- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Automated Sleep Deprivation Setup Using a Shaking Platform in Mice
Published: Vol 13, Iss 4, Feb 20, 2023 DOI: 10.21769/BioProtoc.4620 Views: 2009
Reviewed by: Christine Muheim Shaorong MaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Using Fiber Photometry in Mice to Estimate Fluorescent Biosensor Levels During Sleep
Mie Andersen [...] Celia Kjaerby
Aug 5, 2023 2045 Views

Locomotor Activity Monitoring in Mice to Study the Phase Shift of Circadian Rhythms Using ClockLab (Actimetrics)
Andrea Brenna [...] Urs Albrecht
Feb 20, 2025 1785 Views

A Protocol to Assess Time-of-Day-Dependent Learning and Memory in Mice Using the Novel Object Recognition Test
Jordan Mar [...] Isabella Farhy-Tselnicker
Sep 20, 2025 2497 Views
Abstract
The functions of sleep remain largely unclear, and even less is known about its role in development. A general strategy to tackle these questions is to disrupt sleep and measure the outcomes. However, some existing sleep deprivation methods may not be suitable for studying the effects of chronic sleep disruption, due to their lack of effectiveness and/or robustness, substantial stress caused by the deprivation method, or consuming a large quantity of time and manpower. More problems may be encountered when applying these existing protocols to young, developing animals, because of their likely heightened vulnerability to stressors, and difficulties in precisely monitoring sleep at young ages. Here, we report a protocol of automated sleep disruption in mice using a commercially available, shaking platform–based deprivation system. We show that this protocol effectively and robustly deprives both non-rapid-eye-movement (NREM) sleep and rapid-eye-movement (REM) sleep without causing a significant stress response, and does not require human supervision. This protocol uses adolescent mice, but the method also works with adult mice.
Graphical abstract

Automated sleep deprivation system. The platform of the deprivation chamber was programmed to shake in a given frequency and intensity to keep the animal awake while its brain and muscle activities were continuously monitored by electroencephalography and electromyography.
Background
The functions of sleep and its long-term impact on behavior and physiology have been largely understudied. Studies focusing on the acute effect of sleep deprivation (SD) in juvenile mice, rats, and cats revealed that sleep affects synaptic structure and plasticity during adolescent development (Frank et al., 2001; Dumoulin Bridi et al., 2015; Shaffery et al., 2006; Shaffery et al., 2002; Maret et al., 2011; Yang et al., 2014; W. Li et al., 2017; de Vivo et al., 2017). However, whether these changes result in long-lasting behavioral changes remains unknown. To evaluate the impact of long-term sleep perturbation, especially during development, chronic sleep disruption is required, and so is longitudinal sleep monitoring using electroencephalogram (EEG) along the manipulation process.
Automated SD in rodents often uses physical stimulations or setups that keep the animal in constant motion, such as using continuously moving treadmills or rotating wheels (Colavito et al., 2013). In an alternating platform setup, the animal was forced to move between two small platforms that continuously and alternatively move above and below water (Pierard et al., 2007). In a setup of spinning disk above a water tank, the disk rotated whenever sleep onset was detected, and the animal would need to stay awake and mobile in order not to fall into the water (they woke up in the case they did fall) (Rechtschaffen et al., 1983). Another method is the so-called “grid over water,” where placing the animal on a grid floor suspended over the water surface significantly reduced NREM and REM sleep and increased sleep latency, but the reduction of sleep was only in half (Shinomiya et al., 2003). REM-selective deprivation can be achieved by placing the animal on a small platform surrounded by and slightly above the water surface (Morden et al., 1967). The animal was allowed to have NREM sleep by crouching on the platform; however, upon transition to a REM episode, the loss of muscle tone caused the animal to touch or fall into the water and thus aroused the animal (Morden et al., 1967). These automated SD paradigms all produce stress, anxiety, and/or excessive physical stimuli, making them unsuitable for adolescent mice, which likely have heightened vulnerability to stressors (Romeo, 2013). In comparison, more mild methods such as the “gentle touch” protocol requires constant monitoring of the mouse behavior or EEG by an experimenter, as whenever the mouse exhibits signs of sleep (e.g., staying still for longer than 5 s, or low-frequency EEG), the experimenter directly intervenes by touching the animal using a soft tool (e.g., a brush), keeping the animal awake (Eban-Rothschild et al., 2016; W. Li et al., 2017; Yang et al., 2014). However, this method is labor-intensive, and may not be easily replicated between experimenters; thus, it is not ideal if high throughput or chronic intervention is desired. Another “gentle” protocol requires exposing the animal to novel objects, a different environment, and/or nesting materials, which leads to exploratory or nest-building behaviors, respectively, and helps maintain wakefulness. This method does not cause significant stress (Kopp et al., 2006), but the animal tends to become habituated quickly, and the forced wakefulness usually does not last longer than 2 h. In addition, pre-exposure to the enrichment materials may be a confounder, if a behavior task such as the novel object recognition test is performed subsequently.
Here, we report an alternative protocol for automated SD in mice, which is effective for 4 h or longer and robust across many days, does not cause significant stress even in adolescent animals, and can be performed in high throughput (up to four mice at a time for each chamber) without human intervention. The protocol is ideal for chronic sleep disruptions over days, in both developing and adult mice. In combination with a closed loop algorithm, it is also possible to achieve REM-selective automated deprivation.
Materials and Reagents
Paper towels
Male or female mice with a C57BL/6J background produced by breeding pairs purchased from Jackson Laboratory, or with a 129S2/SvPasCrl background produced by breeding pairs purchased from Charles River. They were born and housed at constant temperature (22 ± 1 °C) and humidity (40%–60%), under a 12/12 h light:dark cycle [lights-on: 7:00–19:00, zeitgeber time (ZT) 0–12; lights-off: 19:00–7:00, ZT 12–24], with access to food and water ad libitum. Animals were single-housed in customized home-cages after implantation of the electrodes for EEG and electromyography (EMG) (see Equipment).
(Optional) An ultra-light extension cord (~50 cm in length, Cooner wires, catalog number: CW8183) was used for reducing the cable weight on adolescent mice during EEG/EMG recording; however, this is not required for adult mice.
Corticosterone ELISA kit (Enzo, catalog number: ADI-900-097, storage temperature: 4 °C)
Microvette® CB 300 Lithium heparin (Sarstedt Inc., catalog number: 16.443.100)
Hydrogel (ClearH2O, catalog number: 70-01-5022)
70% ethanol
Equipment
Sleep Deprivation System (ViewPoint Life Sciences, Inc., Lyon, France), composed of a deprivation chamber (PVC cylinder, height: 46 cm, width: 30 cm, weight: 5 Kg) with a shaking platform at the bottom, a control box, and a computer with the controlling program Shaker Driver (Figure 1A, B). A video from the vendor illustrating the setup and its use can be found in https://youtu.be/LNTc7c5yFZM.
A customized home-cage (Figure 1C) was made by taping two standard mouse cages together (without the lid, dimensions, 28 cm × 17.5 cm × 12.5 cm). A big opening (approximately 22 × 11 cm) was made on the top for the extension cord/recording cable to pass through, and a small hole was drilled on the side of bottom part, for water access. Regular mouse food pellets were placed inside the cage together with the bedding.
Eppendorf centrifuge 5424 (Eppendorf, Hamburg, Germany).
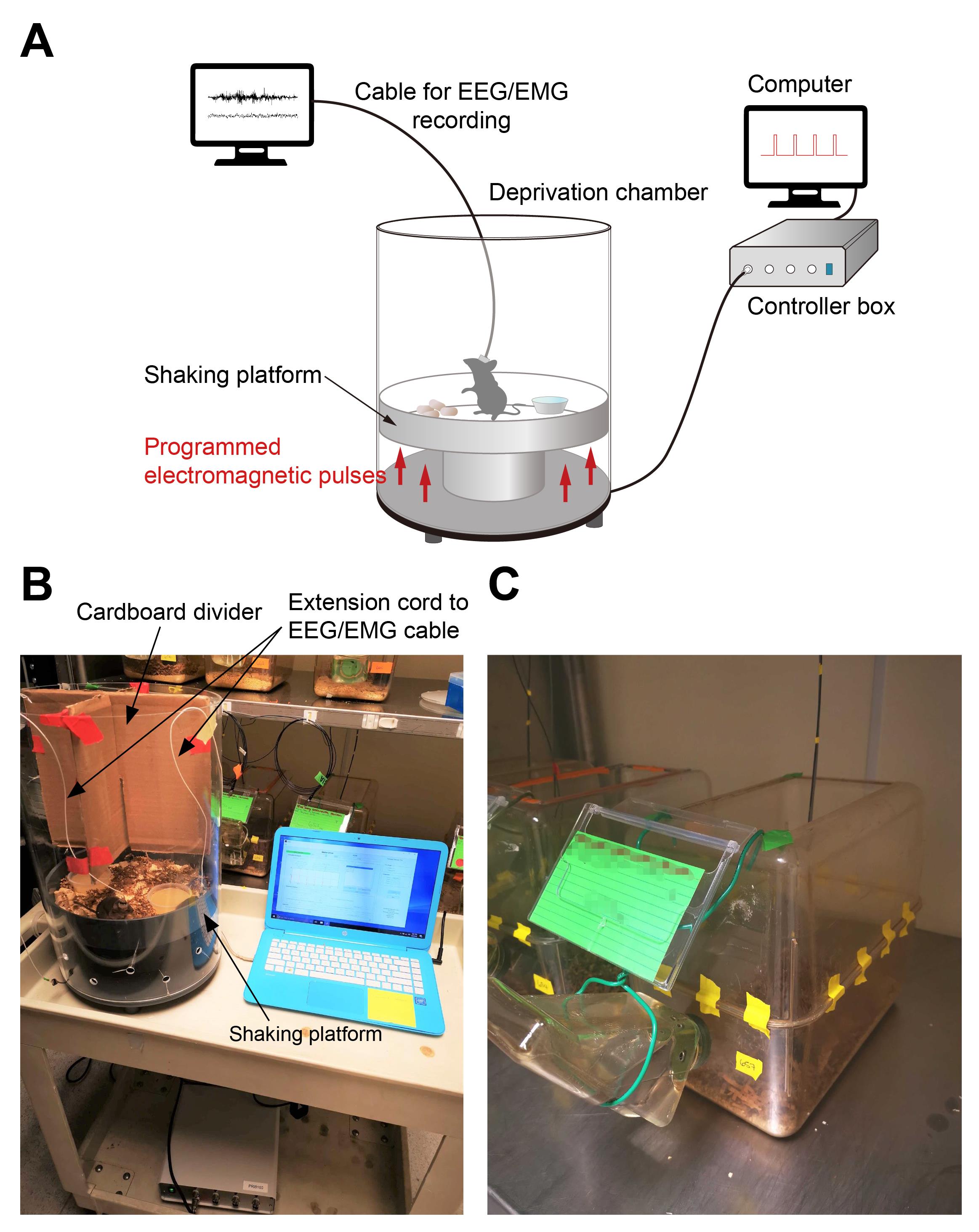
Figure 1. Automated sleep deprivation system and the customized home-cage. A. Schematics of the automated sleep deprivation system with simultaneous EEG/EMG recording. B. Sleep deprivation chamber. C. Customized home-cage.
Software
Shaker Driver v1.9.4 (ViewPoint Life Sciences) (Figure 2)
Standard data processing and statistics software: Excel, Prism (GraphPad; version 8 or higher), etc.
Procedure
The procedures of electrode implantation surgery, EEG/EMG recoding, and data processing have been described in detail in our previous studies (Eban-Rothschild et al., 2016; S. B. Li et al., 2018; S. B. Li et al., 2020; Bian et al., 2022; S. B. Li et al., 2022), and therefore will not be a focus of this protocol. The SD system can accommodate up to four mice with EEG/EMG recording at the same time, using a custom-made divider (cardboard, Makrolon plastic, or other materials) which prevents the tangling of recording cables (Figure 1B).
Wean the mouse pups on postnatal day (P) 21 and assign them randomly to Sleep Deprivation (SD) or Control (Ctrl) groups.
Implant the electrodes for EEG/EMG recording at P28–P30 (Bian et al., 2022).
After the surgery, individually house the animals in the customized home-cage (Figure 1C).
At least one day before EEG/EMG recording, habituate the animal to the ultra-light extension cord for at least 24 h, by connecting the cord to the mouse headset. Fix the other end of the extension cord above the home-cage. Allow an ample length of the extension cord, for the free moving of the animal, but avoid leaving too much of the cord in the cage to prevent the animal from biting it. Mice will be kept connected for the entire experimental period.
Baseline recording of spontaneous sleep/wake cycle in the home-cage (P36, Day 0).
Connect the extension cord to the main EEG/EMG cable and the recording setup.
Start recording at 7:00 (ZT 0) and stop recording at 7:00 the next morning (ZT 24).
Set up the SD and Ctrl protocols in the Shaker Driver (Figure 2 and Video 1).
In Stimulation Designer, set High Level Duration (ms) to 15, Total Duration (ms) to 500, and Delay (ms) to 0. Test the communication amongst the computer, the control box, and the deprivation chamber by clicking the Test button in Shaker Driver. It will generate a pulse as you programmed in Stimulation Designer, and the platform should shake accordingly.
Set up the delivery sequence of pulses for the SD and Ctrl protocols.
For the SD protocol, set Protocol duration (hours) to 4 in Sequence Designer, check “Use a randomized number of stimulations,” and set Number of Stimulations per sequence from 2 to 8. Check “Use a randomized Time between 2 sequences (min)” and set Time from 0.2 to 0.8. This will generate a train of randomized 2–8 pulses (of 15 ms in duration) delivered at 2 Hz every randomized 0.2–0.8 minutes, for a total duration of 4 h.
For the Ctrl protocol, set Protocol duration (hours) to 4 in Sequence Designer, uncheck “Use a randomized number of stimulations,” and set Number of Stimulations per sequence to 5. Uncheck “Use a randomized Time between 2 sequences (min)” and set Time to 0.5. This will generate a train of five pulses (of 15 ms in duration) delivered at 2 Hz every 0.5 min, for a total duration of 4 h.
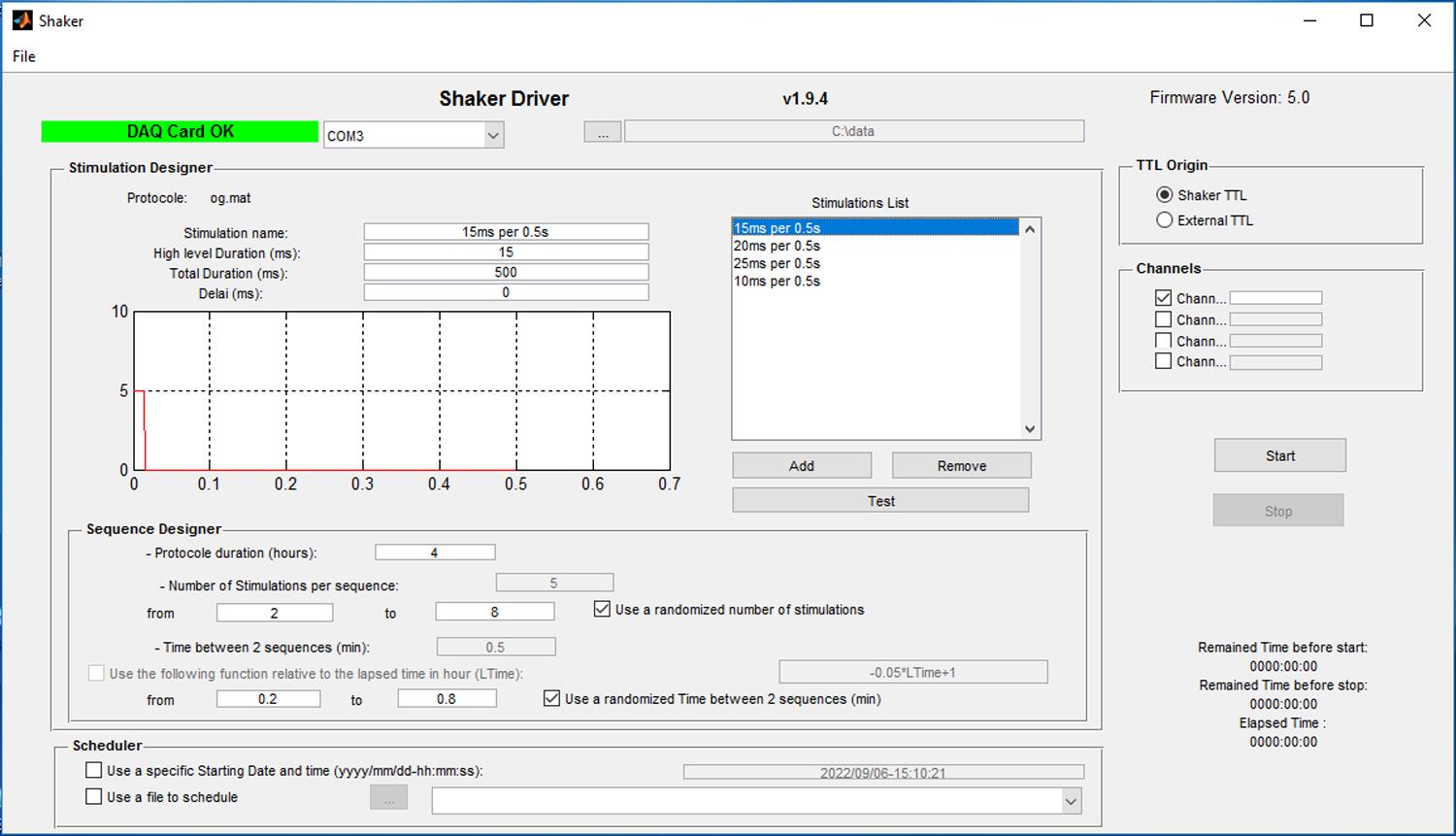
Figure 2. Software interface of Shaker DriverVideo 1. Setting up Shaker Driver for the SD and Ctrl protocols
Perform the Ctrl/SD protocol (P37–P41, Days 1–5)
Place bedding materials on the platform. Other materials/objects (e.g., shredded paper, nestlets, or paper tubes) may also be added as environmental enrichment, as they help to prevent distress in animals. Place a few food pellets and hydrogels (see Notes) on the platform, so the animals have access to food and water ad libitum.
Transfer the SD animals to the deprivation chamber at 9:00 (ZT 2). No prior acclimation or habituation to the chamber is needed. The novel environment of the chamber also helps to maintain the animals awake. Place each animal in each compartment separated by the cardboard divider and allow the extension cord to pass through from the top of the apparatus (Figure 1A, B and Video 2). Start the SD procedure in Shaker Driver. After 4 h (ZT 2–6), the procedure will stop, or it can be manually terminated by clicking the Stop button.
Video 2. Programmed platform shaking during an SD sessionFor Ctrl animals, transfer them to the deprivation chamber and start the Ctrl protocol at 19:00 (ZT 12) and stop at 23:00 (ZT 16).
After either an SD or Ctrl session, immediately return the animals to their home-cages.
Discard the bedding, food pellets, and hydrogels. Clean the apparatus with 70% ethanol.
Repeat steps a–e for the remaining days of the Ctrl/SD protocol.
EEG/EMG recording is continuously performed on Day 3 from 7:00 (ZT 0) to 7:00 the next morning (ZT 24). It can also be performed in other days during the five days of Ctrl/SD (see Notes).
One day after Ctrl/SD (P42, Day 6), record the 24-h EEG/EMG signals in the home-cage.
To examine the stress response potentially caused by the SD or Ctrl protocols, measure the plasma corticosterone level by enzyme-linked immunosorbent assay (ELISA):
Use a different cohort of animals without EEG/EMG recording and subject them to steps 6 and 7. On Days 1 and 5, immediately after the Ctrl/SD session, anesthetize the animal using isoflurane and use a capillary to collect a small quantity of blood (~100 μL) from the retro-orbital sinus into heparin-treated microvette tubes (Sarstedt Inc., 16.443.100). The animal should recover from at least one blood collection. Collect no-shake control samples from naïve animals without any manipulation at the same ZT.
Separate the plasma from the whole blood sample by centrifugation (Eppendorf, Centrifuge 5424) at 1,000 × g and room temperature for 15 min. Transfer the supernatant (plasma) to a clean tube, avoiding contamination with the blood cells in the precipitate.
Measure the corticosterone level in the plasma samples using a corticosterone ELISA kit (Enzo, ADI-900-097), according to the manufacturer’s instructions.
Data analysis
The processing and quantification of EEG/EMG data was described in our previous studies (Eban-Rothschild et al., 2016; S. B. Li et al., 2018; S. B. Li et al., 2020; Bian et al., 2022; S. B. Li et al., 2022), and is subject to change in different EEG/EMG systems—thus, it is not characterized here. States of Wake, NREM, and REM were defined based on the following criteria: Wake: desynchronized small-amplitude EEG and extensive EMG activity; NREM: large-amplitude, delta (0.25–4 Hz)-dominant oscillations in EEG, and reduced EMG activity compared to wakefulness; REM: high frequency (4–9 Hz-dominant) and relatively small and uniform amplitude of EEG, often associated with a flat EMG (muscle atonia). Any epoch that lasts longer than 4 s in SleepSign (Kissei Comtec Co.) (Eban-Rothschild et al., 2016) or 5 s in a custom MATLAB script (S. B. Li et al., 2018; S. B. Li et al., 2020; Bian et al., 2022; S. B. Li et al., 2022) was counted as a state.
Calculate the percentage of time in Wake, NREM, and REM states in each hour, and plot it against the ZT to evaluate the effect of SD. Perform repeated-measure two-way analysis of variance (RM 2-way ANOVA) followed by Bonferroni's multiple comparisons to examine the difference between the groups. Representative data can be found in Extended Data Figure 1d–f of our previous study (Bian et al., 2022).
Calculate the parameters of sleep architecture, such as the total time of each state during the entire 4-h SD session, total number of bouts of each state, average bout length, sleep rebound in the following 6 h (Bian et al., 2022), and reduction of NREM and REM sleep caused by the SD protocol compared to the Ctrl protocol (Figure 3A). Other parameters can be calculated form EEG as well, such as the power spectrum of each state, before or after SD. Representative data can be found in Extended Data Figure 1g–m of our previous study (Bian et al., 2022).
The sleep recording one day after SD (P42, Day 6) or on additional following days can be used to evaluate any long-lasting alterations in sleep architecture, by comparing it to baseline recording, or to the Ctrl animals.
The plasma corticosterone measurements from mice receiving no shaking, or immediately after the SD session on Days 1 or 5 showed no significant difference, confirming that the SD protocol does not cause significant acute stress. These results can be found in Extended Data Figure 1n of our previous study (Bian et al., 2022). The corticosterone levels on Day 5 were also not significantly different between Ctrl and SD groups (Figure 3B).
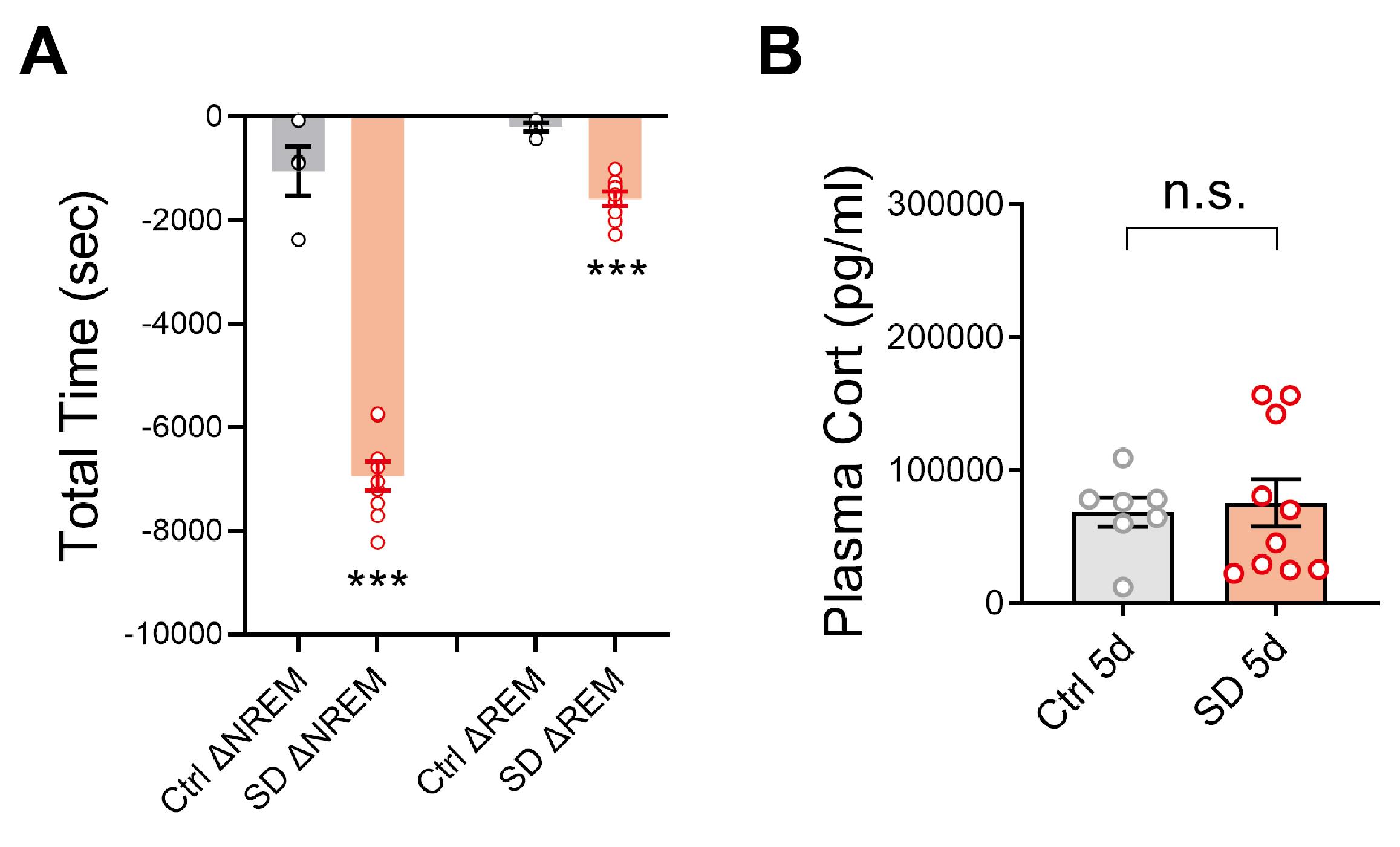
Figure 3. Representative data. A. Reduction of NREM or REM sleep amount (s) during 4 h Ctrl/SD relative to their respective baseline sleep during the same ZT period. The Ctrl protocol began immediately at dark phase onset and lasted for 4 h (ZT 12–16), and the SD protocol was performed in early light phase onset (ZT 2–6) in adolescent mice (P35–P42). N = 4 mice in Ctrl; 9 mice in SD. Welch’s t-test, ΔNREM, t = 10.63, df = 5.13, P = 0.0001; ΔREM, t = 8.74, df = 10.99, P = 0.000003. B. Five days of SD did not induce significant stress compared to the Ctrl protocol. N = 7 mice in Ctrl; 10 mice in SD. Welch’s t-test, t = 0.3331, df = 14.14, P = 0.74. Data are shown as means ± S.E.M. * P < 0.05; ** P < 0.01; *** P < 0.001; n.s., not significant. SD values were extracted from the data published in Bian et al. (2022).
Notes
The system robustly deprives the animals from sleep and can accommodate up to four mice at a time with EEG recording by using a divider.
The randomness in the SD protocol helps to reduce habituation to the shaking, and thus prevents sleep, while the non-random design in the Ctrl protocol does the opposite, i.e., minimizing the sleep disturbance as much as possible. Indeed, during Ctrl sessions we found that some mice can retain a small amount of sleep, which is not significantly different from the spontaneous sleep during the early dark hours (ZT 12–16).
We recommend avoiding using the same animals for corticosterone measurement and EEG/behavioral tests, due to potential confounding effects and poor physical conditions after repeated blood collections.
The corticosterone measurements reported in this protocol do not examine the acute/transient stress during the treatment. If that is a concern for the study, additional stress controls or more timepoints for stress measurements should be added.
Due to the short age window for manipulations in adolescent mice, we used a minimum of 24 h for habituating the animal to the recording cable. However, longer habituation time is strongly encouraged when doing less age-sensitive experiments. We have compared the EEG recordings in adolescent mice after 24 h (1 d) and 5 days (5 d) of habituation, respectively, and found that the overall sleep/wake pattern and the total time of each state were not significantly different between 1 d and 5 d (Supplementary Figure 1A and 1B). However, the average bout length of Wake and NREM episodes did appear to be increased as the habituation time increased, although only the difference in the dark-phase NREM category was statistically significant (Supplementary Figure 1C). These results suggest that 1 d habituation is sufficient for restoring the normal sleep/wake cycle, although the animal might go through slight sleep fragmentation, which is likely improved with extended habituation.
The deprivation chamber comes with a water bottle held on the cylinder. However, the water bottle has serious leaking issues during shaking, so we did not use it; instead, we just placed hydrogels on the chamber floor.
ViewPoint also offers a SleepScore software that, according to the vendor, enables real-time detection of vigilance states and specific deprivation of NREM and REM sleep (https://www.viewpoint.fr/en/p/software/sleepscore). However, we have not yet tested the performance of this software.
Acknowledgments
This work was supported by Human Frontier Science Program fellowship LT000338/2017-L (W.-J.B.), Brain & Behavior Research Foundation NARSAD Young Investigator grant 29952 (W.-J.B.) and National Institutes of Health grants R01 MH102638 (L.d.L.), R01 MH087592 (L.d.L.), R01 MH116470 (L.d.L.). Data derived from the use of the protocols described here have been published recently (Bian et al., 2022).
Competing interests
The authors declare no competing interests.
Ethics
All experimental protocols were approved by the Stanford University Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
References
- Bian, W. J., Brewer, C. L., Kauer, J. A. and de Lecea, L. (2022). Adolescent sleep shapes social novelty preference in mice. Nat Neurosci 25(7): 912-923.
- Colavito, V., Fabene, P. F., Grassi-Zucconi, G., Pifferi, F., Lamberty, Y., Bentivoglio, M. and Bertini, G. (2013). Experimental sleep deprivation as a tool to test memory deficits in rodents. Front Syst Neurosci 7: 106.
- de Vivo, L., Bellesi, M., Marshall, W., Bushong, E. A., Ellisman, M. H., Tononi, G. and Cirelli, C. (2017). Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355(6324): 507-510.
- Dumoulin Bridi, M. C., Aton, S. J., Seibt, J., Renouard, L., Coleman, T. and Frank, M. G. (2015). Rapid eye movement sleep promotes cortical plasticity in the developing brain. Sci Adv 1(6): e1500105.
- Eban-Rothschild, A., Rothschild, G., Giardino, W. J., Jones, J. R. and de Lecea, L. (2016). VTA dopaminergic neurons regulate ethologically relevant sleep-wake behaviors. Nat Neurosci 19(10): 1356-1366.
- Frank, M. G., Issa, N. P. and Stryker, M. P. (2001). Sleep enhances plasticity in the developing visual cortex. Neuron 30(1): 275-287.
- Kopp, C., Longordo, F., Nicholson, J. R. and Luthi, A. (2006). Insufficient sleep reversibly alters bidirectional synaptic plasticity and NMDA receptor function. J Neurosci 26(48): 12456-12465.
- Li, S. B., Borniger, J. C., Yamaguchi, H., Hedou, J., Gaudilliere, B. and de Lecea, L. (2020). Hypothalamic circuitry underlying stress-induced insomnia and peripheral immunosuppression. Sci Adv 6(37): eabc2590.
- Li, S. B., Damonte, V. M., Chen, C., Wang, G. X., Kebschull, J. M., Yamaguchi, H., Bian, W. J., Purmann, C., Pattni, R., Urban, A. E., et al. (2022). Hyperexcitable arousal circuits drive sleep instability during aging. Science 375(6583): eabh3021.
- Li, S. B., Nevarez, N., Giardino, W. J. and de Lecea, L. (2018). Optical probing of orexin/hypocretin receptor antagonists. Sleep 41(10): zsy141.
- Li, W., Ma, L., Yang, G. and Gan, W. B. (2017). REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci 20(3): 427-437.
- Maret, S., Faraguna, U., Nelson, A. B., Cirelli, C. and Tononi, G. (2011). Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nat Neurosci 14(11): 1418-1420.
- Morden, B., Mitchell, G. and Dement, W. (1967). Selective REM sleep deprivation and compensation phenomena in the rat. Brain Res 5(3): 339-349.
- Pierard, C., Liscia, P., Philippin, J. N., Mons, N., Lafon, T., Chauveau, F., Van Beers, P., Drouet, I., Serra, A., Jouanin, J. C. and Beracochea, D. (2007). Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacol Biochem Behav 88(1): 55-63.
- Rechtschaffen, A., Gilliland, M. A., Bergmann, B. M. and Winter, J. B. (1983). Physiological correlates of prolonged sleep deprivation in rats. Science 221(4606): 182-184.
- Romeo, R. D. (2013). The Teenage Brain: The Stress Response and the Adolescent Brain. Curr Dir Psychol Sci 22(2): 140-145.
- Shaffery, J. P., Lopez, J., Bissette, G. and Roffwarg, H. P. (2006). Rapid eye movement sleep deprivation revives a form of developmentally regulated synaptic plasticity in the visual cortex of post-critical period rats. Neurosci Lett 391(3): 96-101.
- Shaffery, J. P., Sinton, C. M., Bissette, G., Roffwarg, H. P. and Marks, G. A. (2002). Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience 110(3): 431-443.
- Shinomiya, K., Shigemoto, Y., Okuma, C., Mio, M. and Kamei, C. (2003). Effects of short-acting hypnotics on sleep latency in rats placed on grid suspended over water. Eur J Pharmacol 460(2-3): 139-144.
- Yang, G., Lai, C. S., Cichon, J., Ma, L., Li, W. and Gan, W. B. (2014). Sleep promotes branch-specific formation of dendritic spines after learning. Science 344(6188): 1173-1178.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Bian, W. J. and de Lecea, L. (2023). Automated Sleep Deprivation Setup Using a Shaking Platform in Mice . Bio-protocol 13(4): e4620. DOI: 10.21769/BioProtoc.4620.
Category
Neuroscience > Behavioral neuroscience > Sleep and arousal
Neuroscience > Development
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









