal hip joints. The surgical incision region is shown as a double-headed arrow. B. Make a 0.5 cm incision and carefully inject the AAV into multiple spots in the fat pad. C. Suture the incision using 4-0 braided silk.
Notes
All materials used in this experiment must be sterilized or autoclaved to prevent contamination.
Observe the mice daily to ensure there are no surgical complications such as infection, bleeding, or poor wound healing. Apply the ointment to the incision region or prolong the Rimadyl feeding if particular surgical complications arise.
Acknowledgments
We thank the National RNAi Core Facility at Academia Sinica in Taiwan for providing AAV8 reagents and related services. This work was financially supported by the “Cancer Progression Research Center, National Yang Ming Chiao Tung University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan. This work was supported by the MOST 111-2740-B-A49-001 and NSTC 111-2634-F-A49-014. We have successfully applied this protocol to our current publication (Wu et al., 2022).
Competing interests
The authors declare that no competing financial interests exist.
Ethics
All the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of National Yang Ming Chiao Tung University. (IACUC #1090706, 1090706r, 1100332) and housed on a 12:12 h light/dark cycle at 22 °C.
References
- Atchison, R. W., Casto, B. C. and Hammon, W. M. (1965). Adenovirus-Associated Defective Virus Particles. Science 149(3685): 754-756.
- Bates, R., Huang, W. and Cao, L. (2020). Adipose Tissue: An Emerging Target for Adeno-associated Viral Vectors. Mol Ther Methods Clin Dev 19: 236-249.
- Cao, H., Molday, R. S. and Hu, J. (2011). Gene therapy: light is finally in the tunnel. Protein Cell 2(12): 973-989.
- Flotte, T. R., Afione, S. A. and Zeitlin, P. L. (1994). Adeno-associated virus vector gene expression occurs in nondividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol 11(5): 517-521.
- Haczeyni, F., Bell-Anderson, K. S. and Farrell, G. C. (2018). Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev 19(3): 406-420.
- Haslam, D. W. and James, W. P. (2005). Obesity. Lancet 366(9492): 1197-1209.
- Jimenez, V., Munoz, S., Casana, E., Mallol, C., Elias, I., Jambrina, C., Ribera, A., Ferre, T., Franckhauser, S. and Bosch, F. (2013). In vivo adeno-associated viral vector-mediated genetic engineering of white and brown adipose tissue in adult mice. Diabetes 62(12): 4012-4022.
- Jo, J., Gavrilova, O., Pack, S., Jou, W., Mullen, S., Sumner, A. E., Cushman, S. W. and Periwal, V. (2009). Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol 5(3): e1000324.
- O'Neill, S. M., Hinkle, C., Chen, S. J., Sandhu, A., Hovhannisyan, R., Stephan, S., Lagor, W. R., Ahima, R. S., Johnston, J. C. and Reilly, M. P. (2014). Targeting adipose tissue via systemic gene therapy. Gene Ther 21(7): 653-661.
- Pipe, S., Leebeek, F. W. G., Ferreira, V., Sawyer, E. K. and Pasi, J. (2019). Clinical Considerations for Capsid Choice in the Development of Liver-Targeted AAV-Based Gene Transfer. Mol Ther Methods Clin Dev 15: 170-178.
- Salans, L. B., Cushman, S. W. and Weismann, R. E. (1973). Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. J Clin Invest 52(4): 929-941.
- Spalding, K. L., Arner, E., Westermark, P. O., Bernard, S., Buchholz, B. A., Bergmann, O., Blomqvist, L., Hoffstedt, J., Naslund, E., Britton, T., et al. (2008). Dynamics of fat cell turnover in humans. Nature 453(7196): 783-787.
- Swinburn, B. A., Sacks, G., Hall, K. D., McPherson, K., Finegood, D. T., Moodie, M. L. and Gortmaker, S. L. (2011). The global obesity pandemic: shaped by global drivers and local environments. Lancet 378(9793): 804-814.
- Uhrig-Schmidt, S., Geiger, M., Luippold, G., Birk, G., Mennerich, D., Neubauer, H., Grimm, D., Wolfrum, C. and Kreuz, S. (2014). Gene delivery to adipose tissue using transcriptionally targeted rAAV8 vectors. PLoS One 9(12): e116288.
- Vishvanath, L. and Gupta, R. K. (2019). Contribution of adipogenesis to healthy adipose tissue expansion in obesity. J Clin Invest 129(10): 4022-4031.
- Wabitsch, M., Funcke, J. B., von Schnurbein, J., Denzer, F., Lahr, G., Mazen, I., El-Gammal, M., Denzer, C., Moss, A., Debatin, K. M., et al. (2015). Severe Early-Onset Obesity Due to Bioinactive Leptin Caused by a p.N103K Mutation in the Leptin Gene. J Clin Endocrinol Metab 100(9): 3227-3230.
- Wang, D., Tai, P. W. L. and Gao, G. (2019). Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov 18(5): 358-378.
- Wu, S. C., Lo, Y. M., Lee, J. H., Chen, C. Y., Chen, T. W., Liu, H. W., Lian, W. N., Hua, K., Liao, C. C., Lin, W. J., et al. (2022). Stomatin modulates adipogenesis through the ERK pathway and regulates fatty acid uptake and lipid droplet growth. Nat Commun 13(1): 4174.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Wu, S. C. and Lin, C. H. (2023). Direct Adeno-associated Viruses Injection of Murine Adipose Tissue. Bio-protocol 13(10): e4674. DOI: 10.21769/BioProtoc.4674.
Category
Biological Engineering > Biomedical engineering
Biological Sciences
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link











