- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishment of an in vitro Differentiation and Dedifferentiation System of Rat Schwann Cells
Published: Vol 13, Iss 5, Mar 5, 2023 DOI: 10.21769/BioProtoc.4631 Views: 1802
Reviewed by: Gal HaimovichPhilipp WörsdörferAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Differentiation, Maintenance, and Contraction Profiling of Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes
Matthijs Snelders [...] Jeroen Essers
Mar 5, 2025 3858 Views
Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2395 Views
Accurate Identification of Cell Cycle Stages in RPE1 Cells Using the ImmunoCellCycle-ID Method
Syon Reddy [...] Aussie Suzuki
Aug 5, 2025 1843 Views
Abstract
In the peripheral nervous system, Schwann cells are the primary type of glia. This protocol describes an in vitro differentiation and dedifferentiation system for rat Schwann cells. These cultures and systems can be used to investigate the morphological and biochemical effects of pharmacological intervention or lentivirus-mediated gene transfer on the process of Schwann cell differentiation or dedifferentiation.
Graphical abstract

Background
Schwann cells are the primary glial cells of the peripheral nervous system. During axonal sorting and myelination in the peripheral nerves, Schwann cells originate from neural crest cells that differentiate into mature phenotypes (Cristobal and Lee, 2022). Moreover, Schwann cells’ incredible plasticity is one of the most important characteristics following nerve damage or demyelination (Nocera and Jacob, 2020). Schwann cells undergo dedifferentiation after injury and redifferentiate to promote nerve regeneration and complete functional recovery (Jessen and Mirsky, 2019). Therefore, it is important to study Schwann cells’ differentiation and dedifferentiation status to understand their role in nerve development and injury. As reported previously, dibutyryl adenosine 3',5'-cyclic monophosphate (dbcAMP) induces Schwann cells to acquire a differentiated phenotype (Yamauchi et al., 2011). After stimulation with 1 mM dbcAMP, Schwann cells from rats change from bipolar or tripolar to flat within 24 h. By contrast, mouse Schwann cells retain their bipolar or tripolar morphology after dbcAMP treatment (Arthur-Farraj et al., 2011). And a previous study described in vitro assays of scalable differentiation and dedifferentiation of Schwann cell in the absence of neurons (Monje PV., 2018). Based on previous studies, we established a detailed and simplified system for differentiation and dedifferentiation of Schwann cells. In order to clearly distinguish morphological changes, we performed the differentiation and dedifferentiation assay using rat Schwann cells. In brief, the first step was to obtain Schwann cells from the spinal nerves of rats based on a previous study (Wen et al., 2017). During the second step, differentiation and dedifferentiation assays were conducted. Schwann cells were purified and passaged, and experiments were conducted on the third passage. Finally, Schwann cell status was determined by morphological examination, western blotting analysis, and immunofluorescence detection.
Materials and reagents
Cell culture dish (3.5 and 6 cm) (JET BIOFILT, catalog numbers: CD000035, TCD-010-060)
50 mL centrifuge tubes (Corning, catalog number: 430828)
15 mL centrifuge tubes (Corning, catalog number: 430790)
1.5 mL tube (JET BIOFILT, catalog number: CFT000015)
Polyvinylidene fluoride membrane (PVDF) (Bio-Rad, catalog number: 1620177)
Cell glass coverslips (diameter: 12 mm, thickness: 0.13–0.17 mm) (Fisherbrand, catalog number: FIS12-545-80)
Neonatal Sprague-Dawley (SD) rat [postnatal 1–2 days (P1–P2)]
Distilled water
75% ethanol
Phosphate buffered saline (PBS) (Gibco, catalog number: 10010023)
Poly-L-lysine hydrobromide (PLL) (Sigma-Aldrich, catalog number: P1274)
0.25% trypsin-EDTA (Gibco, catalog number: 25200072)
Fetal bovine serum (FBS) (Corning, catalog number: 35-076-CV)
DMEM/F12 (Gibco, catalog number: 11330057)
Cytosine arabinoside (Ara-C) (Sigma-Aldrich, catalog number: C1768)
Recombinant human heregulin β-1 (PeproTech, catalog number: 100-03)
Forskolin (Sigma-Aldrich, catalog number: F6886)
4% paraformaldehyde (PFA) (Biosharp, catalog number: BL539A)
Dimethyl sulfoxide (DMSO), suitable for cell culture (Beyotime, catalog number: ST038)
Dibutyryl adenosine 3',5'-cyclic monophosphate (dbcAMP) (Sigma-Aldrich, catalog number: D0627)
Triton X-100 (Sigma-Aldrich, catalog number: V900502)
Tween-20 (Sigma-Aldrich, catalog number: P1379)
Phalloidin (Abcam, catalog number: ab176759)
RIPA lysis buffer (FUDE Biological Technology, catalog number: FD009)
Rabbit monoclonal (EP1039Y) anti-p75 (Abcam, catalog number: ab52987)
Rabbit anti-Krox20 (Novus Biologicals, catalog number: 13491-1-AP)
Mouse monoclonal anti-c-Jun (BD Biosciences, catalog number: 610326)
Mouse monoclonal (9-9-3) anti-Sox2 (Abcam, catalog number: ab79351)
Alexa Fluor® 488 goat anti-mouse IgG (H+L) (Thermo Fisher Scientific, catalog number: A11001)
Alexa Fluor® 568 goat anti-rabbit IgG (H+L) (Thermo Fisher Scientific, catalog number: A11011)
4’,6-diamidino-2’-phenylindole (DAPI) (Sigma-Aldrich, catalog number: D8417)
Penicillin–streptomycin (Gibco, catalog number: 15140-122)
Omni-ECLTM Light Chemiluminescence kit (EpiZyme, catalog number: SQ201)
10% dodecyl sulfate sodium salt-polyacrylamide gel (EpiZyme, catalog number: PG112)
5% non-fat milk (Solarbio, catalog number: D8340)
Tris-buffered saline (Sigma-Aldrich, catalog number: 93318)
Horseradish peroxidase (HRP)-conjugated secondary antibody (Invitrogen, catalog numbers: 31430, 31460)
1,000× Ara-C (10 mM in distilled water, see Recipes)
1,000× dbcAMP (1,000 mM in DMSO, see Recipes)
30 mM forskolin stock solution (see Recipes)
Complete growth medium of rat Schwann cells (see Recipes)
10% FBS (see Recipes)
3% FBS (see Recipes)
1% FBS (see Recipes)
1 mM dbcAMP (see Recipes)
0.1% Triton X-100 (see Recipes)
5% gelatin (see Recipes)
PBST (see Recipes)
Blocking buffer (see Recipes)
Equipment
Pipettes (Thermo Fisher Scientific, 10, 200, and 1,000 μL)
CO2 incubator (Thermo Fisher Scientific, model: Heracell 150i)
Surgical scissors and forceps (Shenzhen RWD, catalog numbers: S14014 and F12029-09)
Spring scissors (Shenzhen RWD, catalog number: S11001)
Superfine forceps (Shenzhen RWD, catalog number: F13002)
Stereomicroscope (Jiangnan Novel, model: SZ6060)
Centrifuge (Eppendorf, model: Micro21)
Phase contrast microscope (Zeiss, model: Primovert)
Fluorescence microscope (Zeiss, model: Axio Imager A2)
Software
ImageJ (Version 1.8.0, https://imagej.en.softonic.com/)
Procedure
Preparation for primary Schwann cell cultures from neonatal rat
Prepare the culture plate: coat 3.5 cm cell culture dishes with 1 mL of PLL solution (0.1 mg/mL). Incubate overnight at 37 °C in a CO2 incubator. This step is only used to increase cell adhesion to the culture dish. Therefore, the CO2 content is not relevant at this point.
The next day, remove the PLL solution, rinse the dish surface thoroughly with distilled water, and air dry before use. Add 1.5 mL of cold PBS to each dish and place on ice.
Note: Do not use PBS to clean the dishes as this will cause salt crystallization after drying and disrupt cell adhesion.
Prepare culture media and solutions:
Medium for cell proliferation (culture medium to expand Schwann cells) (Medium I): DMEM/F12 containing 3% FBS, 3 μM forskolin, 10 ng/mL heregulin β-1, and 100 mg/mL penicillin–streptomycin.
Medium for cell starvation (Medium II): DMEM/F12 containing 1% FBS and 100 mg/mL penicillin–streptomycin.
Medium for Schwann cell differentiation (Medium III): DMEM/F12 containing 1% FBS, 100 mg/mL penicillin–streptomycin, and 1 mM dbcAMP.
Medium for Schwann cell dedifferentiation (Medium IV): DMEM/F12 containing 1% FBS and 100 mg/mL penicillin–streptomycin.
Culture of primary Schwann cells from rat spinal nerves
Note: This part refers to a previous study (Wen et al., 2017).
Prepare surgical equipment (see Figure 1).
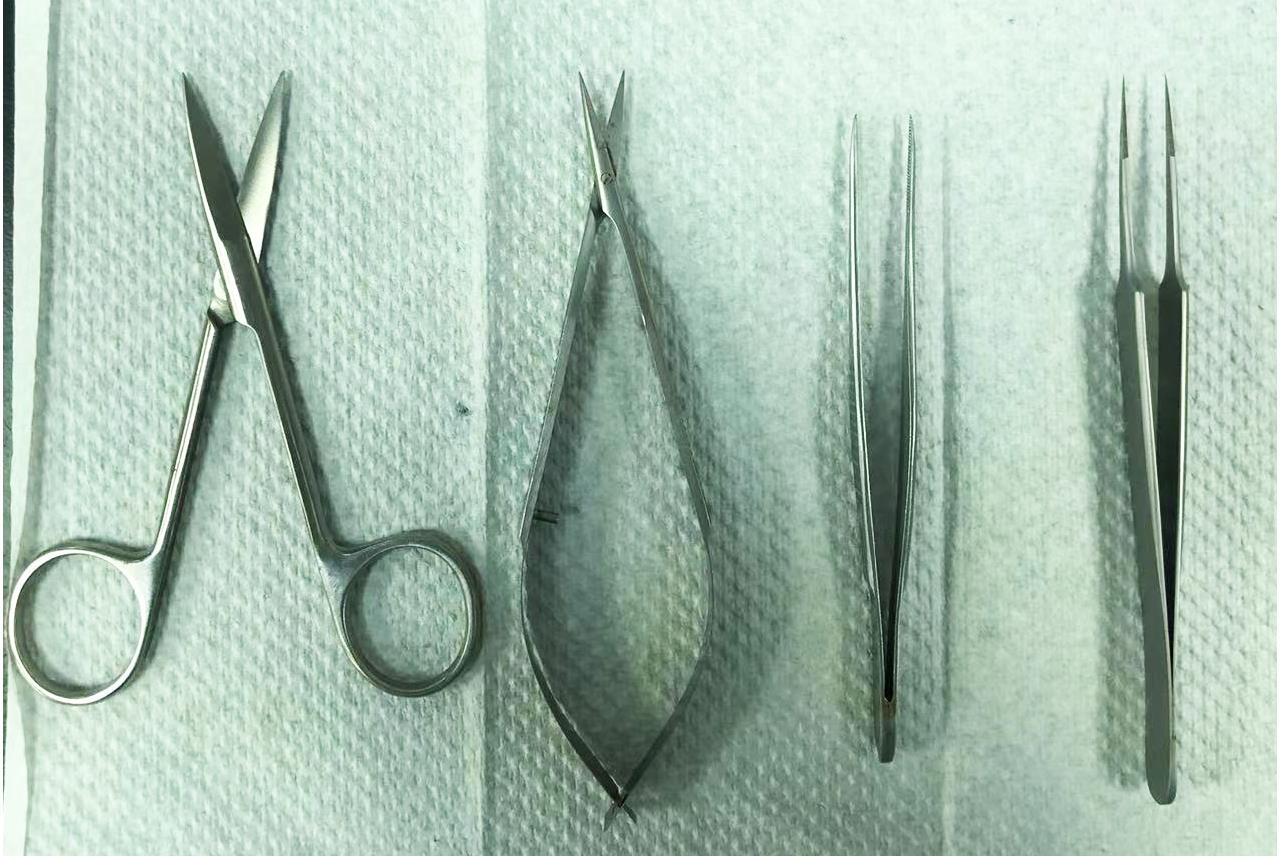
Figure 1. Surgical equipment used in this protocolTissue collection: anesthetize the neonatal rat by putting it on ice for 2–3 min, sterilize them with 75% ethanol, remove the head with surgical scissors and forceps, and collect the sciatic nerves and spinal nerves with superfine forceps.
Tissue digestion: prepare one 1.5 mL tube, add 1 mL of PBS, and cool on ice. Transfer these nerves to a 1.5 mL tube and use spring scissors to cut them into 1-mm-longsegments. Then, add 1 mL of pre-warmed 0.25% trypsin-EDTA to the tube. Cap the tube and incubate at 37 °C in a CO2 incubator for 30 min with occasional shaking every 10 min.
Collect cells by centrifugation: stop digestion by adding 100 μL of FBS, then prepare a single cell suspension by gently digesting the cell sample 30 times with a 1 mL pipette. Then, centrifuge the cell suspension at 100× g for 5 min at room temperature and discard the supernatant. Resuspend the cell sediment in 200 μL of DMEM/F12 containg 10% FBS, place the cell suspension in a 3.5 cm PLL-coated culture dish, and culture the cells in a CO2 incubator for 1–2 h to ensure cell adhesion. Add 1 mL of 10% FBS to the culture plated and culture the cells for 24 h.
Note: At this time, the cells suspension is a mixture of Schwann cells and fibroblasts (see Figure 2A).
Cell purification: replace the culture medium with DMEM/F12 containing 10% FBS and 10 μM Ara-C (add 1 μL of 10 mM Ara-C stock solution per 1 mL of medium) to eliminate the fibroblasts. After 48 h, replace the culture medium with Medium I of rat Schwann cells.
Note: After purification, approximately 1 × 104 Schwann cells per rat can be obtained. When 100% confluence is reached after expansion, the number of Schwann cells per dish will be approximately 1 × 105–2 × 105 (seeFigure 2B).
Cell passage: when the culture reaches 90% confluence, wash the cells with 1 mL of PBS, discard the PBS wash, add 1 mL of 0.25% trypsin-EDTA to the cell dish, and leave at room temperature for 2 min. Observe the cells under a phase contrast microscope and gently shake the dish. When the cells begin to detach, add 1 mL of 10% FBS to stop digestion, collect the cells in a 1.5 mL tube, centrifuge at 100× g for 5 min at room temperature, discard the supernatant, and gently resuspend the cells in 1 mL complete growth medium of rat Schwann cells.
Note: Schwann cells are passaged and expanded at a ratio of 1:3–1:4, and the third passage cells are used for further experiments (see Figure 2C). At this time, cells are actively proliferating and fibroblasts are absent.
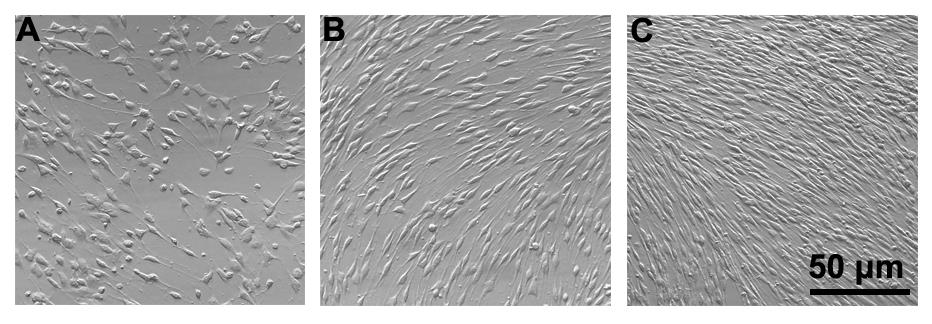
Figure 2. Purification and expansion of rat Schwann cells. A. 24 h after cell dissection, the culture should consist of Schwann cells and fibroblasts. Schwann cells have an elongated spindle-like morphology, whereas fibroblasts are very flat. B. On day 3, purified rat Schwann cells became confluent. C. After 2 days of growth in Medium I, the culture is routinely passaged to passage 3 in complete growth medium in the presence of forskolin and heregulin β-1, and the culture is filled with Schwann cells (>95% confluence) with typical bipolar spindle cell bodies.
Differentiation and dedifferentiation of Schwann cells (Figure 3)
Seed and starvation: seed 5,000 Schwann cells in a 3.5 cm dish (covered with glass coverslips, for immunofluorescence analysis) or seed 1 × 105 Schwann cells in a 6 cm dish (for western blotting analysis) in Medium I to allow a fast proliferation of rat Schwann cells. After one day, remove the complete growth medium and immediately replace it with an equal volume of Medium II overnight to adapt to the lack of mitotic stimulation.
Note: Complete growth medium (Medium I) of rat Schwann cells containing forskolin can induce Schwann cells to acquire a differentiated phenotype, and 10% FBS will induce Schwann cell proliferation but not differentiation. Therefore, before inducing Schwann cell differentiation, we starved the cells by culturing them in Medium II at low serum concentrations and without forskolin.
Differentiation: remove the starvation medium (Medium II) from each dish, replace with an equal volume of Medium III, and culture the cells in a CO2 incubator for 72 h. Before the dedifferentiation process, observe the cultures of dbcAMP-induced differentiated Schwann cells under a phase contrast microscope to confirm the expected morphological changes.
Note: After about 6 h, the rat Schwann cells began to show morphological changes. We recommended starting with cultures without forskolin and dbcAMP as controls. To extend the differentiation time of Schwann cells, simply replace the culture medium with fresh differentiation conditioned medium (Medium III).
Dedifferentiation: after 72 h, Schwann cells differentiated under the influence of dbcAMP. To induce dedifferentiation, simply change the culture medium to Medium IV, and culture the cells in a CO2 incubator for 24 h. Continue collecting or analyzing cultures the day after dbcAMP withdrawal, or as required by the experimental design.
Note: dbcAMP-induced differentiated Schwann cells does not dedifferentiate upon exposure to growth factors such as forskolin and heregulin β-1. Thus, we recommend using a medium that does not contain forskolin and heregulin β-1 throughout the differentiation and dedifferentiation experiments. Approximately 6 h after removal of dbcAMP, almost half of the rat Schwann cells return to typical bipolar or tripolar shape. You can add drugs or perform virus transfections before dedifferentiation experiment.
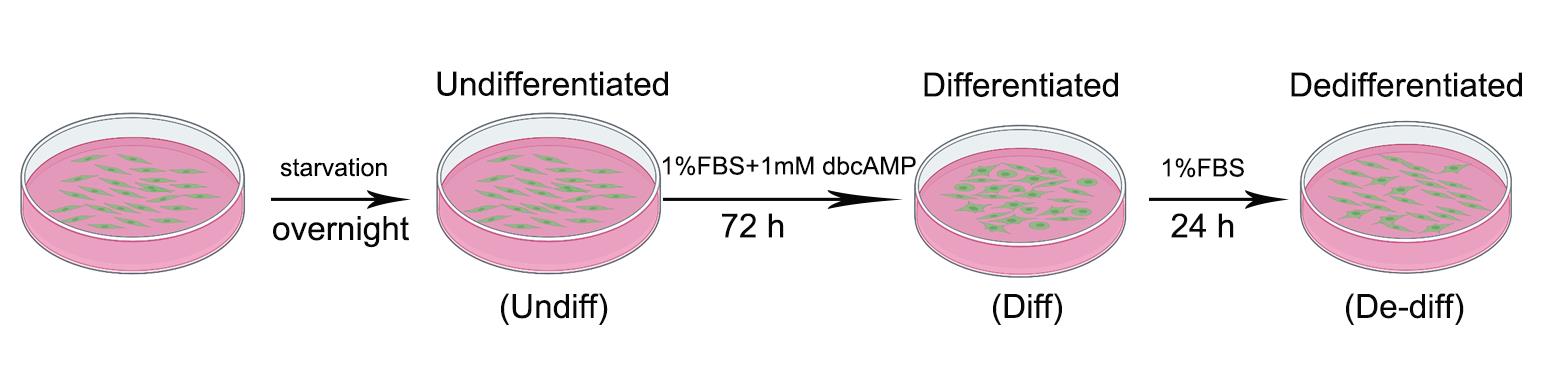
Figure 3. Differentiation and dedifferentiation system of rat Schwann cells. Schwann cells are cultured in Medium I for several days, then the third-passage cells are plated at an appropriate density and starved overnight in Medium II. The next day, the Schwann cells are treated with 1 mM dbcAMP, which induces morphological change and upregulation of differentiation markers, which could be examined by phase contrast microscope observation, immunofluorescence, or western blotting. After 72 h, Schwann cell dedifferentiation can be induced by simply changing the culture medium to Medium IV. Undiff: Undifferentiated; Diff: Differentiated; De-diff: Dedifferentiated.
Data analysis
Phase contrast microscopy (Figure 4A)
To identify the phenotype of Schwann cells, observe their morphology under a phase contrast microscope. Schwann cells were treated with dbcAMP to obtain a differentiated phenotype with morphological transition from an elongated spindle-like shape to a flattened shape; simple dbcAMP removal can reverse the differentiated Schwann cells into an elongated bipolar morphology.
Immunofluorescence (Figure 4B)
Cell fixation: fix cells with 4% PFA for 30 min at room temperature.
Permeabilization and blocking: penetrate cells with 0.1% Triton X-100 (see Recipe 8) for 30 min and incubate with 5% gelatin (see Recipe 9) at room temperature for 1 h.
Primary antibodies: prepare a 1:200 dilution of mouse anti-c-Jun and a 1:100 solution of rabbit anti-Krox20 in 5% gelation solution. Incubate the cells with primary antibodies at 4 °C overnight.
Secondary antibodies: prepare a 1:400 dilution of Alexa Fluor® 488 goat anti-mouse IgG (H+L) or Alexa Fluor® 568 goat anti-rabbit IgG (H+L) in PBST (see Recipe 10) secondary antibodies. Incubate cells with the corresponding secondary antibodies at room temperature for 2 h, wash cells with PBST twice, and then incubate cells with 1 μg/mL DAPI for 5 min at room temperature to stain nuclei.
Western blotting (Figure 4C, 4D)
Cells lysis: add 100 μL of RIPA lysis buffer to the cell dish, blow, and collect lysates into a 1.5 mL tube.
Protein electrophoresis: prepare 10% dodecyl sulfate sodium salt-polyacrylamide gel, pipette 10 μg of protein into each well, and run the electrophoresis using the following parameters: 80 V and 300 mA for 1.5 h. When the electrophoresis is completed, remove the gel carefully and transfer proteins to a PVDF membrane.
Incubation of primary and secondary antibodies: after blocking with blocking buffer for 2 h at room temperature, prepare a 1:800 dilution of mouse anti-Sox2 and a 1:500 solution of rabbit anti-p75 in blocking buffer, incubate the membrane in primary antibody at 4 °C overnight, and incubate Horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 2 h.
Visualization and calculation: visualize the membrane using Omni-ECLTM Light Chemiluminescence kit and calculate protein quantity using ImageJ.
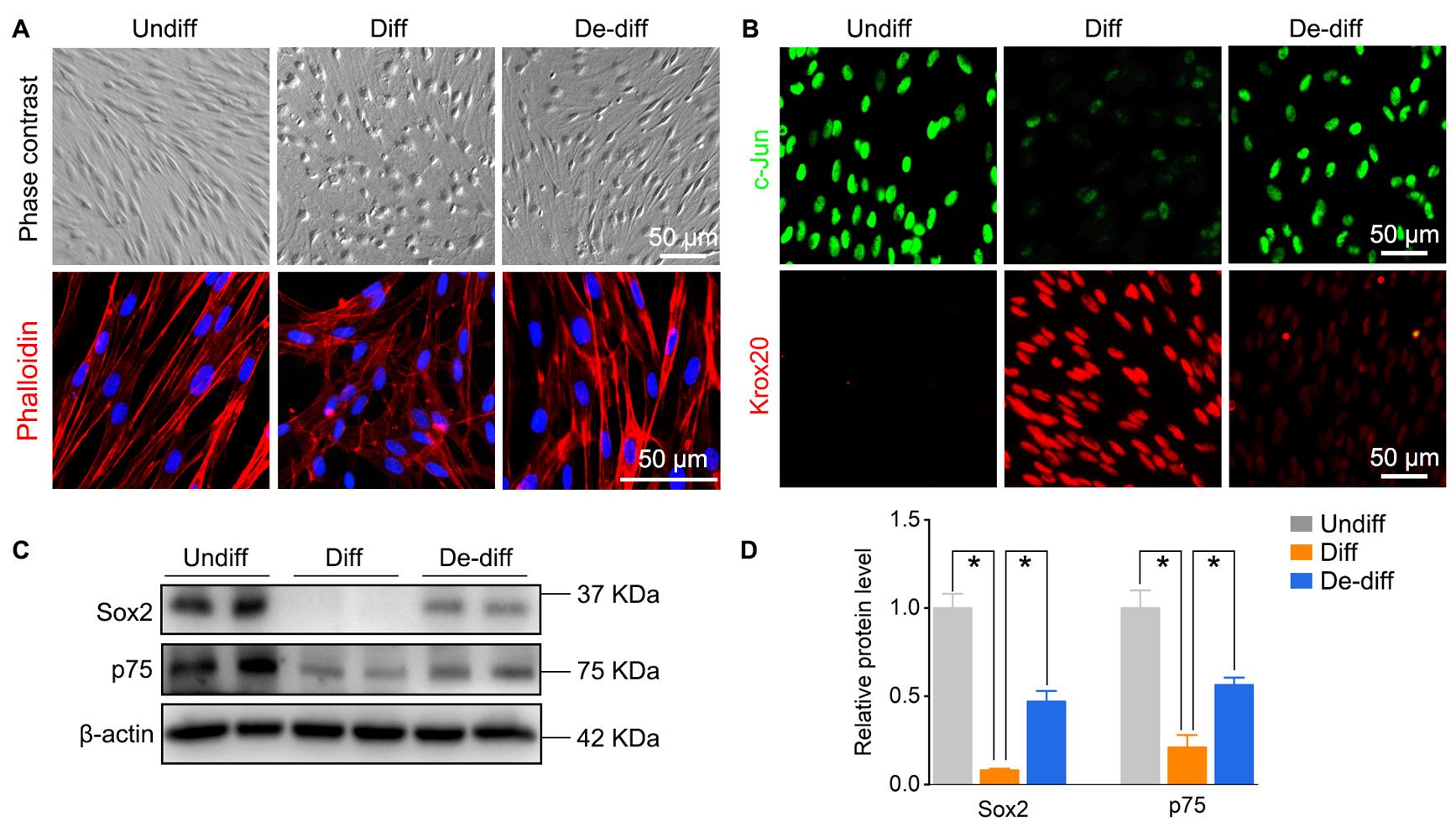
Figure 4. Identification of rat Schwann cells’ phenotype. (A) Cell morphology and cytoskeleton stained with phalloidin were observed under a microscope. (B) Immunofluorescence of c-Jun (indicates immature or dedifferentiated Schwann cells) and Krox20 (indicates mature or differentiated Schwann cells). (C, D) Western blotting analysis and data statistics of Sox2 and p75 (indicates immature or dedifferentiated Schwann cells). Undiff: Undifferentiated; Diff: Differentiated; De-diff: Dedifferentiated.
Recipes
1,000× Ara-C
Dissolve 4.86 mg of Ara-C in 2 mL of distilled water to make a 1,000× stock solution of 10 mM and sterilize the solution by filtration. Store at -20 °C.
1,000× dbcAMP
Dissolve 50 mg of dbcAMP in 101.756 μL of distilled DMSO to make a 1,000× stock solution of 1,000 mM and store at -20 °C.
30 mM forskolin stock solution
Dissolve 10 mg of forskolin in 812 μL of distilled DMSO to prepare a 30 mM forskolin stock solution. Store at -20 °C.
Complete growth medium of rat Schwann cells
48.5 mL of DMEM/F12 containing 1.5 mL of FBS, 3 μM forskolin, 10 ng/mL heregulin- β-1, and 100 mg/mL penicillin–streptomycin.
10% FBS
45 mL of DMEM/F12 containing 5 mL of FBS supplemented with 1% penicillin–streptomycin.
3% FBS
48.5 mL of DMEM/F12 containing 1.5 mL of FBS supplemented with 1% penicillin–streptomycin.
1% FBS
49.5 mL of DMEM/F12 containing 0.5 mL of FBS supplemented with 1% penicillin–streptomycin.
1 mM dbcAMP
Add 1 μL of 1,000× dbcAMP (1,000 mM) to 1 mL of 1% FBS.
0.1% Triton X-100
Dilute 1 mL of Triton X-100 into 1,000 mL of PBS.
5% gelatin
Dissolve 0.5 g of gelatin in 10 mL of PBS. Add 300 μL of Triton X-100 (0.3%) to the buffer.
PBST
Add 1 mL of Tween-20 to 1,000 mL of PBS. Store at room temperature.
Blocking buffer
5% non-fat milk in Tris-buffered saline containing 0.5% Tween-20
Acknowledgments
This protocol is adapted from the previous published papers (Zou et al., 2022), (Wen et al., 2017) and (Monje PV., 2018). Thanks to Professor Jiasong Guo (Southern Medical University, Guangzhou, China).
Competing interests
There are no conflicts of interest or competing interests.
Ethics
All animal procedures were performed in accordance with the guidelines for the ethical treatment of animals.
References
- Arthur-Farraj, P., Wanek, K., Hantke, J., Davis, C. M., Jayakar, A., Parkinson, D. B., Mirsky, R. and Jessen, K. R. (2011). Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia 59(5): 720-733.
- Cristobal, C. D. and Lee, H. K. (2022). Development of myelinating glia: An overview. Glia 70(12): 2237-2259.
- Jessen, K. R. and Mirsky, R. (2019). The Success and Failure of the Schwann Cell Response to Nerve Injury. Front Cell Neurosci 13: 33.
- Nocera, G. and Jacob, C. (2020). Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci 77(20): 3977-3989.
- Wen, J., Tan, D., Li, L. and Guo, J. (2017). Isolation and Purification of Schwann Cells from Spinal Nerves of Neonatal Rat. Bio Protoc 7(20): e2588.
- Yamauchi, J., Miyamoto, Y., Hamasaki, H., Sanbe, A., Kusakawa, S., Nakamura, A., Tsumura, H., Maeda, M., Nemoto, N., Kawahara, K., Torii, T. and Tanoue, A. (2011). The atypical Guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination. J Neurosci 31(35): 12579-12592.
- Zou, Y., Zhang, J., Liu, J., Xu, J., Fu, L., Ma, X., Xu, Y., Xu, S., Wang, X. and Guo, J. (2022). SIRT6 Negatively Regulates Schwann Cells Dedifferentiation via Targeting c-Jun During Wallerian Degeneration After Peripheral Nerve Injury. Mol Neurobiol 59(1): 429-444.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Ying, Z. (2023). Establishment of an in vitro Differentiation and Dedifferentiation System of Rat Schwann Cells. Bio-protocol 13(5): e4631. DOI: 10.21769/BioProtoc.4631.
Category
Neuroscience > Peripheral nervous system > Schwann cell
Cell Biology > Cell isolation and culture > Cell differentiation
Cell Biology > Cell imaging > Fixed-cell imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link