- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Myonecrosis Induction by Intramuscular Injection of CTX
(*contributed equally to this work) Published: Vol 13, Iss 1, Jan 5, 2023 DOI: 10.21769/BioProtoc.4587 Views: 3605
Reviewed by: Vivien Jane Coulson-ThomasDieu-Huong HoangMarielle Saclier

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Induction of Skeletal Muscle Injury by Intramuscular Injection of Cardiotoxin in Mouse
Xin Fu [...] Ping Hu
May 5, 2023 3323 Views

An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
Jeremy R. Pearson [...] Eduardo Rosa-Molinar
Apr 20, 2025 2510 Views

Workflow for Fluorescence-Targeted Lamella Milling From Vitrified Cells With a Coincident Fluorescence, Electron, and Ion Beam Microscope
Elise G. Perton [...] Jacob P. Hoogenboom
Jul 20, 2025 2834 Views
Abstract
Skeletal muscle, one of the most abundant tissue in the body, is a highly regenerative tissue. Indeed, compared to other tissues that are not able to regenerate after injury, skeletal muscle can fully regenerate upon mechanically, chemically, and infection-induced trauma. Several injury models have been developed to thoroughly investigate the physiological mechanisms regulating skeletal muscle regeneration. This protocol describes how to induce muscle regeneration by taking advantage of a cardiotoxin (CTX)-induced muscle injury model. The overall steps include CTX injection of tibialis anterior (TA) muscles of BL6N mice, collection of regenerating muscles at different time points after CTX injury, and histological characterization of regenerating muscles. Our protocol, compared with others such as those for freeze-induced injury models, avoids laceration or infections of the muscles since it involves neither surgery nor suture. In addition, our protocol is highly reproducible, since it causes homogenous myonecrosis of the whole muscle, and further reduces animal pain and stress.
Graphical abstract

Background
The final goal of regenerative medicine is to reconstitute tissue and organ functionality after injury or disease (Mao and Mooney, 2015). Tissue regeneration is a physiological process orchestrated by different infiltrating and tissue-resident cell types. Skeletal muscle is one of the most dynamic and plastic tissues of the human body and represents approximately 40% of total body weight in humans (Frontera and Ochala, 2015). Skeletal muscle not only controls posture maintenance and locomotion but plays a fundamental role in the maintenance of metabolic homeostasis, being involved in heat production and carbohydrate and amino acid storage (Schiaffino et al., 2013). In this context, muscle degeneration due to acute and chronic conditions leads to reduced mobility and strength, and to metabolic disorders (Schiaffino et al., 2013). Regeneration of skeletal muscle relies on a tightly regulated gene expression program and on the activation of specific signaling pathways that characterize muscle embryonic development (Chargé and Rudnicki, 2004). The ability of muscle to regenerate is principally due to a specific population of normally quiescent muscle stem cells called satellite cells that are strictly associated with muscle fibers (Chargé and Rudnicki, 2004). Satellite cells are myogenic precursor cells (MPCs) that reside in a quiescent state beneath the basal lamina that surrounds muscle fibers (Chargé and Rudnicki, 2004). In detail, soon after muscle damage, satellite cells escape quiescence and start to proliferate. A subset of these activated cells proliferate and differentiate, whereas others return to quiescence to reconstitute the pool of satellite cells that will be crucial for further rounds of skeletal muscle regeneration (Chargé and Rudnicki, 2004).
Although satellite cells play a key role in muscle regeneration, their presence is not sufficient for efficient skeletal muscle repair, and many additional cell types play an active role in promoting tissue repair (Joe et al., 2010; Uezumi et al., 2010). Among them, key players in the complex scenario of skeletal muscle regeneration are inflammatory cells that invade muscle soon after injury and, together with satellite cells, contribute to a complete muscle regeneration (Tidball, 2017). Among the inflammatory cells, monocytes/macrophages play a major role in the repair process (Wang and Zhou, 2022). For example, macrophages release cytokines and growth factors that contribute to satellite cell activation, proliferation, and differentiation, according to their polarization state (Saclier et al., 2013). In particular, soon after muscle damage, macrophages adopt a pro-inflammatory profile, releasing pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), interleukine-6 (IL-6) and insulin-like growth factor (IGF-1) that, in turn, promote satellite cell proliferation (Tidball, 2017). Starting from three to five days post injury, these macrophages skew from a pro- to an anti-inflammatory profile, releasing anti-inflammatory cytokines that not only dampen the inflammatory response but also play trophic function by promoting satellite cell differentiation and fusion, thus contributing to the formation of new myofibers (Tidball, 2017). To date, different mouse models of acute muscle injury are available, as they represent an attractive system for investigating interactions between the immune system and satellite cells during skeletal muscle regeneration. Indeed, the onset of tissue damage is well defined and the time course of inflammation and regeneration predictable (Tidball, 2017). The most used injury models are freeze injury (FI), barium chloride injection, notexin (NTX) injection, and cardiotoxin (CTX) injection. The last one provides a useful model for sterile inflammation and, importantly, induces homogeneous damage to the whole muscle. Furthermore, it triggers the infiltration of many monocytes and macrophages participating in muscle repair, regeneration, and growth (Rigamonti et al., 2014). Here, we describe a protocol to induce muscle sterile injury by CTX injection. This method allowed us to study the influence of mitochondrial calcium uptake on macrophage metabolic profiles and on the skeletal muscle regeneration process (Feno et al., 2021).
Myonecrosis is induced by intramuscular injection of CTX in tibialis anterior (TA) muscles, as performed in many other laboratories (Mounier et al., 2013; Perdiguero et al., 2011; Saclier et al., 2013). Compared with other protocols, our method has the great advantage of avoiding muscle laceration (Feno et al., 2021). Specifically, we can directly inject CTX in TA muscles without exposing them, thus avoiding surgery and consequent damage. Muscles are collected for analysis at different time points after injury (at 3, 7, and 14 days) to precisely characterize the time course of skeletal muscle regeneration. After muscle collection, regenerating TA muscles are frozen, and muscle sections are characterized by hematoxylin and eosin (H&E) staining to evaluate the efficiency of the CTX- induced skeletal muscle regeneration process.
Materials and Reagents
8 weeks C57BL6N male mice (Charles River)
Isoflurane 1000 mg/g (Piramal Critical Care, catalog number: 803249)
500 μM CTX (Merk, catalog number: 217503-1MG)
9% NaCl2 (Merk, catalog number: S3014)
UltraPure DNase/RNase-Free distilled water (Thermo Fisher Scientific, catalog number: 10977049)
70% v/v ethanol (Merk, catalog number: 64-17-5)
Isopentane (2-Methylbutane anhydrous ≥99%-1l) (Merck, catalog number: 277258)
Liquid nitrogen
Hematoxylin & Eosin (H&E) kit staining (Bio-Optica, catalog number: 04-061010)
Optimal cutting temperature compound (O.C.T.) (Tissue-Tek, catalog number: 4583)
Equipment
VetFlo veterinary anesthesia apparatus
Dissection tools:
Fine scissors (Fine Science Tools, catalog number: 14160-10)
Fine forceps (Fine Science Tools, catalog number: 11412-11)
Standard forceps (Fine Science Tools, catalog number: 11150-10)
Microcentrifuge tubes 1.5 mL (Sarstedt, catalog number: 72706)
Cryovials (Sarstedt, catalog number: 72.694.006)
Glass slides (Vetrotecnica, catalog number: 01.4230.34)
500 μL syringe 30 G (Vetrotecnica, catalog number: 11.3525.45)
Plastic scoop
Dewar bottle for liquid nitrogen
Cryostat (Leica, model: CM1850)
Procedure
Depending on the experimental design, the number of mice required for experiments varies. To obtain statistically significant and reproducible results we used at least four adult (approximately 8 weeks old) male mice for each condition (e.g., not injected, and injected for 3, 7, and 14 days).
In sterile conditions, prepare CTX stock solution by dissolving 1 mg of CTX powder (MW: 6.8 g/mol) in 294 μL (3.4 mg/mL) of sterile 0.9% NaCl2 to obtain a 500 µM solution of CTX. Aliquot the CTX stock solution and store it at -20 °C. To avoid freeze-thaw cycles, prepare aliquots of 10 μL. For TA injection, dilute the stock solution in sterile 0.9% NaCl2 to obtain a final solution of 10 μM CTX in a total volume of 50 μL for each TA muscle. Mix the solution by vortexing and keep it on ice until use.
Note: CTX is not classified as dangerous, so no particular care must be taken. It is important to prepare and store the solution in sterile conditions.
Anesthetize the animals with isoflurane, until they fall asleep. Isoflurane is administered in 100% O2 . Use an induction concentration of isoflurane of approximately 3%–4%. Maintenance concentrations are 1.25%–1.75% to avoid the overall alteration of mouse physiology. In this condition, mice can be anesthetized for up to 20–30 min.
Fill a syringe (500 μL syringe 30 G, Vetrotecnica) with 50 μL of 10 μM CTX and keep it ready for use.
Restrain the mouse properly and insert its head inside the anesthetic tube (Figure 1A).
Shave the hindlimbs of the mouse with an electric razor and swab the area with 70% ethanol. Hold the foot of the hindlimb firmly, insert the needle of the syringe prepared in step 3 in the TA mid-belly region parallel to the muscle, and then gently pull the syringe plunger before injecting CTX to ensure that the needle has not entered a blood vessel (Figure 1B). The optimal positioning of injection sites is shown in Figure 2 by inserting the needle of the syringe parallel to the TA muscle. If no blood enters the syringe, 50 μL of CTX is to be slowly injected into TA muscle. Special care should be taken when injecting into TA to avoid going past the muscle tissue.
Let the mouse recover from anesthesia and place it back in its cage.
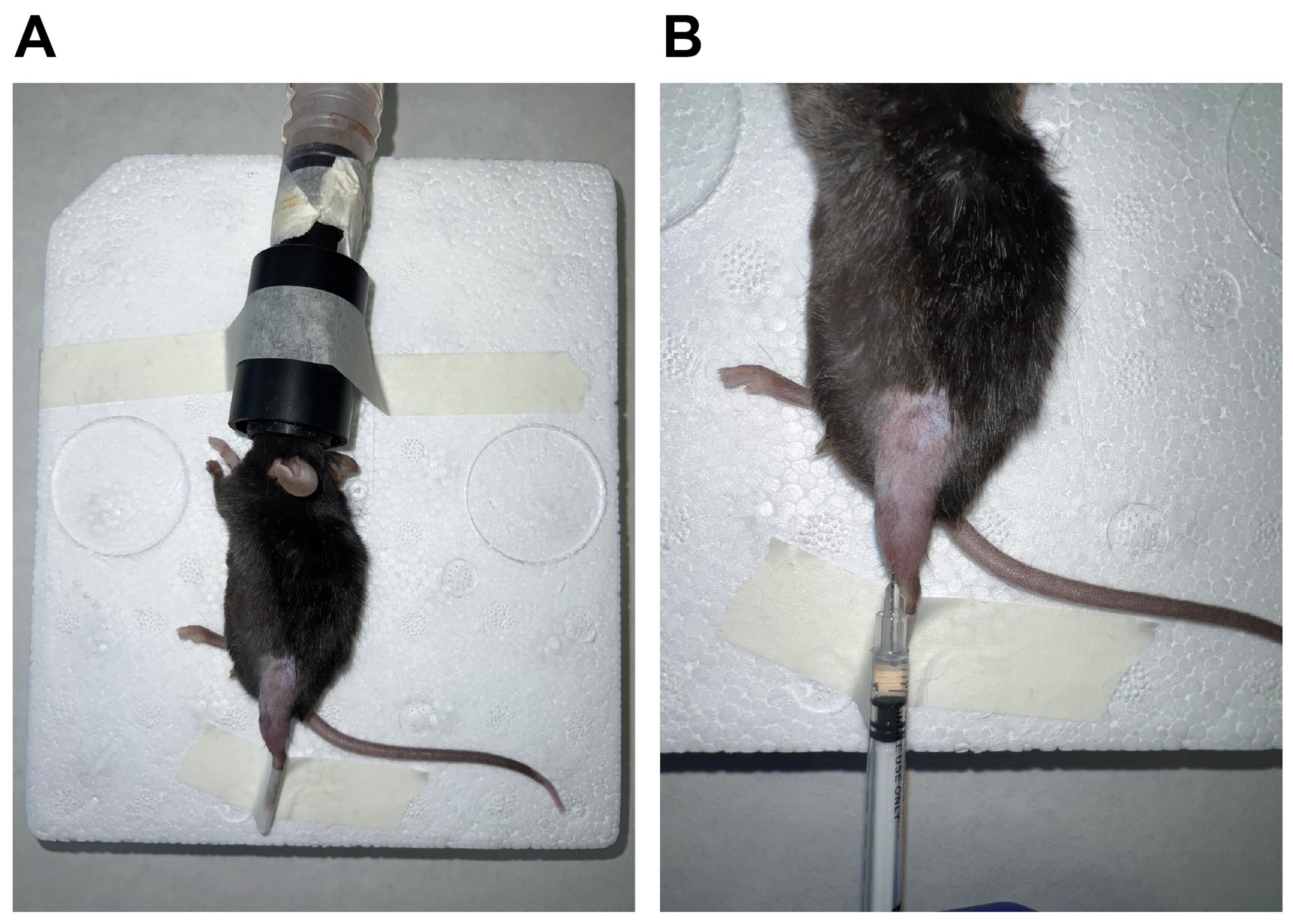
Figure 1. Anesthesia administration and CTX injection. After the induction with sufficient anesthesia in the animal, the leg is restrained (A), and injury by CTX injection can be inflicted by injection in the TA muscle (B).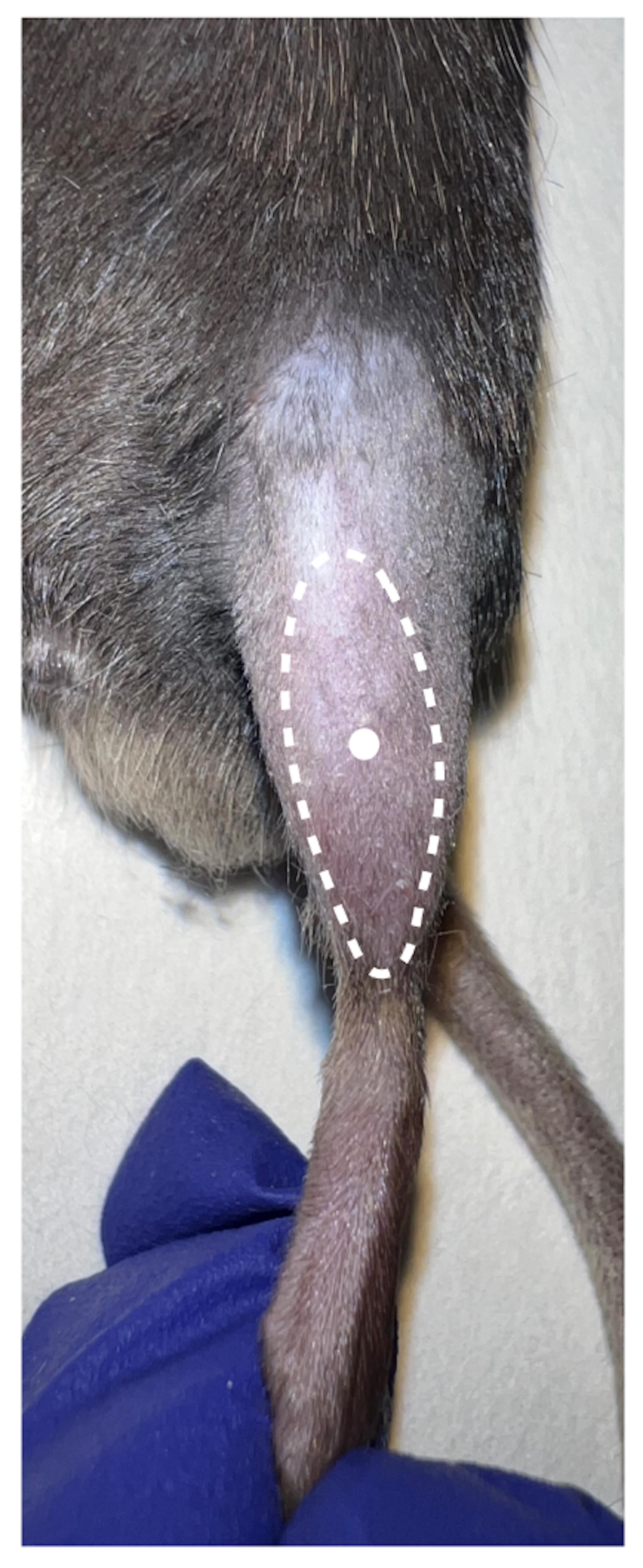
Figure 2. Optimal positioning of the CTX injection site (white dot) in the TA muscleAt the selected time points post CTX injection, anesthetize the recipient mouse through isoflurane inhalation and sacrifice it by cervical dislocation.
Regenerating TA can be collected as follows:
Cut the skin below the ankle.
Cut through the skin along the inner hindlimb.
Expose the muscle fascia and patella by pulling the skin up, and remove the epimysium (Figure 3A).
Separate the tendon of TA muscle using forceps along the inner margin of the shank (Figure 3B).
Snip the tendons with the fine scissors (Figure 3C). Gently pull the TA muscle from the bone (Figure 3C). Below the TA you can see the extensor digitorum longus (EDL) muscle (Figure 3C).
Cut the upper part of the TA near the patella (Figure 3D). You can proceed with TA freezing (Figure 3E and Figure 4).
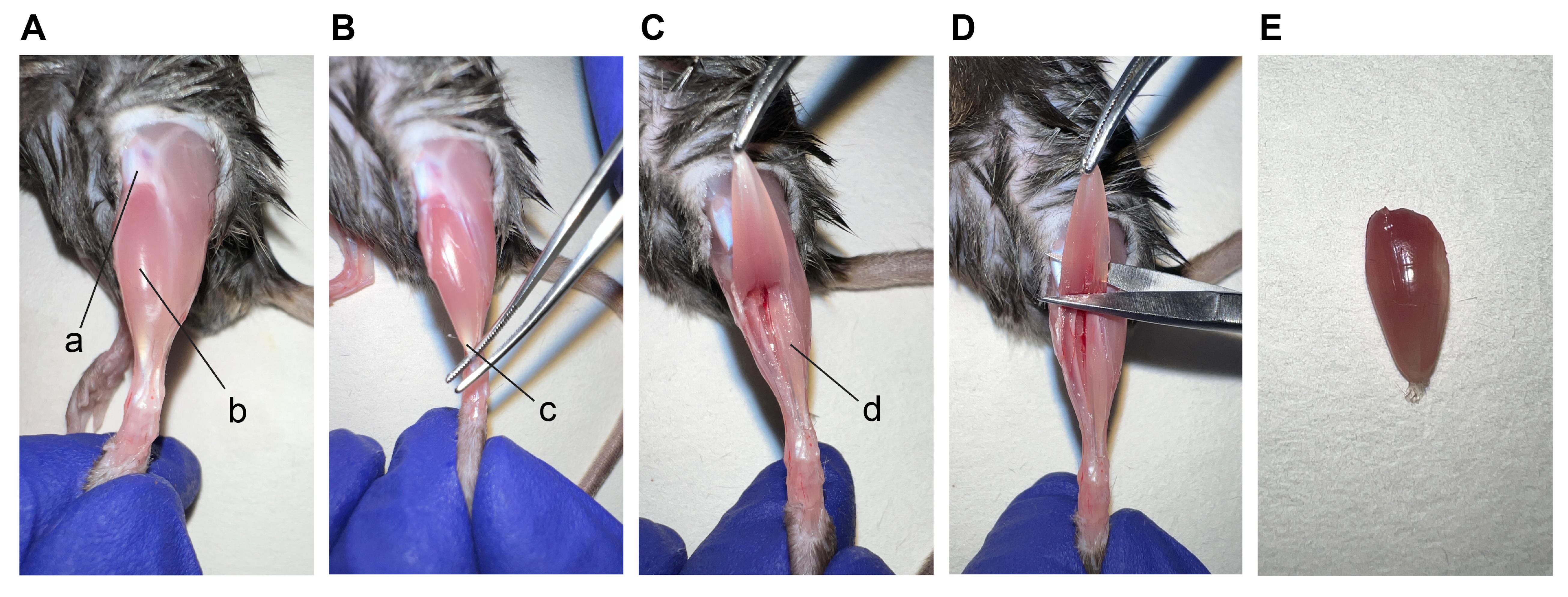
Figure 3. Procedures to isolate the TA muscle. Pictures depict (A) TA muscle after the removal of epimysium, (B) isolation of TA tendon, (C) cut of the TA tendon, (D) cut of the distal part of the TA, and (E) TA muscle. a. patella; b. TA; c. tendon of TA muscle; d. EDL muscle.Fill up the Dewar with liquid nitrogen and take all the material necessary for freezing muscles (isopentane, plastic scoop, standard forceps, and cryovials).
Spray dissection tools and workspace with 70% ethanol. Then fill up a becher with isopentane (Figure 4A) and place it on the surface of the Dewar full of nitrogen to cool it down (to avoid the risk of solidification, the becher is laid on the surface of the liquid nitrogen) (Figure 4B).
Place the isolated TA muscles on the plastic scoop and then inside the becher containing cold isopentane until the muscle is completely frozen (it takes approximately 30 s) (Figure 4C and 4D). Take a cryovial with a standard tweezer and cool it by dipping it inside liquid nitrogen (Figure 4E). Once the cryogenic vial is cold (5 s), remove the scoop from the isopentane, immediately detach the muscle from the plastic scoop, and place it into the cryovial (Figure 4F). This operation must be performed quickly to avoid thawing the muscles. The cryovials are conserved at -80 °C until use.
Note: You can consider the muscle completely frozen when it whitens homogeneously.
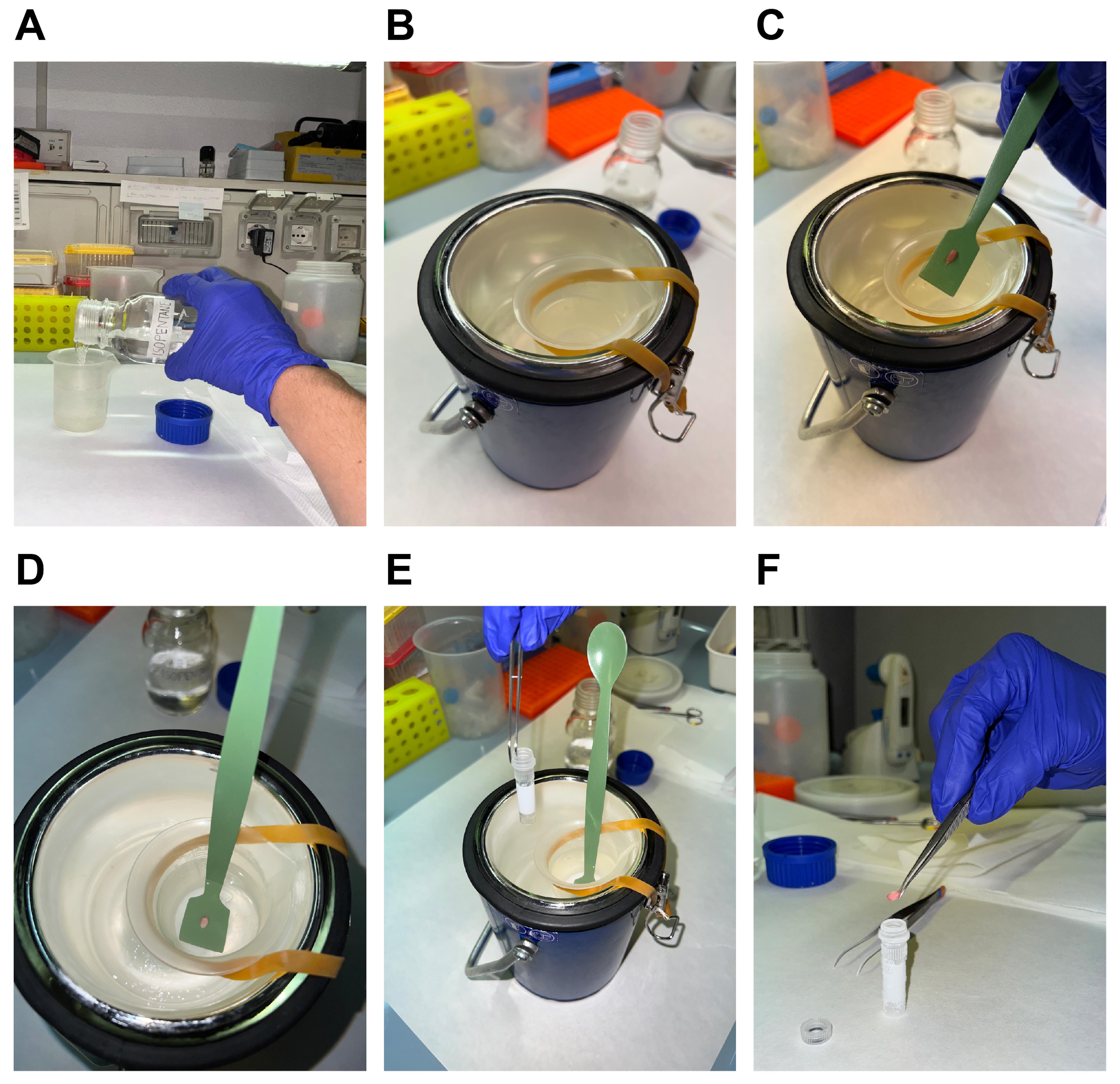
Figure 4. Procedure for freezing TA muscles for Hematoxylin and Eosin staining. Fill a small becher with isopentane (A), place it on the surface of a Dewar containing liquid nitrogen (B), place the isolated TA muscle on the plastic scoop (C), and then inside isopentane (D), immerge the cryovial in liquid nitrogen using a tweezer (E) and detach the muscle from the plastic scoop and place it into the cryovial (F).For sectioning, cut the frozen muscles transversely and embed one half, at the tendon site, on the cryostat chuck by using two drops of OCT. Allow the tissue block to equilibrate to the cryostat temperature (-20 °C) before cutting the sections. Cut 6 μm muscle slides with the cryostat and make them adhere on positively charged microscope glass slides. Dry the glass slides at room temperature until the sections are firmly adherent to the slide.
Perform H&E staining, following the manufacturer’s instructions (Bio-Optica) (Figure 5).
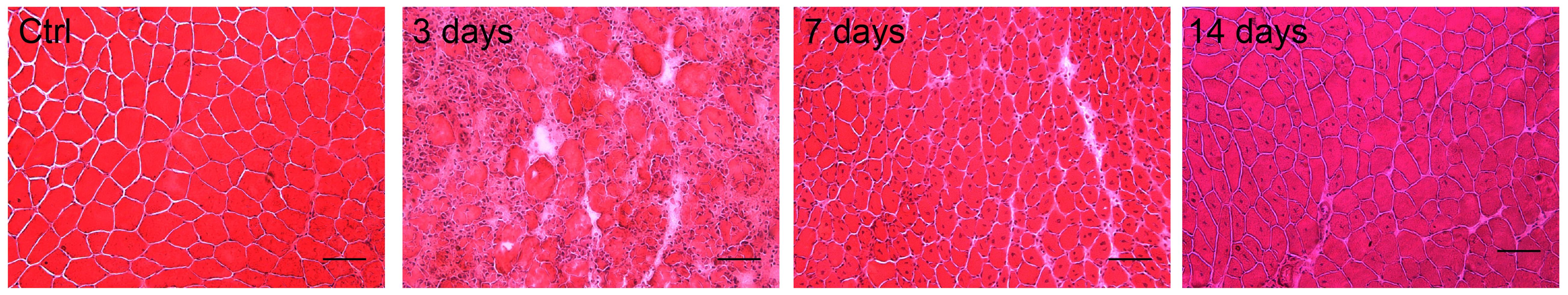
Figure 5. Hematoxylin and Eosin (H&E) staining of WT tibialis anterior (TA) muscles (Ctrl) noninjected and 3, 7, and 14 days after CTX injection. Scale bars, 50 μm.
Data analysis
To compare two data sets, as in Feno et al. (2021), in which we assessed the effect of mitochondrial Ca2+ on macrophage polarization during skeletal muscle regeneration, a parametric Student’s t-test and a parametric one-way analysis of variance (ANOVA) with post hoc Tukey’s multiple comparisons test were applied. Whenever the sample size was lower than five, nonparametric Mann-Whitney or nonparametric Kruskal-Wallis tests were performed to confirm the statistical analysis. P ≤ 0.05 was considered significant.
Notes
Intramuscular injection:
During intramuscular injection, the mouse must be properly anesthetized and restrained. If the mouse is allowed to kick or struggle, this may injure the muscle or the sciatic nerve that runs along the length of the femur. Insert the needle of the syringe parallel to the TA muscle, and once injected, check muscle status. If the CTX injection has been done correctly, you should observe muscle swelling.
Anesthetic procedure:
Mice that are anesthetized must be monitored during the procedure to assure that they are maintained in the proper anesthetic level. Monitor the respiration and color of the mucosae membrane and exposed tissue. An increased respiration rate is a sign that the anesthesia is too light. On the contrary, a deep or irregular respiration rate means that anesthesia is too high.
Acknowledgments
We thank the Italian Association for Cancer Research (AIRC) (IG18633 to R.R.), the University of Padova (STARS@UNIPD WiC grant 2017 to R.R.), the Italian Telethon Association (GGP16029 to R.R. and GGP16026 to A.R.), the Italian Ministry of Health (RF-2016-02363566 to R.R. and GR-2016-02362779 to A.R.), the Cariparo Foundation (to R.R.), and ERC (grant number 3228. v23 to A.V.) for supporting this work.
This protocol is derived from Feno et al. (2021), DOI: 10.1126/scisignal.abf3838.
Competing interests
The authors declare that there is no conflict of financial or research interests.
Ethics
In vivo experiments were performed in accordance with the Italian law D. L.vo n.26/2014.
References
- Chargé, S. B. P. and Rudnicki, M. A. (2004). Cellular and molecular regulation of muscle regeneration. Physiol Rev 84(1): 209-238.
- Feno, S., Munari, F., Reane, D. V., Gissi, R., Hoang, D. H., Castegna, A., Chazaud, B., Viola, A., Rizzuto, R. and Raffaello, A. (2021). The dominant-negative mitochondrial calcium uniporter subunit MCUb drives macrophage polarization during skeletal muscle regeneration. Sci Signal 14(707): eabf3838.
- Frontera, W. R. and Ochala, J. (2015). Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 96(3): 183-195.
- Joe, A. W. B., Yi, L., Natarajan, A., Le Grand, F., So, L., Wang, J., Rudnicki, M. A. and Rossi, F. M. V (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12(2): 153-163.
- Mao, A. S. and Mooney, D. J. (2015). Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A 112: 14452-14459.
- Mounier, R., Théret, M., Arnold, L., Cuvellier, S., Bultot, L., Göransson, O., Sanz, N., Ferry, A., Sakamoto, K., Foretz, M., et al. (2013). AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab 18(2): 251-264.
- Perdiguero, E., Sousa-Victor, P., Ruiz-Bonilla, V., Jardí, M., Caelles, C., Serrano, A. L. and Muñoz-Cánoves, P. (2011). p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol 195(2): 307-322.
- Rigamonti, E., Zordan, P., Sciorati, C., Rovere-Querini, P. and Brunelli, S. (2014). Macrophage plasticity in skeletal muscle repair. Biomed Res Int 2014: 560629.
- Saclier, M., Yacoub-Youssef, H., Mackey, A. L., Arnold, L., Ardjoune, H., Magnan, M., Sailhan, F., Chelly, J., Pavlath, G. K., Mounier, R., et al. (2013). Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells 31(2): 384-396.
- Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B. and Sandri, M. (2013). Mechanisms regulating skeletal muscle growth and atrophy. FEBS J 280(17): 4294-4314.
- Tidball, J. G. (2017). Regulation of muscle growth and regeneration by the immune system. Nat Rev Immunol 17(3): 165-178.
- Uezumi, A., Fukada, S., Yamamoto, N., Takeda, S. and Tsuchida, K. (2010). Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12(2): 143-152.
- Wang, X. and Zhou, L. (2022). The Many Roles of Macrophages in Skeletal Muscle Injury and Repair. Front Cell Dev Biol 10: 952249.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Feno, S., Munari, F., Gherardi, G., Vecellio Reane, D., D’Angelo, D., Viola, A., Rizzuto, R. and Raffaello, A. (2023). Myonecrosis Induction by Intramuscular Injection of CTX. Bio-protocol 13(1): e4587. DOI: 10.21769/BioProtoc.4587.
- Feno, S., Munari, F., Reane, D. V., Gissi, R., Hoang, D. H., Castegna, A., Chazaud, B., Viola, A., Rizzuto, R. and Raffaello, A. (2021). The dominant-negative mitochondrial calcium uniporter subunit MCUb drives macrophage polarization during skeletal muscle regeneration. Sci Signal 14(707): eabf3838.
Category
Cell Biology > Tissue analysis > Injury model
Cell Biology > Cell imaging > Cryosection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









