- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis
Published: Vol 13, Iss 5, Mar 5, 2023 DOI: 10.21769/BioProtoc.4625 Views: 2053
Reviewed by: Wenrong HeYao XiaoYe XuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures
Marisa Conte [...] Alfredo Ambrosone
Mar 5, 2025 2219 Views

High-Performance Liquid Chromatography Quantification of Glyphosate, Aminomethylphosphonic Acid, and Ascorbate in Culture Medium and Microalgal Cells
Juan Manuel Ostera [...] Gabriela Malanga
Apr 5, 2025 1182 Views

Rapid Miniprep of Intact Chloroplasts from Arabidopsis thaliana Leaves
Brenda A. Carranza-Correa [...] Manuel Gutiérrez-Aguilar
May 20, 2025 2646 Views
Abstract
The vacuole is one of the most conspicuous organelles in plant cells, participating in a series of physiological processes, such as storage of ions and compartmentalization of heavy metals. Isolation of intact vacuoles and elemental analysis provides a powerful method to investigate the functions and regulatory mechanisms of tonoplast transporters. Here, we present a protocol to isolate intact vacuoles from Arabidopsis root protoplasts and analyze their elemental content by inductively coupled plasma mass spectrometry (ICP-MS). In this protocol, we summarize how to prepare the protoplast, extract the vacuole, and analyze element concentration. This protocol has been applied to explore the function and regulatory mechanisms of tonoplast manganese (Mn) transporter MTP8, which is antagonistically regulated by CPK4/5/6/11 and CBL2/3-CIPK3/9/26. This protocol is not only suitable for exploring the functions and regulatory mechanisms of tonoplast transporters, but also for researching other tonoplast proteins.
Graphical abstract
Background
The central vacuole is the largest compartment of a mature plant cell and participates in plenty of physiological processes. The determination of its elemental concentrations is essential for researching the function and regulation of plant tonoplast transporters. The transport rates of different anions and the factors affecting the uptake of chloride ions across the tonoplast were detected in isolated barley vacuoles (Martinoia et al., 1986). Transport of phosphate across the tonoplast was also detected in intact vacuoles, which were isolated from suspension-cultured cells of Catharanthus roseus (L.) G. Don (Massonneau et al., 2000). Shimaoka et al. (2004) modified the method for extracting intact vacuoles, which is summarized in this article, and detected the tonoplast proteins combined with proteomic analysis. The function and regulatory mechanisms of the tonoplast manganese (Mn) transporter MTP8 were resolved via this method (Eroglu et al., 2016; Zhang et al., 2021; Ju et al., 2022). In conclusion, this protocol is feasible and also applied for the research to investigate tonoplast transporters in the future.
Materials and Reagents
40 μm cell strainer (Corning, catalog number: 431750)
50 mL tubes (Sangon Biotech, catalog number: F602788)
Cellulase R10 (Yakult, catalog number: L0012)
Macerozyme R10 (Yakult, catalog number: L0021)
D-mannitol (Sigma-Aldrich, catalog number: M9647)
MES hydrate (Sigma-Aldrich, catalog number: M2933)
Tris-base (Thermo Fisher Scientific, catalog number: BP152-5)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C5670)
Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333)
Percoll (GE Healthcare, catalog number: 17-0891-09)
HEPES (Sigma-Aldrich, catalog number: H3375)
EGTA (Sigma-Aldrich, catalog number: H3889)
Sucrose (Sigma-VETEC, catalog number: V900116)
K-gluconate (Sigma-Aldrich, catalog number: G4500)
D-sorbitol (Sigma-Aldrich, catalog number: S1876)
MnSO4 (Sigma-Aldrich, catalog number: M7634)
HNO3 (GHTECH, catalog number: 1.14003.018)
H2O2 (Sinopharm Chemical Reagent, catalog number: 10011208)
Murashige and Skoog (MS) base salts with vitamins (Phytotech Labs, catalog number: M519)
Agar (Sigma-Aldrich, catalog number: A1296)
KNO3 (Sinopharm Chemical Reagent, catalog number: 10017218)
Ca(NO3)2·4H2O (Sigma-Aldrich, catalog number: SC278601)
NH4H2PO4 (Sinopharm Chemical Reagent, catalog number: 10002808)
MgSO4·7H2O (Sinopharm Chemical Reagent, catalog number: 10013018)
H3BO3 (Sinopharm Chemical Reagent, catalog number: 10004808)
MnCl2·4H2O (Sigma-Aldrich, catalog number: SM500501)
(NH4)6Mo7O24·4H2O (Sinopharm Chemical Reagent, catalog number: 10002318)
ZnSO4·7H2O (Sinopharm Chemical Reagent, catalog number: 10024018)
CuSO4·5H2O (Sinopharm Chemical Reagent, catalog number: 10008218)
EDTA-Fe(III)Na (Biotopped, catalog number: Q0028-100g)
BSA (Sigma-Aldrich, catalog number: V900933)
β-mercaptoethanol (14.3 M) (Millipore, catalog number: 444203)
1/2 MS medium (see Recipes)
1/5 Hoagland solution (see Recipes)
Protoplasting solution (see Recipes)
Medium B (see Recipes)
Medium I (see Recipes)
Medium II (see Recipes)
Medium III (see Recipes)
Medium IV (see Recipes)
Medium V (see Recipes)
Medium VI (see Recipes)
Medium VII (see Recipes)
Medium VIII (see Recipes)
Equipment
Artificial illumination incubator (PERCIVAL, model: LT-36VL)
Horizontal centrifuge (Eppendorf, model: Centrifuge 5810R)
Dissolver (LabTech, model: DigiBlock ED54)
ICP-MS (Thermo Fisher Scientific, model: ICAP Qc)
Perfluoroalkoxy alkane (PFA) vessels (LabTech, model: GC-36-L)
Fuchs-Rosenthal hemacytometer (Marienfeld, model: Dark-Line 0650010)
Procedure
Protoplast preparation
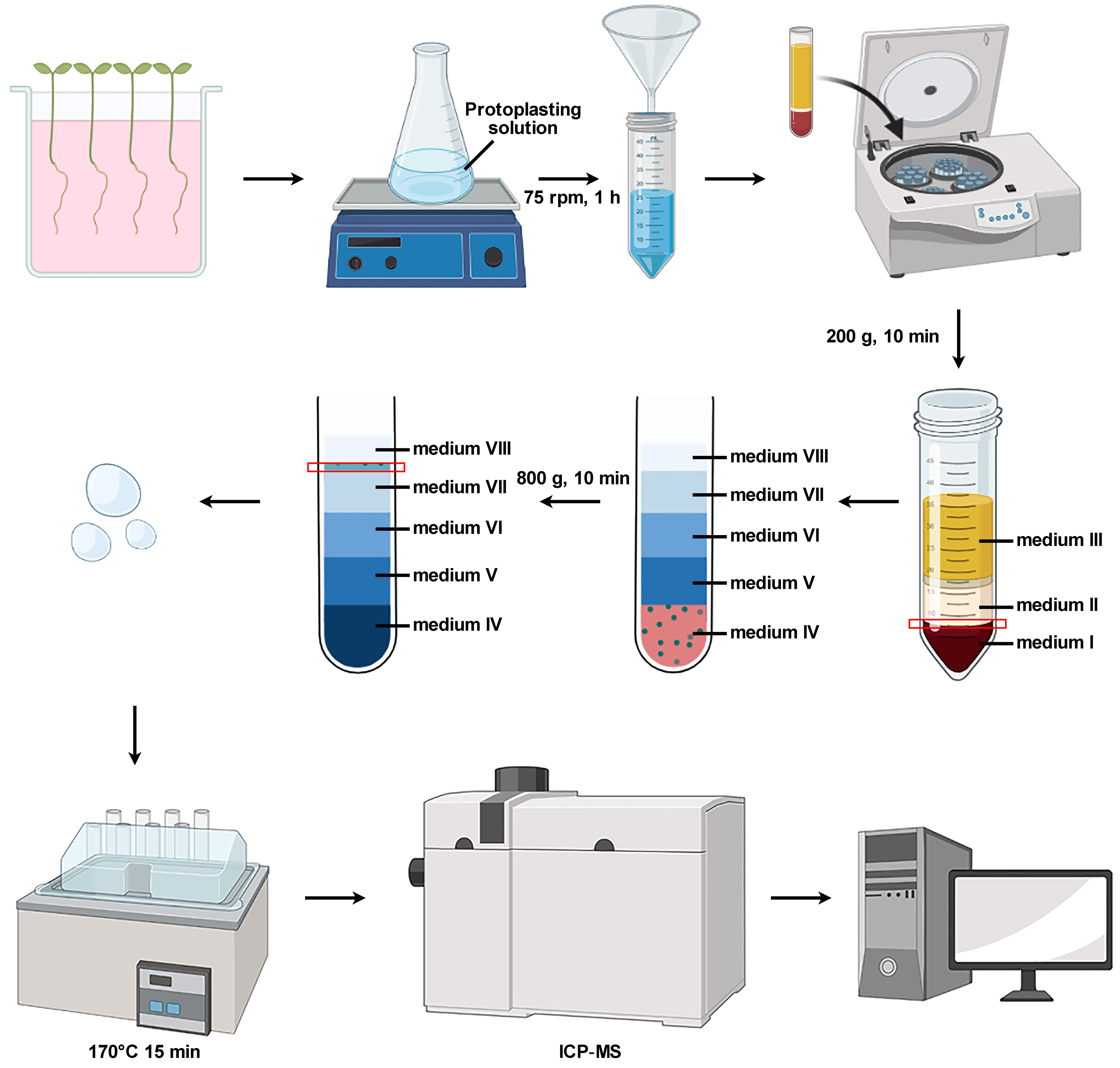
Sterilize approximately 50 Arabidopsis seeds, plant on 1/2 MS medium, and then grow in an artificial illumination incubator under normal light conditions (100 µmol m-2 s-1) with a long-day cycle (16:8 h light/dark) at 22 °C. After seven days, clamp the seedlings with a small sponge and place them on a reticulated floating board; immerse the roots in 1/5 Hoagland solution and then grow in an artificial illumination incubator under normal light conditions (100 µmol m-2 s-1) with a short-day cycle (8:16 h light/dark) at 22 °C. Change the solution every three days. After seven weeks, transfer the seedlings to the same solution containing 240 μM MnSO4 for another seven days.
Take 10–15 seedling roots and cut them into small pieces with a blade, depositing them into a 100 mL flask containing the protoplasting solution (see Recipes). Generally, 20 mL of protoplasting solution is used per 15 seedling roots.
Shake the flasks gently (75 rpm) at room temperature for 1 h. A longer incubation time may increase the protoplast yield.
Filter the protoplast solution with a 40 μm cell strainer and carefully pour into a 50 mL tube.
Vacuole extraction
In order to reduce the disruption of protoplasts, first add 5 mL of medium I to the bottom of the tube and then gently mix the filtered protoplasts. After centrifugation (200 × g, 10 min), discard the supernatant with a pipette to obtain sedimented protoplasts.
Slowly add 5 mL of medium I into the tube and gently mix with the sedimented protoplasts.
Using a pipette, add 5 mL of medium II to the tube against the wall, and then 5 mL of medium III. Each layer of liquid should be added slowly to ensure that the layers do not break apart. After adding medium III, the gradient is formed and can be clearly seen in the tube. After centrifugation (800 × g, 10 min), purified colorless protoplasts are obtained in the interfaces between medium I and II or between medium II and III. Discard the latter protoplasts (between medium II and III) with the pipette to avoid contamination of the vacuolar fractions with protoplasts.
Gently mix approximately 2 mL of the remaining purified protoplasts with 2 mL of medium B and incubate on ice for 5 min.
Gently mix the mixture of protoplasts, vacuoles, and lysate (from step B4) with 5 mL of medium IV first. Then, a gradient is formed by consecutive overlaying with 5 mL each of medium V, medium VI, medium VII, and medium VIII.
After centrifugation (800 × g, 10 min), discard medium VIII with a pipette. Carefully aspirate approximately 1 mL of liquid between medium VII and medium VIII as the purified vacuoles.
Elemental analysis
Determine the number of vacuoles using a Fuchs-Rosenthal hemacytometer. (Approximately 10 vacuoles per square millimeter of the Fuchs-Rosenthal hemacytometer, whose height is 0.1 mm through microscope observation; so, there are 100 vacuoles in each cubic millimeter of solution.) The total number of vacuoles in the solution is obtained by multiplying the number of vacuoles per cubic millimeter by the volume of the solution.
Digest the vacuoles in PFA vessels with 1.5 mL of HNO3 (65%) and 0.6 mL of H2O2 (30%) for 15 min in the dissolver at 170 °C.
After cooling down to room temperature, transfer the digestion solution to a plastic volumetric flask and dilute to 5 mL with ultrapure water (18.25 MΩ cm−1).
Determine Mn in the digestion solution using an inductively coupled plasma mass spectrometer (ICP-MS).
Data analysis
The vacuolar elemental concentration of each plant material requires three biological replicates to be measured. The mean value of three biological replicates was calculated and statistically analyzed (Student’s t-test). Detailed data and statistical methods could be found in the previously published articles (Figure 5B in Zhang et al., 2021 and Figure 4B in Ju et al., 2022).
Recipes
1/2 MS medium (adjust the pH to 5.7 using Tris)
Reagent Final concentration Amount MS base salts with vitamins 0.222% 2.22 g Sucrose 1% 10 g Agar
ddH2O
Total
1%
n/a
n/a
10 g
Up to 1 L
1 L
1/5 Hoagland Solution
Reagent Final concentration Amount KNO3 1 μM 0.51 g Ca(NO3)2·4H2O 1 μM 1.18 g NH4H2PO4
MgSO4·7H2O
H3BO3
MnCl2·4H2O
(NH4)6Mo7O24·4H2O
ZnSO4·7H2O
CuSO4·5H2O
EDTA-Fe(III)Na
ddH2O
Total
0.2 μM
0.4 μM
3 nM
0.5 nM
1 nM
0.4 nM
0.2 nM
20 nM
n/a
n/a
0.12 g
0.49 g
1.85 μg
0.99 μg
12.36 μg
1.15 μg
0.05 μg
8.42 μg
Up to 1 L
1 L
MES-Tris buffer
Reagent Final concentration Amount MES hydrate 0.5 M 9.76 g Tris-base Adjust pH to 5.7 ddH2O n/a Up to 100 mL Total n/a 100 mL Protoplasting solution
Reagent Final concentration Amount Cellulase R10 1.25% 1.25 g Macerozyme R10 0.3% 0.3 g D-mannitol (0.8 M) 0.4 M 50 mL MES-Tris buffer (0.5 M, pH 5.7) 20 mM 4 mL KCl (1 M) 20 mM 2 mL Heat the solution to 55 °C for 10 minutes and let it cool down to room temperature BSA 0.1% 0.1 g CaCl2 (1 M) 10 mM 1 mL β-mercaptoethanol (14.3 M) 5 mM 35 μL ddH2O n/a Up to 100 mL Total n/a 100 mL Medium B
Reagent Final concentration Amount HEPES-Tris (1 M, pH 7.2) 30 mM 3 mL K-gluconate 30 mM 0.7 g MgCI2 (1 M) 2 mM 0.2 mL EGTA (0.5 M) 2 mM 0.4 mL ddH2O n/a Up to 100 mL Total n/a 100 mL Medium I
Reagent Final concentration Amount Sucrose 400 mM 13.69 g Percoll 50% 50 mL Medium B n/a Up to 100 mL Total n/a 100 mL Medium II
Reagent Final concentration Amount Sucrose 400 mM 13.69 g Percoll 7.5% 7.5 mL Medium B n/a Up to 100 mL Total n/a 100 mL Medium III
Reagent Final concentration Amount D-sorbitol 400 mM 7.27 g Medium B n/a Up to 100 mL Total n/a 100 mL Medium IV
Reagent Final concentration Amount D-sorbitol 200 mM 3.64 g Percoll 25% 25 mL Medium B n/a Up to 100 mL Total n/a 100 mL Medium V
Reagent Final concentration Amount Sucrose 200 mM 6.85 g Percoll 7.5% 7.5 mL Medium B n/a Up to 100 mL Total n/a 100 mL Medium VI
Reagent Final concentration Amount Sucrose 200 mM 6.85 g Percoll 5% 5 mL Medium B n/a Up to 100 mL Total n/a 100 mL Medium VII
Reagent Final concentration Amount Sucrose 200 mM 6.85 g Percoll 2.5% 2.5 mL Medium B n/a Up to 100 mL Total n/a 100 mL Medium VIII
Reagent Final concentration Amount Sucrose 200 mM 6.85 g Medium B n/a Up to 100 mL Total n/a 100 mL
Acknowledgments
This research was funded by a grant from the National Natural Science Foundation of China (31770289 to C.W.), Northwest A&F University (Z111021604 to C.W.), and the National Natural Science Foundation of China (31900236 to Z.Z.).
The initial publication on preparation of the plant material is published in Eroglu et al. (2016); the initial publication on protoplast preparation of the plant material is published on Bargmann et al. (2010); the initial publication on vacuole extraction is published on Shimaoka et al. (2004).
Competing interests
Non-financial competing interests on behalf of all authors.
References
- Bargmann, B. O. and Birnbaum, K. D. (2010). Fluorescence activated cell sorting of plant protoplasts. J vis exp (36).
- Eroglu, S., Meier, B., von Wirén, N. and Peiter, E. (2016). The vacuolar manganese transporter MTP8 determines tolerance to iron deficiency-induced chlorosis in Arabidopsis. Plant Physiol 170(2): 1030-1045.
- Ju, C., Zhang, Z., Deng, J., Miao, C., Wang, Z., Wallrad, L., Javed, L., Fu, D., Zhang, T., Kudla, J., Gong, Z. and Wang, C. (2022). Ca2+-dependent successive phosphorylation of vacuolar transporter MTP8 by CBL2/3-CIPK3/9/26 and CPK5 is critical for manganese homeostasis in Arabidopsis. Mol Plant 15(3): 419-437.
- Martinoia, E., Schramm, M. J., Kaiser, G., Kaiser, W. M. and Heber, U. (1986). Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol 80(4): 895-901.
- Massonneau, A., Martinoia, E., Dietz, K. J. and Mimura, T. (2000). Phosphate uptake across the tonoplast of intact vacuoles isolated from suspension-cultured cells of Catharanthus roseus (L.) G. Don. Planta 211(3): 390-395.
- Shimaoka, T., Ohnishi, M., Sazuka, T., Mitsuhashi, N., Hara-Nishimura, I., Shimazaki, K., Maeshima, M., Yokota, A., Tomizawa, K. and Mimura, T. (2004). Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant Cell Physiol 45(6): 672-683.
- Zhang, Z., Fu, D., Sun, Z., Ju, C., Miao, C., Wang, Z., Xie, D., Ma, L., Gong, Z. and Wang, C. (2021). Tonoplast-associated calcium signaling regulates manganese homeostasis in Arabidopsis. Mol Plant 14(5): 805-819.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Ju, C., Fu, D., Wang, C. and Zhang, Z. (2023). Isolation of Intact Vacuoles from Arabidopsis Root Protoplasts and Elemental Analysis. Bio-protocol 13(5): e4625. DOI: 10.21769/BioProtoc.4625.
Category
Plant Science > Plant cell biology > Organelle isolation
Plant Science > Plant biochemistry > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










