- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Establishment of Restraint Stress–induced Anorexia and Social Isolation–induced Anorexia Mouse Models
Published: Vol 13, Iss 2, Jan 20, 2023 DOI: 10.21769/BioProtoc.4597 Views: 1837
Reviewed by: Edgar Soria-GomezAbraam YakoubMiao He
Abstract
Anorexia nervosa (AN) is a devastating neuropsychiatric disease with a prevalence rate of approximately 0.3%–1% among women and morbidity and mortality rates among the highest of all neuropsychiatric disorders. The disease etiology is complex but primarily characterized by reduced food intake and body weight, and intense anxiety and fear associated with gaining weight. Existing rodent models of AN are useful and capture features of the disease, but either require specialized genetic mouse models or are difficult to implement in mice. Here, we describe two simple mouse models of stress-induced anorexia that are easy to implement in basic research labs, and capture core features associated with AN, such as reduced food intake in the context of social/physical stress and increased anxiety-related behavior. These protocols provide reproducible and robust assays for stress-induced anorexia and may be implemented with additional assays to probe the neural circuitry mediating the effects of psychological stress on feeding in mice.
Graphical abstract
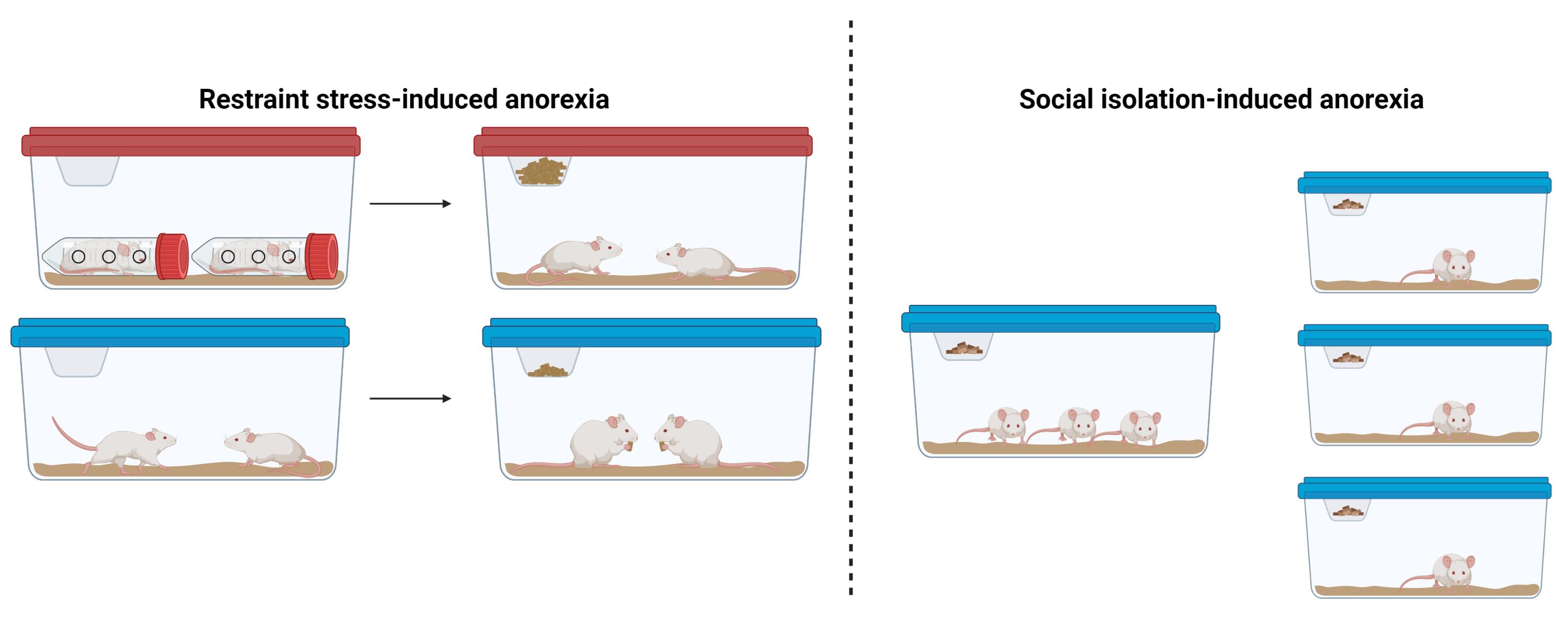
Background
Feeding behavior is controlled by multiplex neural circuitry throughout the brain, including the hypothalamus, hindbrain, and mesolimbic dopamine circuitry (Sohn et al., 2013). While dedicated feeding circuits exist in the brain, substantial experimental evidence indicates that these are bidirectionally connected to neural circuitry controlling stress and emotion (Sweeney and Yang, 2017). These interactions are especially important when considering the biological mechanisms governing neuropsychiatric eating disorders such as anorexia nervosa (AN), since eating disorders are characterized by alterations in both feeding and emotional state (Keski-Rahkonen, 2007). As such, the underlying biological causes of eating disorders may be mediated by neural circuit alterations in brain circuits at the intersection of feeding and emotion. Thus, mouse models of stress-induced anorexia have value in dissecting the neural circuit and molecular mechanisms for normal and pathological feeding responses to stress.
Anorexia nervosa is a serious neuropsychiatric disorder characterized by reduced feeding and body weight and co-morbid neuropsychiatric traits, such as increased anxiety and depressive-related behaviors (Bulik et al., 2006). The disease occurs in approximately 0.3%–1% of women in the United States and is associated with a high rate of morbidity and mortality (Bulik et al., 2006). Since the disease is characterized by reduced feeding in the context of psychological and psychosocial stress, substantial efforts have been made to model aspects of AN in rodents (Scharner and Stengel, 2020; Zhang and Dulawa, 2021). Although AN is a complex disease with human-specific etiology, important components of the disease can be effectively modeled in rodents, such as reduced feeding in the face of stress and anxiety, compulsive exercise, and elevated anxiety and depressive behavior. These models provide an important entry point towards understanding the biological mechanisms mediating dysregulated eating and may provide a pathway towards novel therapeutic options for eating disorders.
One commonly used rodent model of AN is the activity-based anorexia (ABA) mouse model (Carrera et al., 2014). The ABA model subjects rodents (mice or rats) to restricted food access in the presence of a running wheel. In this model, a subset of mice will stop eating and exercise excessively, leading to starvation and death. This model is useful as it incorporates multiple characteristics of AN, such as excessive exercise and voluntary anorexia, and is more effective in female rodents (approximately 90% of AN cases occur in females). However, the model relays on the presence of a running wheel and restricting food access to a user-defined time window (usually between 1 and 4 h/day). Rodents in this model develop gradual hypothermia and may exercise excessively to generate heat. As such, the ABA model does not work when supplemental heat is provided to the rodents (Gutierrez et al., 2008). Thus, although hypothermia is often observed in AN, the ABA model may more closely model physiological forms of anorexia resulting secondarily to hypothermia. Further, the model can be difficult to implement in mice (as opposed to rats), and only a subset of mice are sensitive to the ABA model.
Given the limitations of the ABA model, additional attempts have been made to develop mouse models that capture the AN phenotype more accurately. One model that shows promise is the “GED” (gene, environment, deprivation) model developed in the lab of Lori Zeltser (Madra and Zeltser, 2016; Madra et al., 2015). The GED model uses a transgenic mouse line containing a BDNF-Val66Met mouse mutation that has been associated with neuropsychiatric disorders. In the GED model, the BDNF-Val66Met mice are subjected to social isolation during adolescence (environmental manipulation) and given 20%–30% calorically restricted food access during a user-defined time window of 3 h twice a day. Thus, this model incorporates a genetic component (BDNF-Val66Met mutation), an environmental stressor (social isolation), and food deprivation by both caloric restriction and limited food access to produce anorexia-related behaviors. A subset of mice exposed to the GED model voluntarily stop eating and develop severe anorexia. Like in the ABA model, in the GED model the voluntary starvation phenotype observed in AN is captured and the starvation phenotype is more common in female mice. However, this model relays on a transgenic mouse line, which limits the use of some modern behavioral neuroscience approaches, such as optogenetics and chemogenetics, which often require additional transgenic mouse lines and/or multiple breeding steps. Therefore, there is a need for simplified stress-induced anorexia models which can be implemented in all basic biology labs and transgenic mouse lines (Table 1).
Here, we describe two simple models of stress-induced anorexia that are easy to implement in mice: restraint stress–induced anorexia and social isolation–induced anorexia. These models do not require specialized equipment or transgenic mouse strains and can be implemented in most biology and neuroscience laboratories. Both of these assays may serve as useful models for disorders characterized by reduced feeding in the face of physical or psychosocial stress, such as the reduced appetite associated with stress-related mood disorders or the impaired food-seeking and consumption observed in AN.
Table 1. Strengths and weaknesses of common models of stress-induced anorexia
| Method | Advantages | Disadvantages |
|---|---|---|
| Activity-based anorexia | -Easy to implement -Self-induced anorexia and starvation -Excessive exercise -More severe in female rodents | -Model may rely on physiological anorexia secondary to hypothermia, as opposed to psychologically induced anorexia -Difficult to implement in mice (relative to rats) |
| G.E.D. model | -More severe in females -Self-induced anorexia and starvation in a subset of mice -Anorexia due to a combination of genetic and environmental factors | -Requires a specialized transgenic mouse line |
| Restraint induced–anorexia | -Easy to implement -Reliably induced anorexia and weight loss in mice -Easily combined with advanced behavioral neuroscience approaches (i.e., optogenetics, chemogenetics) | -Not sexually dimorphic -Usually does not induce self-starvation -May not capture psychologically induced anorexia |
| Social isolation–induced anorexia | -Easy to implement -Anorexia resulting from chronic psychological stress | -Anorexia phenotype is usually mild -Better suited for determining factors that enhance psychological-induced anorexia -Unknown sexual dimorphism |
Subjects and housing: Adult male and female C57/BL6J littermate mice may be used for both social isolation and restraint stress–induced anorexia studies. Given that neuropsychiatric eating disorders are highly sexually dimorphic, it is important to include both sexes in the experimental design and to determine if behavioral phenotypes are differentially expressed in males and females. For restraint stress–induced anorexia assays, littermate mice should be group-housed with 3–4 mice per cage. Lights in the housing facility should be on a 12:12 h light/dark cycle. Since mice are nocturnal and consume most of their food during the dark period, it is recommended to perform feeding assays at the start of the dark cycle, although light cycle measurements may also be performed.
Materials and Reagents
Group-housed mice
50 mL conical tubes with holes cut into the sides (for restraint stress–induced anorexia; Figure 1)
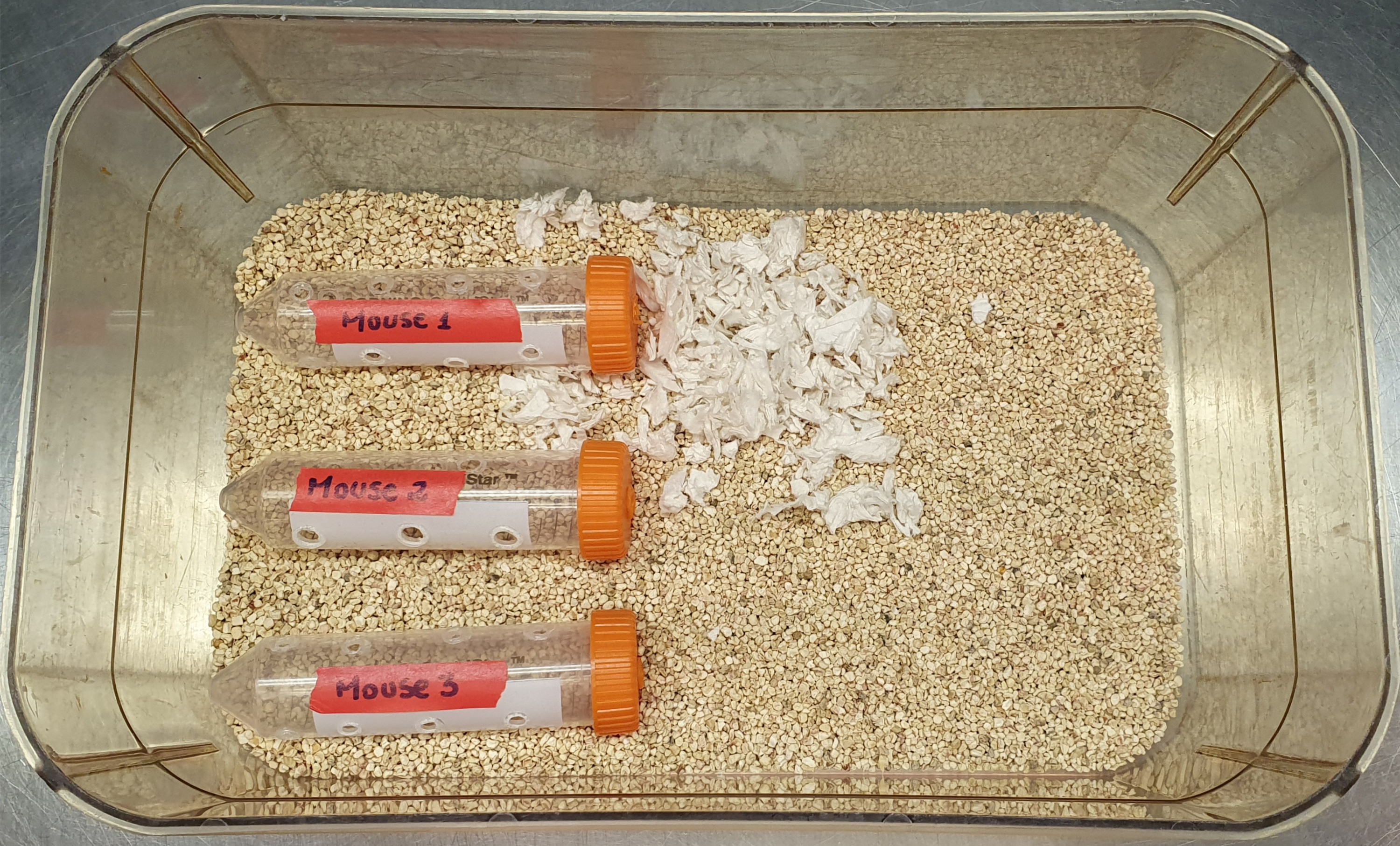
Figure 1. Setup of restraint stress–induced anorexia apparatus. Separate cages are used for male and female mice, respectivelyClean mouse cages
Standard chow mouse diet (Formulab Diet 5008 or similar approved mouse diet)
Standard home cage water bottles
Standard food hoper
Cage enrichment (such as nestlets)
Scale for measuring food intake and body weight (Satorius Entris II Essential Precision Balance or similar)
Lab notebook for recording food intake and body weight
Instructions to make restraint stress apparatus
A simple restraint stress–anorexia apparatus can be constructed from 50 mL conical tubes (Figure 1). Make 6–12 holes, depending on how many you can fit, on the sides of the conical tubes to enable mice to breathe. Care should be taken to make the holes big enough for air flow, but not big enough for the mice to fit their nose into, so they do not hurt themselves in the borders. Holes can be made by using a tool such as a heat pen for melting plastic. The mouse identifying information (i.e., ear tag number and sex) can be written on the side of each tube. During restraint sessions, the mice can be placed into the restrainers within their resident cage (Figure 1). Following each restraint session, the restraint tubes should be cleaned with soap and water and allowed to dry before re-using on each mouse.
Procedure
Restraint stress–induced anorexia
Group house mice, 3–4 per cage and separated by sex; acclimate the mice to housing conditions for one week prior to beginning food intake measurements. During this period, mice should be handled daily by the investigator to acclimate the mice to handling-associated stress.
To acclimate the mice to handling, pick up each mouse and scruff gently. Remove the water bottle and food for one hour prior to baseline feeding measurements (to control for the one-hour restraint period in which mice will not have access to food and water). Place mice in a clean cage immediately following the removal of the food and water so that mice do not have access to any food crumbs that may be on the bottom of the cage.
Note: All feeding measurements should be performed at the same time of day in the same experimental location.
After one hour of food and water removal, add a water bottle to the clean cage with fresh bedding and mouse enrichment (i.e., nestlet or wooden block) and between 20 and 30 g of food to the food hopper (mice eat approximately 3–5 g/day/mouse). Measure the body weight of the mice and amount of food immediately prior to adding the food and water to the cages.
Measure the amount of food left in the food hopper 1, 2, 4, and 24 h after adding the food. Food intake per mouse is calculated at each time point by dividing the amount of food consumed by the number of mice in each cage.
Measure the body weight of the mice 24 h after adding the food.
Repeat steps A2–A4 for 3 to 5 days to establish baseline levels of food intake for each mouse prior to restraint stress.
On restraint stress day (following baseline food intake measurements), grab the mouse by the tail and place each mouse in a 50 mL conical tube (see Figure 1) for sixty min, inserting the head of the animal first followed by the rest of its body, including the tail. For the control animals, only remove the food and water to give them a similar environment of food and water restriction as the restraint mice for sixty minutes.
Notes:
Institutional animal care and use approval (IACUC) must be obtained to perform experimental work on mice. It is essential to monitor each mouse during the restraint period to ensure that mice are breathing adequately.
To obtain the food intake of individual mice with and without restraint stress, the above procedure (steps 1–7) may also be performed on singly housed mice. However, mice should be single-caged for a minimum of one week prior to starting restraint stress–anorexia experiments in order to acclimate mice to single housing.
Following sixty minutes of restraint, return the mice to the home cages with their littermates and add food and water as described in step A3.
Measure food intake as described in step A4.
Repeat steps A7–A9 for an additional 7–10 days to examine the effects of chronic restraint–stress on food intake and body weight.
Social isolation–induced anorexia
Group house mice with 3–4 per cage and acclimate the mice to housing conditions for one week prior to beginning food intake measurements. During this period, mice should be handled daily by the investigator to acclimate the mice to handling-associated stress.
Change mice to a fresh cage, with fresh bedding and mouse enrichment. Measure the body weight of each mouse. Add 20–30 g of food to the cages and measure the amount of food added to each cage. Return to measure the amount of remaining food 1, 2, 4, and 24 h later. Calculate the amount of food consumed by each mouse by dividing the total amount of food consumed by the number of mice in the cage.
Note: All feeding experiments should be performed at the same time of day in the same experimental location. Since mice are nocturnal and consume most of their food during the dark cycle, optimal results will be obtained by performing feeding experiments at the onset of the rodent’s dark cycle.
Repeat step B2 for 3–5 consecutive days to establish a baseline measurement of food intake and body weight prior to social isolation.
At the same time of day as steps B2-B3, single-cage each mouse, measure each mouse’s body weight, and add 10–15 g of food to each cage. Return to measure the amount of food in each cage 1, 2, 4, and 24 h later. For the control group, continue to calculate the amount of food consumed by each mouse by dividing the total amount of food consumed by the number of mice in the cage.
Note: Mice consume food based on a circadian structure. Care must be made to perform all feeding measurements at the same time each day.
Repeat step B4 for an additional 3–14 days.
Data analysis
Exclusion criteria: During stress-induced anorexia experiments, researchers must abide by IACUC approved exclusion criteria for removing animals from experimental conditions. These exclusion criteria will vary at each research institute and individual investigators should discuss exclusion criteria with veterinary staff prior to initiating experiments. Generally, mice are removed from experimental sessions when signs of pain or distress, such as reduced activity or piloerection, are observed or if any of the animal’s body weight drops by more than 20 percent.
Following the completion of restraint stress induced–anorexia or social isolation–anorexia experiments, food intake and body weight is compared for each mouse following no-restraint vs. restraint-stress (paired, within samples comparisons). It is expected that restraint stress will reduce food intake and body weight in mice. For restraint stress–induced and social isolation–induced anorexia, we typically observe 10%–20% changes in food intake in the hours immediately following restraint stress, although results will vary depending on mouse strain, sex, and age of mice. The effects of pharmacological or other manipulations to reduce or enhance stress-induced anorexia can also be evaluated by determining the effect of the manipulation on food intake and body weight. The specific experimental design will vary slightly depending on the specific research question.
Conclusion
In this article, we describe two simple mouse models for inducing stress-induced anorexia. These models provide a valuable experimental tool for researchers interested in determining the neural mechanisms connecting stress with feeding behavior.
Acknowledgments
This work was funded in part by awards R00 DK127065 (P.S.), Brain and Behavior Research Foundation Young Investigator Award (P.S.), and the Foundation for Prader Willi Research (P.S.).
The original methods in this paper are based on our prior publication (Sweeney et al., 2021; DOI: 10.1126/scitranslmed.abd6434).
Competing interests
There are no conflicts of interest or competing interests.
Ethics
All experiments were approved by the University of Illinois Institutional animal care and use committee (IACUC).
References
- Bulik, C. M., Sullivan, P. F., Tozzi, F., Furberg, H., Lichtenstein, P. and Pedersen, N. L. (2006). Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry 63(3): 305-312.
- Carrera, O., Fraga, A., Pellon, R. and Gutierrez, E. (2014). Rodent model of activity-based anorexia. Curr Protoc Neurosci 67: 9 47 41-11.
- Gutierrez, E., Cerrato, M., Carrera, O. and Vazquez, R. (2008). Heat reversal of activity-based anorexia: implications for the treatment of anorexia nervosa. Int J Eat Disord 41(7): 594-601.
- Keski-Rahkonen, A., Hoek, H. W., Susser, E. S., Linna, M. S., Sihvola, E., Raevuori, A., Bulik, C. M., Kaprio, J. and Rissanen, A. (2007). Epidemiology and course of anorexia nervosa in the community. Am J Psychiatry 164(8): 1259-1265.
- Madra, M. and Zeltser, L. M. (2016). BDNF-Val66Met variant and adolescent stress interact to promote susceptibility to anorexic behavior in mice. Transl Psychiatry 6: e776.
- Scharner, S. and Stengel, A. (2020). Animal Models for Anorexia Nervosa-A Systematic Review. Front Hum Neurosci 14: 596381.
- Sohn, J. W., Elmquist, J. K. and Williams, K. W. (2013). Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci 36(9): 504-512.
- Sweeney, P., Bedenbaugh, M. N., Maldonado, J., Pan, P., Fowler, K., Williams, S. Y., Gimenez, L. E., Ghamari-Langroudi, M., Downing, G., Gui, Y., et al. (2021). The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci Transl Med 13(590): eabd6434.
- Sweeney, P. and Yang, Y. (2017). Neural Circuit Mechanisms Underlying Emotional Regulation of Homeostatic Feeding. Trends Endocrinol Metab 28(6): 437-448.
- Zhang, J. and Dulawa, S. C. (2021). The Utility of Animal Models for Studying the Metabo-Psychiatric Origins of Anorexia Nervosa. Front Psychiatry 12: 711181.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Possa-Paranhos, I. C., Catalbas, K., Butts, J., O’Berry, K. and Sweeney, P. (2023). Establishment of Restraint Stress–induced Anorexia and Social Isolation–induced Anorexia Mouse Models. Bio-protocol 13(2): e4597. DOI: 10.21769/BioProtoc.4597.
- Sweeney, P., Bedenbaugh, M. N., Maldonado, J., Pan, P., Fowler, K., Williams, S. Y., Gimenez, L. E., Ghamari-Langroudi, M., Downing, G., Gui, Y., et al. (2021). The melanocortin-3 receptor is a pharmacological target for the regulation of anorexia. Sci Transl Med 13(590): eabd6434.
Category
Neuroscience > Behavioral neuroscience
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









