- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Reliable and Consistent Method to Quantify Percent Lethality and Life Span in Drosophila melanogaster
(*contributed equally to this work) Published: Vol 13, Iss 2, Jan 20, 2023 DOI: 10.21769/BioProtoc.4598 Views: 2293
Reviewed by: Nafisa M. JadavjiChetan PaliwalDjamel Eddine Chafai

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Validating Candidate Congenital Heart Disease Genes in Drosophila
Jun-yi Zhu [...] Zhe Han
Jun 20, 2017 9415 Views

How to Catch a Smurf? – Ageing and Beyond…
In vivo Assessment of Intestinal Permeability in Multiple Model Organisms
Raquel R. Martins [...] Michael Rera
Feb 5, 2018 13837 Views
Abstract
Drosophila melanogaster is a classic model organism to study gene function as well as toxicological effects. To study gene function, the expression of a particular gene of interest is disrupted by using the widely explorable Drosophila genetic toolkit, whereas to study toxicological effects the flies are exposed to a particular toxicant through diet. These experiments often require the quantification of lethality from embryonic to adult stages, as well as the assessment of the life span in order to check the role of the gene/toxicant of interest in Drosophila. Here, we propose an experimental protocol that enables a consistent and rigorous assessment of lethality and life span of cadmium chloride (CdCl2)–exposed or genetically perturbed flies [downregulation and overexpression of the cytosolic Cu, Zn superoxide dismutase (SOD1) gene], consecutively. The protocol insists upon the requirement of one single experimental setup that is unique, distinctive, and cost-effective as it engages minimal laboratory equipment and resources. The described methods lead to the smooth observation of the embryos, their successive stagewise transition, and life span of the adult flies post eclosion. Additionally, these methods also facilitate the assessment of crawling and climbing behavioral parameters of the larvae and adults, respectively, and allow the calculation of lethal concentration (LC50) for the mentioned toxicant as well as median survival of the flies, which can be a determining factor in proceeding with further stages of experiments.
Graphical abstract

Background
Drosophila melanogaster, a holometabolous, invertebrate model organism, has contributed extensively towards analyzing the roles of various phylogenetically conserved genes (Green et al., 2018; Dubey et al., 2020). Toxicological studies utilizing flies have also aided in the assessment of the effects of heavy metals that interfere with important biological processes (Nanda and Firdaus, 2021; Nanda and Firdaus, 2022), including immune system (Nanda et al., 2019). Drosophila research has also paved a way for the exploration of innumerable scientific questions in various branches of biological sciences. Notably, Drosophila melanogaster genome is 60% homologous to humans (Adams et al., 2000).
Analysis of Drosophila health and activity is a fundamental aspect to quantify any adversities either due to genetic manipulation or dietary exposure to toxicant. Among the various life traits, quantifying stagewise lethality, checking life span (Carey et al., 2006), and examining locomotor and reproductive capabilities (Rogina et al., 2007) are the biomarkers most often used (Landis et al., 2020; Olcott et al., 2010). Several behavioral assays also facilitate the analysis of a typical mutant from embryonic to adult stage (Ain and Firdaus, 2021).
The Cu, Zn superoxide dismutase (SOD1) is an antioxidant enzyme that provides protection against excessive reactive oxygen species (ROS) in Drosophila melanogaster. The three isoforms of SOD in Drosophila, namely SOD1, mitochondrial Mn superoxide dismutase SOD 2 (SOD2), and extracellular Cu Zn superoxide dismutase (SOD 3), are encoded by genes located on separate chromosomal loci (Duttaroy et al., 1994; Blackney et al., 2014). In this protocol, we used Drosophila wherein the cytosolic SOD1 gene expression was either overexpressed or downregulated in a ubiquitous manner through genetic crosses between the driver Act-5c-GAL4 and the responder RNAi/overexpression fly lines. This enabled the study of the role of SOD1 gene in life span, lethality, and motility behaviour of Drosophila.
We describe a simple continuous method that requires a single experimental setup to perform both the lethality and life span assays of the genetically altered or toxicant-exposed Drosophila. The protocol is unique and significant, due to the fact that it highlights the usage of minimal laboratory equipment and uses one single experimental setup for continuous monitoring of lethality followed by survival analysis and crawling and climbing assays in larvae and adults, respectively.
Materials and Reagents
Autoclavable glass vials (Borosil, custom made, RIVIERA brand)
Black craft paper (120 GSM)
Brush (soft and synthetic bristles)
Cylinder with spout, CL B (Borosil, catalog number: 3022006)
Conical flask (Borosil)
Graph paper (0.2 mm2)
Measuring cylinder (Borosil)
Non-absorbent cotton
Petri plate (Borosil, catalog number: 3165077
Pipette and microtips (100–1,000 μL) (Thermo Scientific, catalog number: NH27702)
Stirrer (Borosil)
Tapping pad (self-made with soft, cushion material)
Tissue roll, 20 m (Indiamart, catalog number: 128587378)
Watch glass, 75 mm (MRSC Borosilicate)
Agar agar, type I (HiMedia, catalog number: 0000227116)
Agarose low EEO, regular grade (SRL, catalog number: 9303545)
Cadmium chloride (CdCl2) (Rankem, catalog number: N041M14)
Corn flour (single batch) (Dr. RBL’s Corn Flour Atta)
Propionic acid (Qualigens, catalog number: 26955)
Sucrose (TITAN, CAS-57-50-1)
Yeast extract: TM media
Drosophila stocks (Bloomington Drosophila Stock Center):
a) BS #24750: w[1]; P{w[+mC]=UAS-Sod1.A}B37 (express SOD1 under UAS control); mentioned as the simplified name w;+;UAS-SOD1
b) BS #24493: w[*];P{w[+mC]=UAS-Sod1.IR}F103/SM5 (express dsRNA of SOD1 under UAS control); mentioned as w;UAS-SOD1-RNAi;+
c) w;Act-5c-GAL4/CyO-GFP;+ (ubiquitous GAL4)
d) Oregon R (wild-type strain of Drosophila)
Drosophila food media (100 mL) (see Recipes)
Egg-laying media (20 mL) (see Recipes)
Petri plate for crawling assay (2% agarose) (see Recipes)
10 mM CdCl2 stock solution (see Recipes)
30 mM CdCl2 stock solution (see Recipes)
100 mM CdCl2 stock solution (see Recipes)
Equipment
Autoclave (Equitron, catalog number: 7431STWL.AFH.430)
BOD incubator (Thermotech, catalog number: TH-7004)
Fluorescence microscope (RADICAL Scientific, catalog number: RXLr-5-50)
Lab electric heater: eiSCO, 220 V, up to 450°C
Stereomicroscope (Magnus Analytics, MSZ-Bi, catalog number: 16G1070)
Water distillation unit (BOROSIL-HD-XL, Yuwinsil)
Weighing balance (Mettler Toledo, catalog number: ME204/A04)
Thermometer (LABWORLD, mercury thermometer)
Software
GraphPad Prism 9
Procedure
Egg-laying media preparation
Measure the required quantities of components for the egg-laying media (see Recipes).
Boil the media in a conical flask at 100°C. Let the media cool down and then add propionic acid.
Pour 2 mL of media in the required number of autoclaved vials and seal the vials with autoclaved cotton plugs. Add yeast paste (yeast extract dissolved in water) at the corner of each vial in order to enhance egg-laying capacity.
Setting up crosses for egg collection
Collect 10 virgin females from each of the following fly lines: w;+;UAS-SOD1, w;Act-5c-GAL4/CyO-GFP;+ (ubiquitous driver), and w;UAS-SOD1-RNAi;+. Collect the virgins with the help of a stereomicroscope, either in the pupal stage by identifying the male testes as two black dots or in adult stage by detecting meconium and gonads in the posterior.
Set three vials containing egg-laying media as follows: (a) SOD1 overexpression: 2 days old, 10 virgin females of w;+;UAS-SOD1 and 5 males of w; Act-5c-GAL4/CyO-GFP;+, (b) 10 virgin females of w;+;UAS-SOD1 and 5 males of Oregon R, and (c) 10 virgin females of w;Act-5c-GAL4/CyO-GFP;+ and 5 males of Oregon R. The first filial generation (F1) of the above-mentioned crosses (a), (b), and (c) will be referred to as M1, C1, and C2, respectively. Write the date of crossing and genotype in each vial using a marker. Similarly, set up egg collection for SOD1 downregulated progenies: (a) SOD1 downregulation: 10 virgin females of w;UAS-SOD1-RNAi;+ and 5 males of w;Act-5c-GAL4/CyO-GFP;+ (F1 named as M2), and (b) 10 virgin females of w;UAS-SOD1-RNAi;+ and 5 males of Oregon R (C3 progenies).
In order to assess the effect of various concentrations of CdCl2 on Drosophila, set up egg-laying media vials containing Oregon R males and females in a 1:2 ratio.
Drosophila food media preparation
Measure the required quantities (see Recipes) and mix in a flask with a glass stirrer.
Boil the media for 15 min on a heater (up to 100°C). Autoclave the media along with the required number of washed glass vials and non-absorbent cotton.
Allow the media to cool for a few minutes; then, add the required amount of propionic acid with continuous stirring. Pour 3 mL of media to each vial and seal with cotton plugs.
In case of metal media preparation, follow steps C1–C3 without pouring the media in the vials. Prepare CdCl2 stock solutions and pour the required volumes in separate media flasks to obtain working concentrations of 0.05 and 0.1 mM (from a 10 mM stock solution), 0.3, 0.6, and 0.75 mM (from a 30 mM stock), and 1 and 1.5 mM (from a 100 mM stock solution) of CdCl2 (see Recipes). Pour 3 mL of media of each concentration to separate vials and seal with cotton plugs.
Egg collection and rearing
Collect the eggs from the vial by gently stroking with a fine brush soaked in water (Figure 1a). Do not rupture the eggs (check whether they are healthy by microscopic observation of intact micropyle in the egg structure) (Figure 1b). Do not keep any damaged eggs as it may lead to erroneous results in lethality quantification.
Place the collected eggs from the experimental setup B2 and B3 in a small square black paper, in cohorts of 40 eggs per vial. Mark the vials with the number of eggs collected, date of collection, and genotype using a marker.
Maintain the egg-containing vials at a constant temperature in the incubator. Confirm temperatures using a laboratory thermometer. Notably, GAL4 activity gets enhanced and best expressed at 29°C while the life cycle of Drosophila is fast-forwarded to 10 days. Hence, for maximal SOD upregulation and downregulation, raise flies at 29°C.
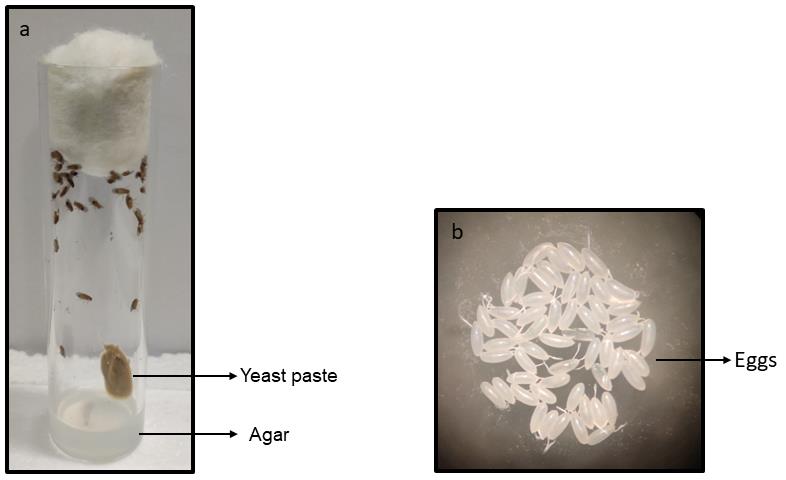
Figure 1. Egg collection setup (Figure 1a) and eggs after collection (Figure 1b)Screening
Genetic crosses often require segregation of markers as per mating schemes given in step B2. Here, green fluorescent protein (GFP)–tagged embryos were separated from non-GFP embryos in experimental setups B2 before performing step D2.
Place the healthy eggs monitored in step D1 in watch glasses. Mark the watch glasses with the genotype, so as not to mix up eggs.
Use the fluorescence microscope to segregate GFP-positive from non-GFP eggs. Remove the GFP-positive eggs gently with a brush and discard them.
Place a black paper containing non-GFP eggs in the respective media vials and follow step D3.
Observation and counting of stagewise lethality
Check the egg-containing vials each day. Observe under the stereomicroscope for lethality in embryonic (yellowish to blackish appearance) or larval stage (no movement of mouth hook).
Transfer 3rd-instar larvae (n = 20) of control and metal-treated (0.3 and 0.6 mM CdCl2) from extra vials kept for performing behavioral assays at larval stage.
Observe and count the number of healthy pupae as well as degenerated pupae. Note down the stage of pupal degeneration: early, mid, or late stage. Encircle pupae with a marker to count their number.
Make a note of number and dates of fly eclosion. Also, mark any lethality in adults during their eclosion.
Maintenance of adult flies for survival assay
Collect flies immediately after their eclosion. Transfer them to fresh media vials. Write the date of eclosion of each genotype on the vial with a marker. Transfer CdCl2-raised flies to normal media post eclosion for survival analysis. Record the death events every day until all flies are dead in one of the groups.
Keep aside 20 flies from CdCl2 and normal media vials for behavioral analysis.
Crawling assay
Dissolve 2% agarose in distilled water and boil it. Pour the dissolved solution quickly in a glass Petri plate and allow to solidify. Place the Petri plate over a 0.2 mm2 graph paper.
Wash 3rd-instar wandering larvae (n = 20), as collected in step F2, one at a time with distilled water; remove the excess water with tissue paper.
Transfer one larva at a time to the center of the Petri plate (Figure 2) and allow it to acclimatize for 1 min. Then, start a one-minute timer and note down the number of grids crossed by the larva in the stipulated time. Return the larva to the center and, after a 1 min interval, note down the second reading for the same larva in terms of number of grids crossed in one minute.
Repeat the above-mentioned process with each of the 20 larvae collected from control, 0.3 mM, and 0.6 mM CdCl2.
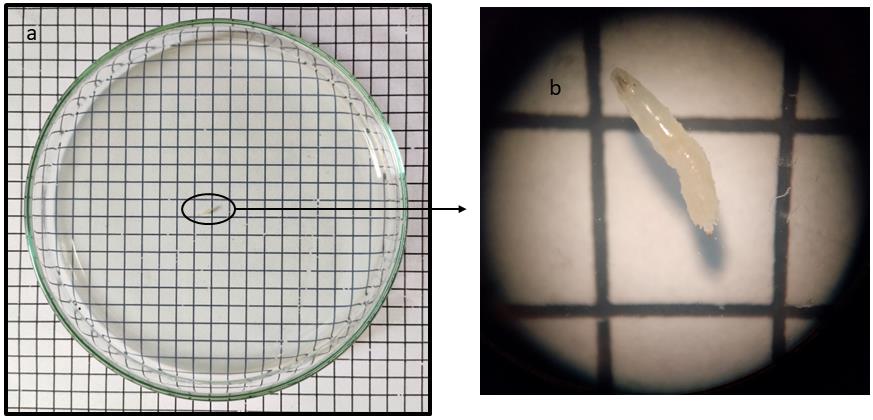
Figure 2. The figure shows a third-instar larva (a) as well as its enlarged view (b), transferred to the center of a Petri plate, coated with 2% agarose and placed over a 0.2 mm2 graph paperClimbing assay
Collect the adults, as mentioned in step G2, two days post eclosion.
Introduce one fly at a time in a 10 mL measuring cylinder, seal it with a cotton plug (Figure 3), and allow the fly to acclimatize for 1 min. Tap the cylinder thrice before recording the time taken by the fly to climb a distance of 10 cm. Repeat after a period of 1 min to get a second reading of the same fly.
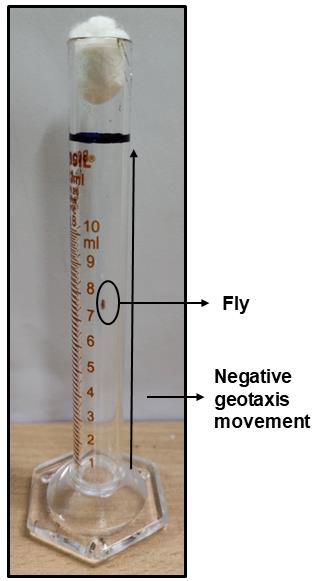
Figure 3. Image of the adult fly climbing assay, wherein a distance of 10 cm has been marked in the cylinder (black line). Time taken for a fly to climb the marked distance was recorded.
Data analysis
Developmental lethality, delayed progression to next molt, shortening of life span, and disrupted locomotor activity are some of the most important indicators of adverse effects of any mutation or toxicant exposure in Drosophila. These can be experimentally analyzed using the protocols described in the above sections.
Quantification of lethality
Open the software GraphPad Prism to plot “Grouped” graph and perform a 2-way ANOVA analysis in order to verify significant lethality of SOD1 mutant flies as well as cadmium-treated flies compared to their respective controls. Lethality plot allows to comprehend the decrease in number of live individuals during egg to pupae transition and then further death events during pupal development, leading to declined emergence of adult fly when exposed to varying concentrations of CdCl2 (Figure 4). In each triplicate, the number of pupae as well as the eclosed fly was counted to quantify the stagewise lethality due to either toxicant exposure or SOD1 level manipulation. LC50 can be calculated for the egg-to-pupae and pupae-to-adult fly transition (Nanda and Firdaus, 2022). The percentage of embryos that displayed lethality during their metamorphosis to pupal stage were 8.3%, 25%, 43.3%, 66.67%, 80%, 88.3%, and 100% for concentrations of 0.05, 0.1, 0.3, 0.6, 0.75, 1, and 1.5 mM of CdCl2, respectively. During pupal metamorphosis, 11%, 35%, 55%, 81.6%, 83.33%, and 95% pupae degenerated from the CdCl2 concentrations of 0.05, 0.1, 0.3, 0.6, 0.75, and 1 mM, respectively. The embryos raised in 1.5 mM CdCl2 media displayed 100% lethality. SOD1-overexpressed flies showed severe lethality during early as well as pupal development; approximately 68% embryos were able to form pupae, of which only 39.6% successfully metamorphosed to adult flies (Figure 5). SOD1 downregulation led to pupal stage lethality with no effect on early development (Figure 4), with only 45.2% embryos successfully surviving until the adult stage.
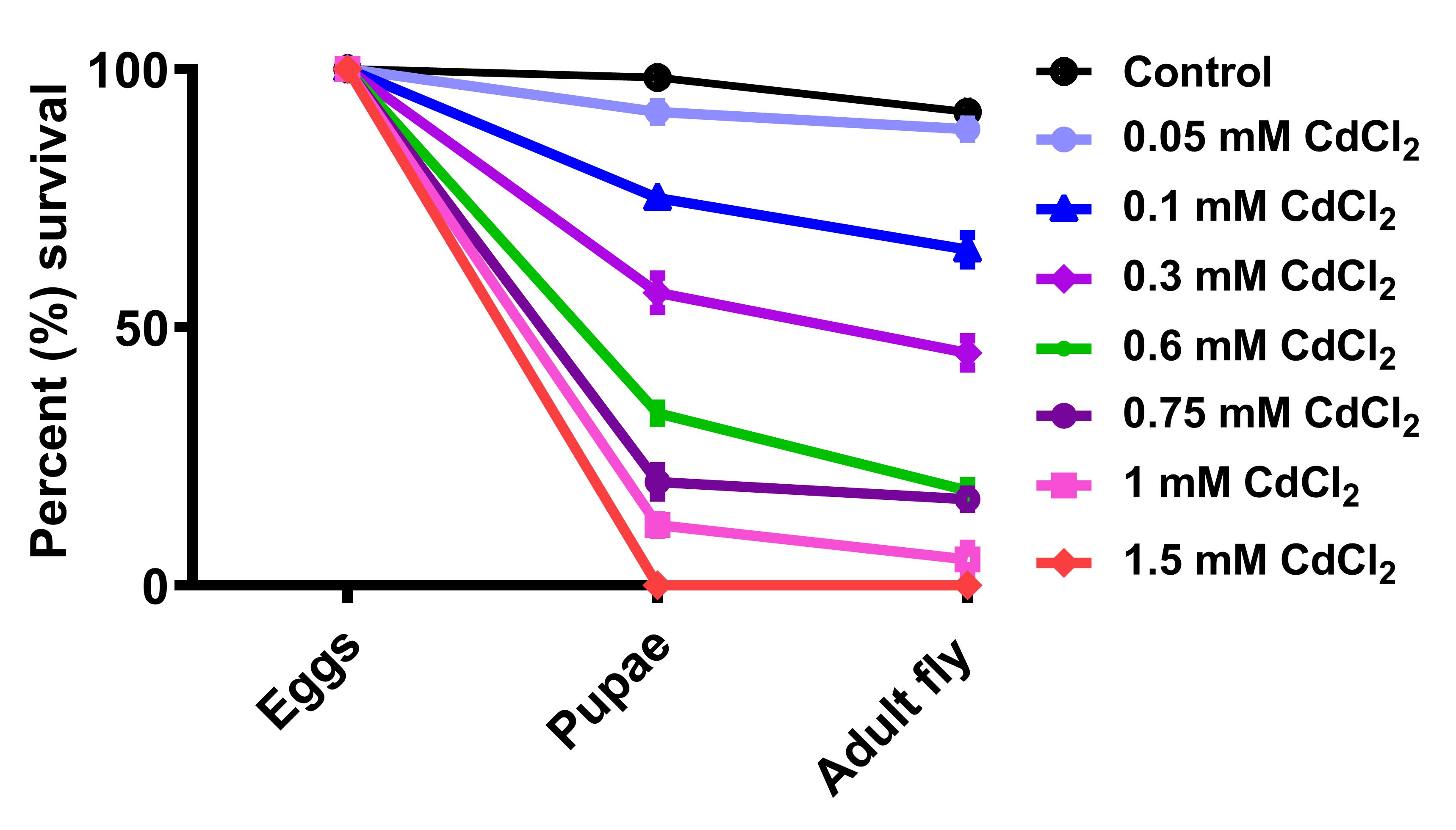
Figure 4. Percent survival of Drosophila in varying CdCl2 concentrations. The graph shows survival percentages of Drosophila at two different developmental stages when eggs were transferred to metal media (taken from Nanda and Firdaus, 2022).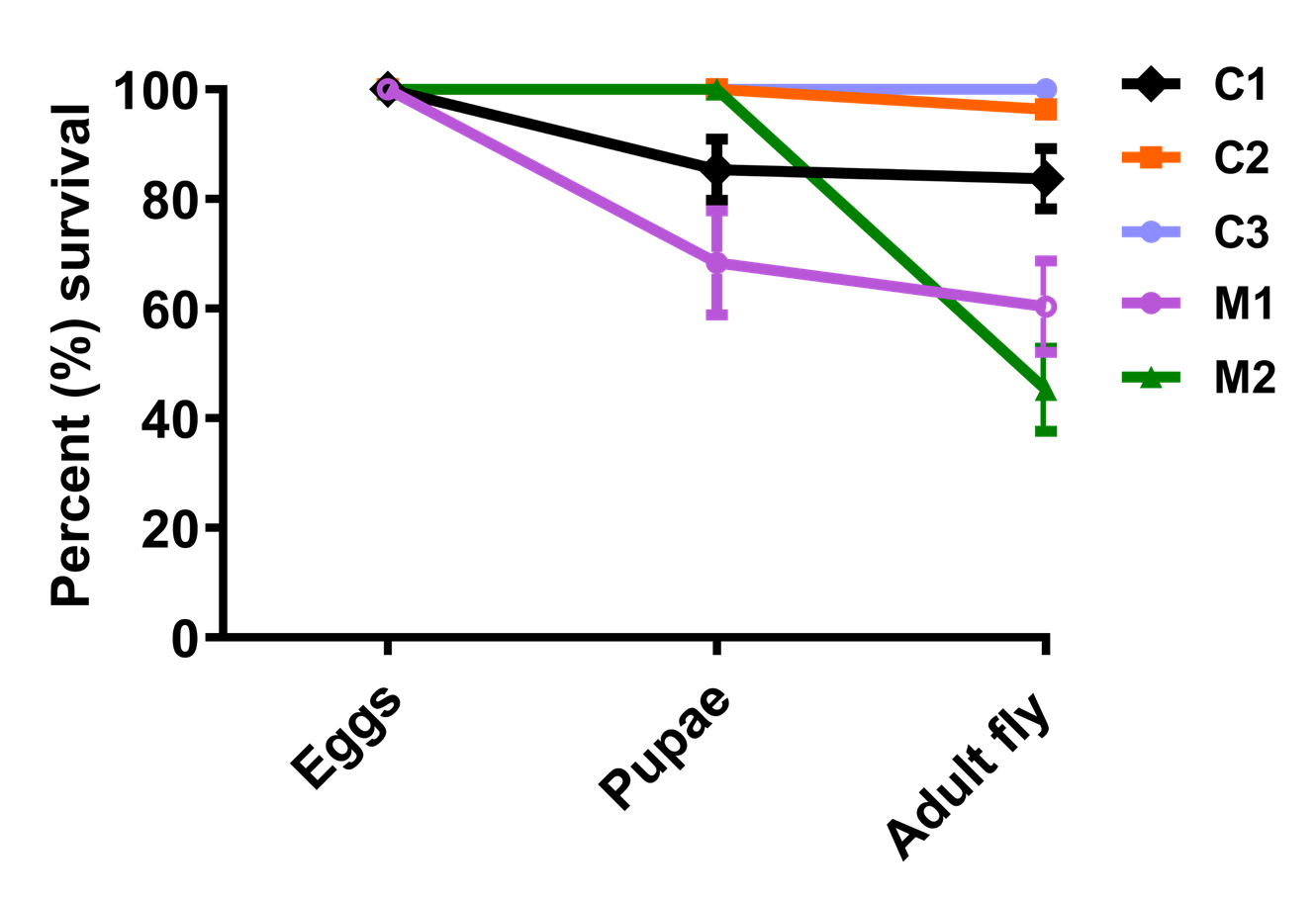
Figure 5. Percent survival of SOD1-overexpressed (M1, w;Act-5c-Gal4/+;UAS-SOD1/+) and downregulated (M2, w;Act-5c-Gal4/UAS-SOD1-RNAi;+) Drosophila in comparison to their respective controls C1 (w/+; +; UAS-SOD1/+), C2 (w/+; Act-5c-GAL4/+;+), and C3 (w/+;UAS-SOD1-RNAi/+;+)Life span assay
To analyze life span of SOD1 mutant flies as well as cadmium-treated flies, plot “Survival” graph and perform “Log-rank (Mantel-Cox test)” by using GraphPad Prism. The life span of the flies post eclosion was observed to be shortened as reflected by the decrease in their median survival with an increase in CdCl2 concentration (Figure 6).
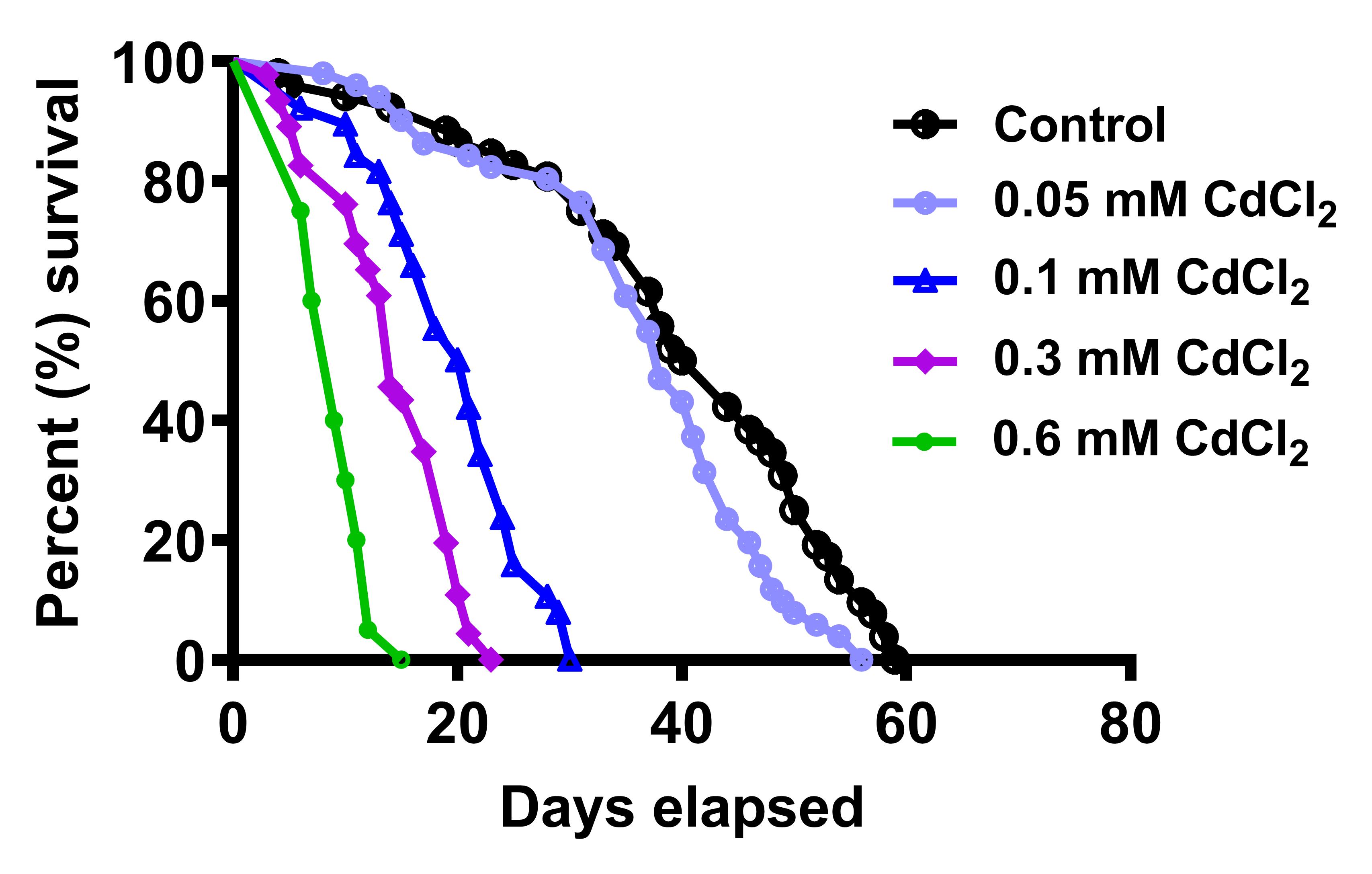
Figure 6. Life span of CdCl2-raised flies. Cd animals succumb earlier than their control counterparts and median survival decreases with increasing metal concentration (Figure taken from Nanda and Firdaus, 2022).SOD1-overexpressed flies (M1) showed median survival of 39 days and its respective controls C1 and C2 had median survival of 51 and 64.5 days, respectively. Although the survival curve of M1 was significantly different from C2, it was comparable to C1. Likewise, SOD1 downregulation (M2) led to a median survival of 56.5 days, whereas its respective controls C2 and C3 median survival was calculated as 64.5 and 66 days, respectively. Herein, M2 showed no significant differences with C2 (Figure 7).
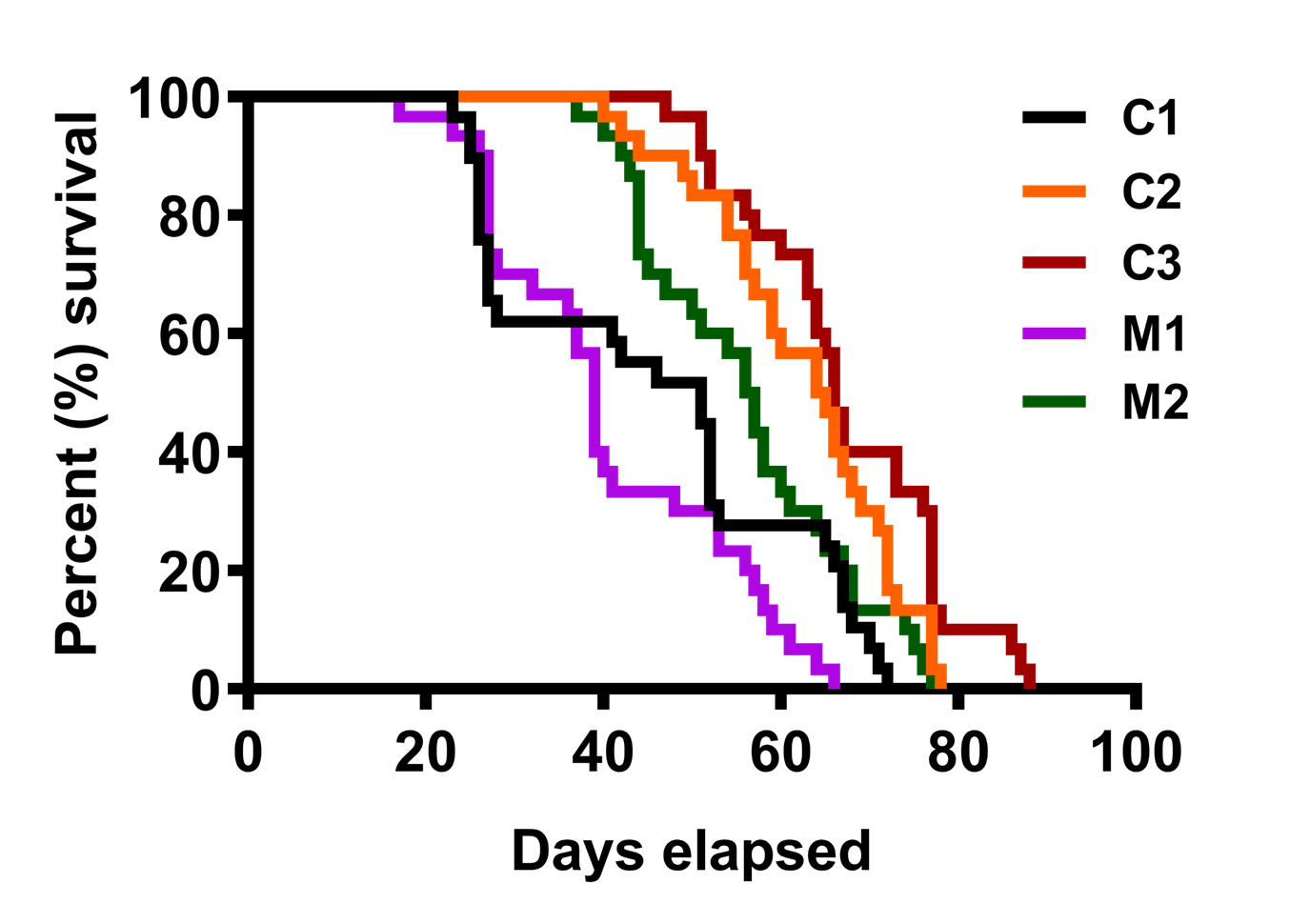
Figure 7. Life span of SOD1-overexpressed (M1) and downregulated (M2) flies with respective controlsLocomotor assays
Larval crawling assay
To analyze locomotor activities of crawling and climbing with CdCl2-fed larvae and adults, respectively, “Column” graphs were plotted in GraphPad Prism and mobility analysis was done using non-parametric t-test. p-values were checked for calculating the significance between the two groups considered.
Control larvae crossed 8.3 grid lines in 1 min on average, whereas 0.3 mM CdCl2-exposed larvae crossed 6.7 grids per minute. Significant decrease in larval mobility was observed in 0.6 mM CdCl2-treated animals, as they were able to cross only 3.55 squares per minute (Figure 8).
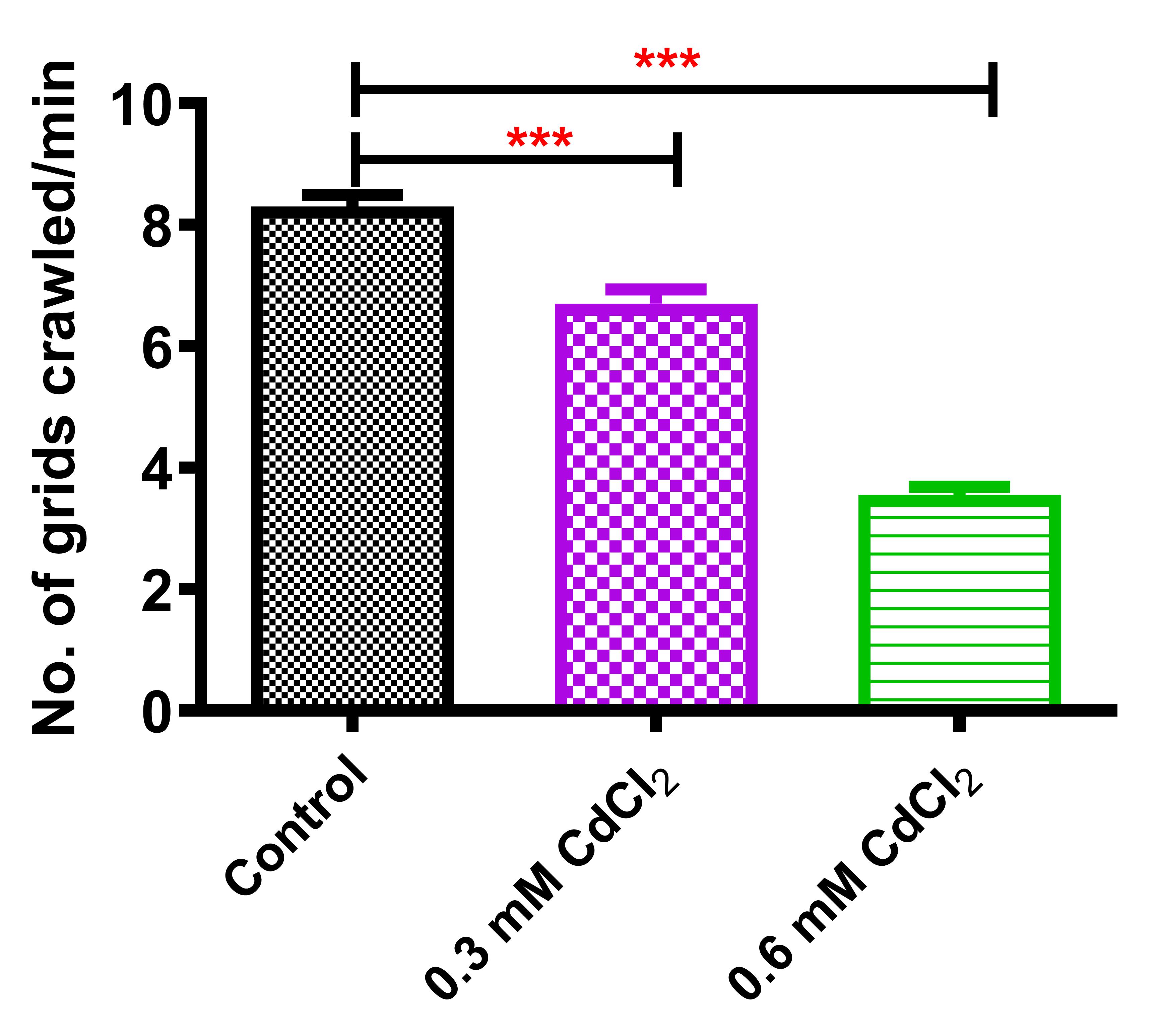
Figure 8. Crawling assay of CdCl2-fed larvaeAdult climbing assay
Average time taken by the control flies to climb a distance of 10 cm was 8.45 s. However, 0.3 and 0.6 mM CdCl2-raised flies’ climbing time was 12.97 and 19.20 s, respectively (Figure 9). Using the above protocol, a significant decline in the climbing activity was observed in the CdCl2 flies.
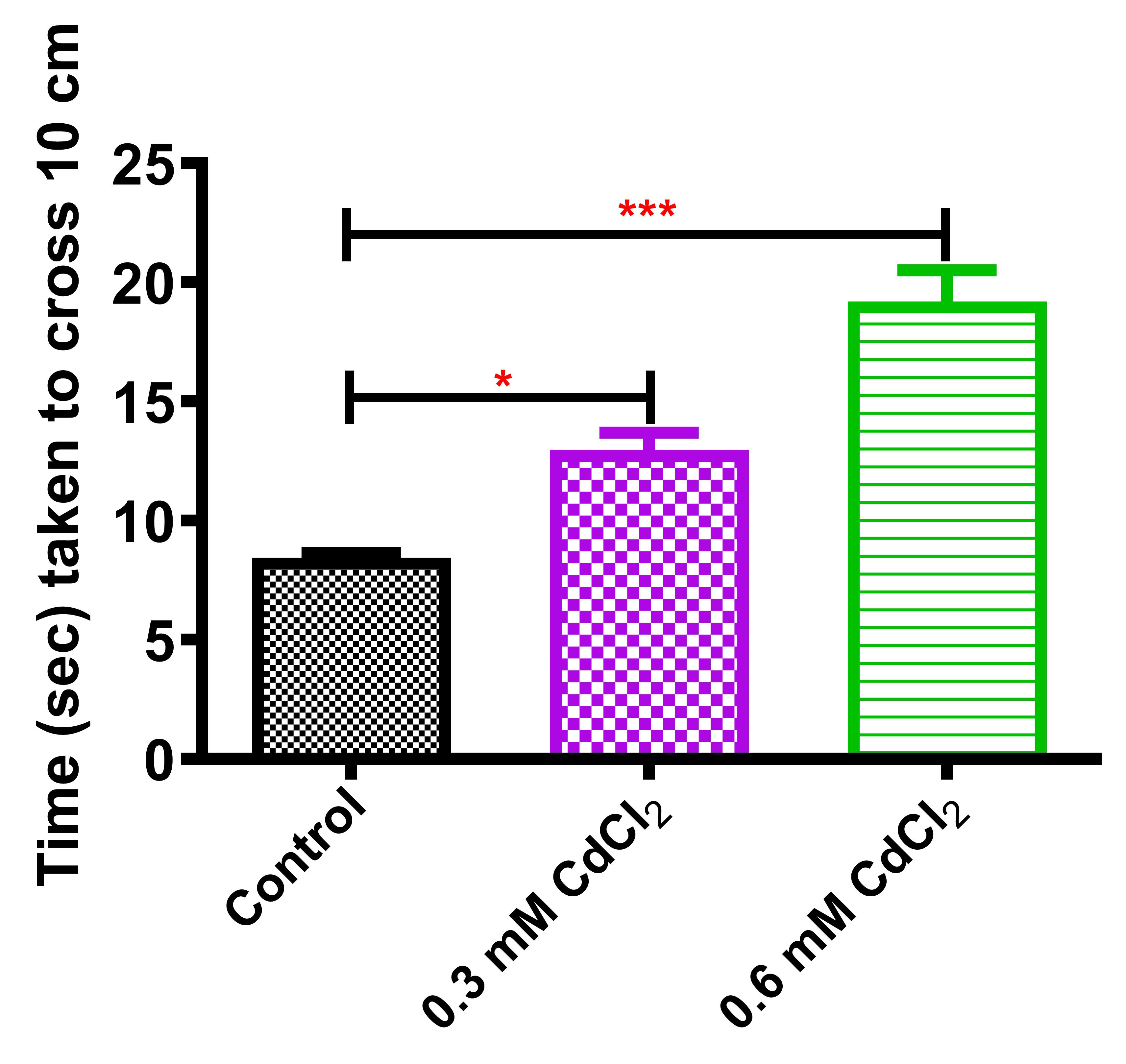
Figure 9. Climbing assay of two-days-old CdCl2 fliesThis protocol enabled Drosophila embryos to be monitored in various concentrations of cadmium salts mixed with media. Successive steps of the protocol led to the conclusive fact that cadmium exposure to the flies caused lethal effects, dispersed throughout their developmental stages (previously published data in Nanda and Firdaus, 2022). The protocol further facilitates the analysis of life span of the surviving animals post eclosion. Life span was observed to be shortened to a greater extent, as reflected by calculation of median survival with an increase in CdCl2 concentration (Nanda and Firdaus, 2022). Thus, cadmium exposure assessment in Drosophila under laboratory conditions indicated its biological toxicity and raises awareness towards its environmental pollution.
The protocol also enabled smooth quantification of stagewise lethality in Drosophila where cytosolic Cu, Zn SOD gene was perturbed using pan-tissue specific actin GAL4 driver. SOD1-overexpressed flies showed lethality dispersed at different developmental stages, while the SOD1-downregulated flies displayed only pupal to adult stage lethality. The experiment exhibited the essentiality of SOD expression towards proper development of flies, a result that has not been published before. Previously published literature showed an increase in the life span of SOD-overexpressed flies (Orr et al., 2003) and a decrease in the life span of SOD-downregulated flies (Oka et al., 2015; Martin et al., 2009). However, in the present study, SOD1 manipulation did not lead to any significant life span differences.
Since 0.3 and 0.6 mM Cd concentrations fall on either side of LC50 (Nanda and Firdaus, 2022), they were selected to further analyze the effect of cadmium on locomotor abilities of Drosophila. Our protocol enabled the collection of larvae and adults from these concentrations to perform crawling and climbing assays, respectively. Crawling ability was significantly decreased upon cadmium treatment and a similar trend was observed in adult flies’ climbing abilities. This incites future research on cadmium-led deregulation of neuromuscular functioning.
Notes
Virgin female isolation should be cautiously done under the stereomicroscope, either in the pupal or adult stage (Ashburner et al., 2005).
Prepare media with appropriately measured quantities and concentrations as mentioned in the recipes.
Take a maximum volume of 500 µL from the CdCl2 stock solution for 10 mL of media. Rigorous stirring after adding CdCl2 solution in the media is extremely essential.
Collect eggs and transfer them to media vials without damaging them by improper handling.
Carefully observe and note each stage of fly development until eclosion.
Identify and select third-instar larvae for motility assay.
Record the exact time taken by a fly to climb a distance of 10 cm, without stopping at any point or jumping over a short distance.
For 100 mL media preparation, use a 250 mL conical flask. This prevents the media from sticking to the cotton plug due to frothing during autoclave.
Write down each day’s data in a notebook; mention stagewise observations with the date, especially the death events post eclosion (Step F4) so as to plot the survival curve.
Change the food vials every fourth day for survival assay to avoid death due to media sogginess.
Recipes
Drosophila food media (100 mL)
Agar agar (0.8 g)
Yeast extract (1.5 g)
Sucrose (5 g)
Corn flour (8 g)
Propionic acid (400 μL)
Egg-laying media (20 mL)
Agar agar (0.4 g)
Sucrose (0.2 g)
Propionic acid (0.15 μL)
Petri plate for crawling assay (2% agarose)
Agarose (0.25 g)
Distilled water (15 mL)
10 mM CdCl2 Stock solution
CdCl2 (0.0228 g)
Distilled water (10 mL)
30 mM CdCl2 Stock solution
CdCl2 (0.0685 g)
Distilled water (10 mL)
100 mM CdCl2 Stock solution
CdCl2 (0.2284 g)
Distilled water (10 mL)
Acknowledgments
We acknowledge Nanda and Firdaus (2022) for optimizing these protocols. Figures 4 and 6 have been reprinted from the aforementioned citation with due permission from the publisher.
Competing interests
The authors declare no competing interest.
References
- Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A., Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P. W., Hoskins, R. A., Galle, R. F., et al. (2000). The genome sequence of Drosophila melanogaster. Science 287(5461): 2185-2195.
- Ain, U. and Firdaus, H. (2021). Behavioural Assays to Analyse the Muscle Mutants of Drosophila melanogaster. 49-63.
- Ashburner, M., Golic, K. G., Hawley, R. S. (2005). Drosophila: A Laboratory Handbook. Second edition. Cold Spring Harbor Laboratory Press. ISBN: 0-87969-706-7
- Blackney, M.J., Cox, R., Shepherd, D., Parker, J.D. (2014). Cloning and expression analysis of Drosophila extracellular Cu Zn superoxide dismutase.Biosci Rep 34(6): e00164.
- Carey, J. R., Papadopoulos, N., Kouloussis, N., Katsoyannos, B., Muller, H. G., Wang, J. L. and Tseng, Y. K. (2006). Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp Gerontol 41(1): 93-97.
- Dubey, M., Ain, U. and Firdaus, H. (2020). An insight on Drosophila myogenesis and its assessment techniques. Mol Biol Rep 47(12): 9849-9863.
- Duttaroy, A., Meidinger, R., Kirby, K., Carmichael, S., Hilliker, A. and Phillips, J. (1994). A manganese superoxide dismutase-encoding cDNA from Drosophila melanogaster. Gene 143 (2): 223-225
- Green, H. J., Griffiths, A. G., Ylanne, J. and Brown, N. H. (2018). Novel functions for integrin-associated proteins revealed by analysis of myofibril attachment in Drosophila. Elife 7: e35783.
- Landis, G. N., Doherty, D. and Tower, J. (2020). Analysis of Drosophila melanogaster Lifespan. Methods Mol Biol 2144: 47-56.
- Martin, I., Jones, M. A. and Grotewiel, M. (2009). Manipulation of Sod1 expression ubiquitously, but not in the nervous system or muscle, impacts age-related parameters in Drosophila. FEBS Lett 583(13): 2308-2314.
- Nanda, K. P. and Firdaus, H. (2022). Dietary cadmium induced declined locomotory and reproductive fitness with altered homeostasis of essential elements in Drosophila melanogaster. Comp Biochem Physiol C Toxicol Pharmacol 255: 109289.
- Nanda, K. P. and Firdaus, H. (2021). Dietary cadmium (Cd) reduces hemocyte level by induction of apoptosis in Drosophila melanogaster. Comp Biochem Physiol C Toxicol Pharmacol 250: 109188.
- Nanda, K. P., Kumari, C., Dubey, M. and Firdaus, H. (2019). Chronic lead (Pb) exposure results in diminished hemocyte count and increased susceptibility to bacterial infection in Drosophila melanogaster. Chemosphere 236: 124349.
- Oka, S., Hirai, J., Yasukawa, T., Nakahara, Y. and Inoue, Y. H. (2015). A correlation of reactive oxygen species accumulation by depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of Drosophila adults. Biogerontology 16(4): 485-501.
- Olcott, M. H., Henkels, M. D., Rosen, K. L., Walker, F. L., Sneh, B., Loper, J. E. and Taylor, B. J. (2010). Lethality and developmental delay in Drosophila melanogaster larvae after ingestion of selected Pseudomonas fluorescens strains. PLoS One 5(9): e12504.
- Orr, W. C., Mockett, R. J., Benes, J. J. and Sohal, R. S. (2003). Effects of overexpression of copper-zinc and manganese superoxide dismutases, catalase, and thioredoxin reductase genes on longevity in Drosophila melanogaster. J Biol Chem 278(29): 26418-26422.
- Rogina, B., Wolverton, T., Bross, T. G., Chen, K., Muller, H. G. and Carey, J. R. (2007). Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech Ageing Dev 128(9): 477-485.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Kumari, P., Ain, U. and Firdaus, H. (2023). A Reliable and Consistent Method to Quantify Percent Lethality and Life Span in Drosophila melanogaster. Bio-protocol 13(2): e4598. DOI: 10.21769/BioProtoc.4598.
Category
Developmental Biology > Cell growth and fate > Ageing
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









