- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Parasitoid Wasp Culturing and Assay to Study Parasitoid-induced Reproductive Modifications in Drosophila
Published: Vol 13, Iss 1, Jan 5, 2023 DOI: 10.21769/BioProtoc.4582 Views: 1391
Reviewed by: Geoffrey C. Y. LauDURAI SELLEGOUNDERAnand Ramesh PatwardhanAnonymous reviewer(s)
Abstract
In nature, parasitoid wasp infections are a major cause of insect mortality. Parasitoid wasps attack a vast range of insect species to lay their eggs. As a defense, insects evolved survival strategies to protect themselves from parasitoid infection. While a growing number of studies reported both host defensive tactics and parasitoid counter-offensives, we emphasize that this parasite–host relationship presents a unique ecological and evolutionary relevant model that is often challenging to replicate in a laboratory. Although maintaining parasitoid wasp cultures in the laboratory requires meticulous planning and can be labor intensive, a diverse set of wasp species that target many different insect types can be maintained in similar culture conditions. Here, we describe the protocol for culturing parasitoid wasp species on Drosophila larvae and pupae in laboratory conditions. We also detail an egg-laying assay to assess the reproductive modification of Drosophila females in response to parasitoid wasps. This behavioral study is relatively simple and easily adaptable to study environmental or genetic influences on egg-laying, a readout for female germline development. Neither the parasitoid culture conditions or the behavioral assay require special supplies or equipment, making them a powerful and versatile approach in research or teaching laboratory settings.
Graphical abstract

Background
Parasitoid wasps attack the Drosophila genus with an infection rate of 90% in the natural population (Fleury et al., 2004). Depending on the species, wasps lay their eggs in the developing stages of fruit fly larvae or pupae. If the parasitized host fails to encapsulate the newly injected parasitoid egg, these developing eggs hatch into larval wasps that devour the fly progeny innards before emerging from a Drosophila pupal case. After mating, the adult female parasitoid searches for a new host and thus begins the parasitoid life cycle (Carton and Nappi, 1997).
Drosophila have evolved various defensive behavioral and cellular strategies to reduce the risk of parasitoid infection. For instance, while fly larvae display a rolling response (Hwang et al., 2007; Robertson et al., 2013) and parasitoid avoidance behavior to escape the wasp attack (Ebrahim et al., 2015), Drosophila adults show accelerated mating behavior (Ebrahim et al., 2021), avoidance response (Ebrahim et al., 2015), oviposition depression (Lynch et al., 2016; Sadanandappa et al., 2021), and ethanol-seeking behavior (Kacsoh et al., 2013; Kacsoh et al., 2015) in the presence of parasitoids. These host defensive strategies lead to parasitoid’s counter-offensive tactics, resulting in more substantial selection pressure on the host. In the wild, both the host and the parasitoid seem to be locked in a perpetual arms race to best one another's newly evolved adaptations (Wertheim, 2022). Beyond the ecological and evolutionary perspective, studying host–parasitoid biology in the laboratory is significant in economic value. Since parasitoids are used in the biocontrol of fruit flies and other insects, understanding the strategies employed by parasitoid wasps helps to reduce agricultural loss by pests.
Compared to Drosophila , parasitoid wasps culturing in the laboratory can be labor intensive and demands careful planning and gentle handling of the wasps (Small et al., 2012). Here, we provide the step-by-step procedure for culturing parasitoid wasp species—Leptopilina and Trichopria—on Drosophila larvae and pupae, respectively, in laboratory conditions that already exist in a typical fruit fly research or teaching laboratory. Once established, the maintenance and scaling up to larger productions of the parasitoid cultures do not present significant challenges; optimized culture conditions are generally transferable to many parasitoid species. We also summarize the egg-laying assay used by Sadanandappa et al. (2021) to study the parasitoid-induced reproductive modifications in female fruit flies and discuss the adaptability of this behavioral prototype to examine the influence of environmental and genetic factors in germline development.
Materials and Reagents
Narrow Drosophila vials (Genesee Scientific, catalog number: 32-116SB)
Flugs® narrow plastic vials (Genesee Scientific, catalog number: 49-102)
Square bottom Drosophila bottle (Genesee Scientific, catalog number: 32-130)
Flugs® plastic fly bottles (Genesee Scientific, catalog number: 49-100)
Round synthetic paintbrush (Lowell hillcrest, size 4/0 or 3/0)
KimtechTM science kimwipes (Kimberly-Clark Professional, catalog number: 34155, 4.4” × 8.4”)
Cheesecloth (Genesee Scientific, catalog number: 53-100)
Host cultures: larvae and pupae of Drosophila melanogaster (strain Canton S, Bloomington Stock Center)
Larval parasitoid wasps: Leptopilina boulardi (strain Lb17) or L. heterotoma (strain Lh14) (obtained from Todd Schlenke’s laboratory at the University of Arizona)
Pupal parasitoid wasp: Trichopria drosophilae (strain Trical) (from Todd Schlenke’s laboratory)
Raw and unfiltered honey (Nature Nate’s Honey Co.)
Industrial grade carbon dioxide (CO2) (Airgas, an Air Liquide company)
Fly morgue (glass beaker containing Wescodyne® Plus diluted in water and plastic funnel)
Ethanol, 70%, laboratory grade
Drosophila medium (cornmeal molasses yeast medium) (see Recipes)
Gelidium agar (MoorAgar, catalog number: 41076)
Yeast (Phileo by Lesaffre, catalog number: 73050 SafPro Relax+YF)
Corn (MP Biomedicals, catalog number: 0290141180)
Molasses (Reinhart Foodservice, catalog number: DW816)
Propionic acid (Fisher Scientific, catalog number: A258-500)
Methyl 4-hydrobenzoate (NIPAGIN) (Sigma-Aldrich, catalog number: H5501)
Equipment
Stereomicroscope (Zeiss, model: Stemi 2000)
Fiber optic light source (Fisher Scientific, model: LaxcoTM PIFOS150IB)
Incubator with controlled temperature (25 °C), humidity (60%), and 12:12 h light/dark cycles (Percival Scientific, model: DR41VL)
Fly room with controlled temperature (25 °C), humidity (60%), and 12:12 h light/dark cycles
Fly pad, CO2 anesthetizing apparatus (Genesee Scientific, catalog number: 59-119)
Software
Prism 9 (GraphPad Software, LLC, San Diego, CA, https://www.graphpad.com)
OriginPro (OriginLab Corporation, Northampton, MA, https://www.originlab.com)
BioRender (BioRender, https://biorender.com)
Procedure
Part I: Culturing parasitoid wasps (Figure 1)
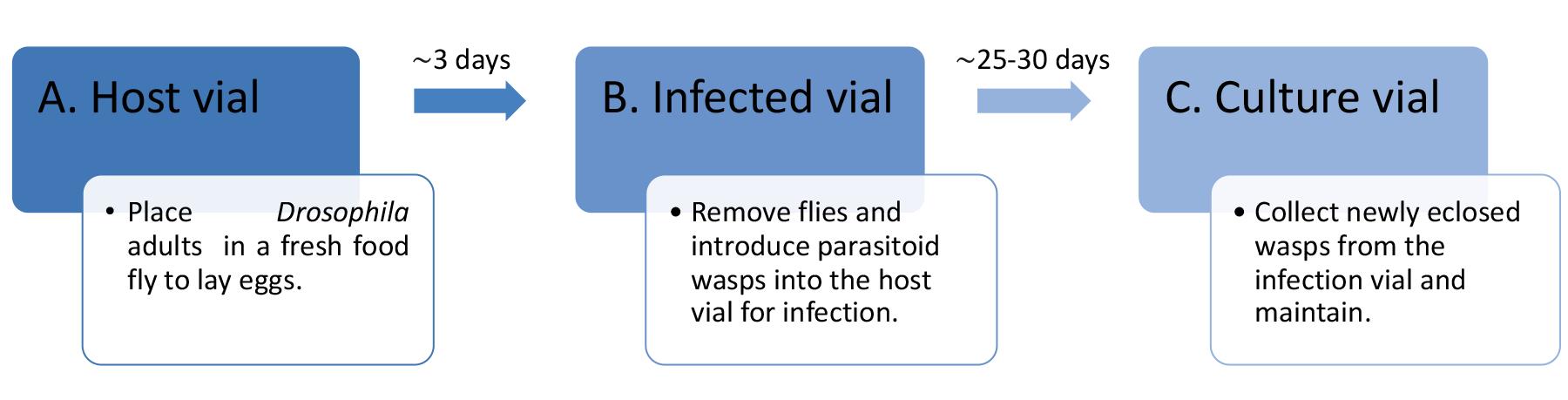
Figure 1. Flow chart summarizing the culturing of parasitoid wasps in laboratory conditions
Preparation of the host vials
In the laboratory, culture wildtype Drosophila (strain Canton S) in plastic fly bottles containing standard cornmeal molasses yeast medium (see Recipes) and maintain in 12:12 h light/dark cycle–controlled incubators at 25 °C and 60% relative humidity, unless otherwise specified.
To avoid overcrowding and stock maintenance, transfer the parental flies (P0, approximately 25 females and 10 males) into fresh fly bottles after four days of egg laying.
At 25 °C, adult Drosophila progeny (F1) begins to eclose from pupal cases in the culture bottles in 9–10 days.
Anesthetize a bottle of F1 adult fruit flies (preferably 3–10-days-old) with CO2 and place the flies on a fly pad.
Using a microscope and a paintbrush, transfer anesthetized F1 adults—15 females and 8 males—into a vial containing fresh fly food (referred to as the host vial ) and allow the flies to lay eggs for three days at 25 °C (Figure 2C–left).
After three days, either discard F1 adults from the host vials into a fly morgue or transfer F1 adults to a fresh food vial for preparing a new host vial.
Culturing larval parasitoid wasp
Following three days of fruit fly egg laying and removal of F1 adults from the host vials, place 3–4 drops of honey diluted 1:1 in distilled water on the inner side of the host vial plugs, to serve as a food source for the adult wasps.
Using CO2, anesthetize 5–10-days-old adult larval parasitoid wasps—Leptopilina boulardi (Lb17) or Leptopilina heterotoma (Lh14)—and sort male and female parasitoids (Figure 2A and 2B). Identifying male and female Leptopilina is relatively easy: male parasitoids have longer antennas and smaller bodies than females. Additionally, on the posterior ventral side of the abdomen, female wasps have a specialized needle-like structure, an ovipositor, that they insert into the host to deposit the eggs.
Gently transfer the adult parasitoids (12 females and 8 males) into the host vial containing the developing Drosophila larvae.
Place the honey-supplemented plugs and leave the host vials (referred to as the infected vial ) on their side until the parasitoids recover from CO2 exposure (Figure 2C–right). Anesthetized wasps could otherwise fall into wet/damp food in an upright vial and, subsequently, be unable to extricate themselves upon waking.
Maintain the wasp-infected vials in a fly room or an incubator with controlled conditions until the emergence of the adult parasitoids (Figure 2D).
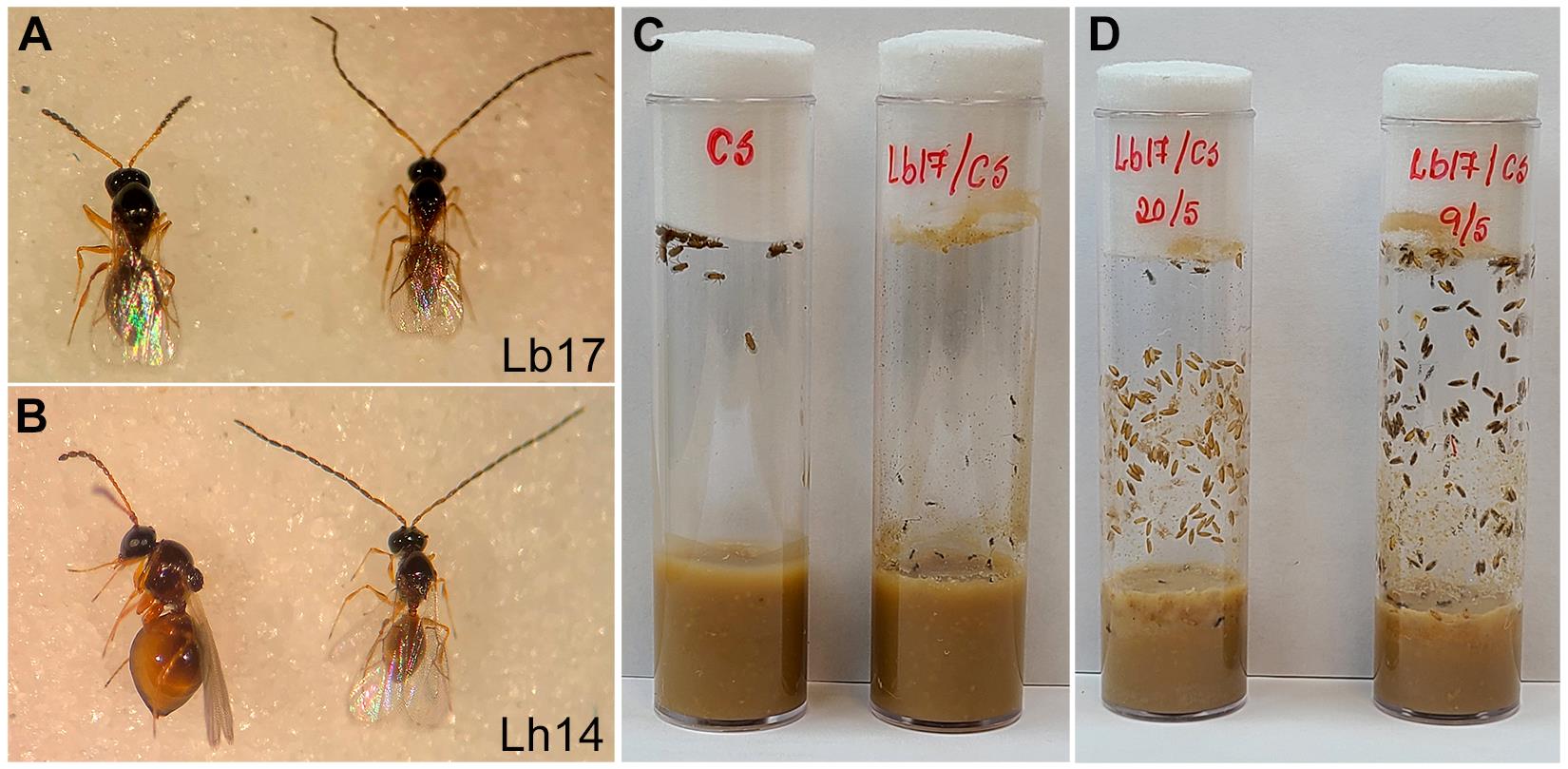
Figure 2. Larval parasitoid–infected host vial. Image of female (left) and male (right) larval parasitoid wasps: (A) L. boulardi (Lb17) and (B) L. heterotoma (Lh14). (C) Image of a freshly prepared host vial for an egg laying (left) and L. boulardi –infected host vial containing the developing larval stages (right). (D) Image of L. boulardi –infected vial with early (left) and late (right) stages of wasp development within a Drosophila pupal case. Note the adult fruit flies that escaped the parasitoid attack or suppressed the infection (left) and newly emerged wasps in the infected vial (right).Culturing pupal parasitoid wasps
After three days of egg laying, either discard or transfer the adult F1 flies from the host vial to a new food vial. Allow the larval stages to develop in the host vial at 25 °C for approximately 3–4 days.
When the third instar larvae initiate pupation (pre-pupae) in the host vials, remove the plugs and add 3–4 drops of honey diluted in distilled water.
Anesthetize 5–10-days-old adult pupal parasitoids [ Trichopria drosophilae (Trical)] using CO2 and sort male and female parasitoids on a fly pad. Like Leptopilina , Trical males also have longer antennas and smaller bodies than females (Figure 3A).
Similar to larval parasitoid infection, carefully transfer the Trical wasps (12 females and 8 males) into the host vial containing Drosophila pre-pupae and developing larval stages.
Cover the freshly infected host vial with honey-supplemented plugs and leave the vials on their side.
After parasitoids wake from CO2 exposure, keep the vials vertically and maintain the wasp-infected vials in a fly room or an incubator with controlled conditions (Figure 3B).

Figure 3. Pupal parasitoid–infected host vial. Image of (A) T. drosophilae female (left) and male (right) pupal parasitoid wasp and (B) the parasitoid-infected vial with early (left) and late (right) stages of development within the fly pupal case. Note the wasps used for infecting the fly pupae (left) and newly emerged ones (right) in the infected vials.Collecting and maintaining parasitoid wasps
Leptopilina females infect the developing Drosophila larval stages, whereas Trichopria females attack fruit fly pupae to lay their eggs (Small et al., 2012). Successful parasitoid infections suppress the host’s immune response and delay fruit fly development. Consequently, the parasitoid egg hatches, develops, and molts, and the adult wasp emerges from the Drosophila pupal case. Depending on parasitoid species and environmental conditions—temperature and humidity—the adult wasp ecloses 25–30 days post-infection.
Alternatively, if the host’s immune response encapsulates the parasitoid egg, it blocks the wasp egg development, and Drosophila adults emerge normally (Carton and Nappi, 1997).
While parasitoid eggs are developing in the host vial, the fruit flies’ progeny that successfully defended against the parasitoid infection or escaped the wasp attack ecloses before the wasp’s emergence (see Figure 2D). Discard these adult fruit flies into a fly morgue.
To collect and maintain adult parasitoid wasps, prepare a culture vial by inserting a folded Kimwipe sheet inside fresh fly food vials to absorb excess moisture and supplement the plugs with diluted honey. Anesthetize the wasps that emerged in the infected vial on a fly pad and gently place them (approximately 50 wasps/vial) in the culture vials (Figure 4).
Repeat the above step once every 4–5 days to collect the newly emerged wasps from the infected vials and maintain the culture vials at room temperature. Then, collected wasps are used for experiments (see below) and to start new wasp cultures by infecting the host vial (Figure 2C and 3B).
To avoid adult parasitoid wasp death due to deteriorating fly food, gradual dryness of the plugs, or accumulation of moisture in the vials, transfer wasps to fresh culture vials as needed.

Figure 4. Parasitoid culture vials. Image of newly emerged male and female L. boulardi (left) and T. drosophilae (right) parasitoids collected from the respectively infected host vials.
Notes
Since parasitoid wasps are sensitive to CO2 anesthetization, we recommend using lower CO2 pressure (approximately 10 psi, depending on the fly pad and setup of CO2 station) than for fruit flies and avoiding prolonged CO2 exposure. Also, handle the wasps gently.
Before its use for parasitizing the host, female wasp mating is crucial. Therefore, allowing newly collected male and female wasps to cohabitate in culture vials prior to infection is important for producing fertilized wasp eggs (Figure 4). Parasitoid wasps follow the haplodiploidy sex determination: males develop from unfertilized eggs and are haploid, whereas females develop from fertilized eggs and are diploid. Allowing time for wasp mating and egg fertilization before infection ensures an appropriate female proportion in the next generation of wasps. This way, the ratio of female to male parasitoids in the host vials during infection is less important.
As mentioned above, the presence of parasitoid wasps triggers various behavioral and physiological modifications in Drosophila (Kacsoh et al., 2013; Robertson et al., 2013; Ebrahim et al., 2015; Kacsoh et al., 2015; Ebrahim et al., 2021; Sadanandappa et al., 2021). Therefore, we recommend limiting exposure by maintaining the wasp and Drosophila cultures in different locations and using a dedicated CO2 station and materials for wasp handling. Otherwise, thoroughly clean the fly station with 70% ethanol after wasp handling.
Part II: Egg-laying assay in Drosophila
Culturing experimental stocks
As stated, culture all experimental fly lines in 12:12 h light/dark cycle–controlled incubators at 25 °C and 60% relative humidity.
To achieve age synchronization of flies for the behavioral analysis, discard all the adult flies into a fly morgue and collect newly eclosed flies (both male and female) within 12 h of clearance into fresh fly bottles (approximately 50 flies/bottle). Avoid experimental fly collection from old, overcrowded, or fungal-infected bottles.
Maintain these bottles, containing 0–12-h-old fruit flies, in an incubator with controlled conditions (25 °C, 60% humidity, and 12:12 h light/dark cycles), followed by five days of aging before subjecting the flies to the reproductive assay.
Performing egg-laying assay
Using a microwave (approximately 30–45 s), liquefy the food in a fresh fly bottle and immediately pour approximately 1 mL of the media into empty Drosophila narrow vials (referred to as egg-laying vial ).
To avoid stray flies entering the egg-laying vial and accumulating water vapor as the food solidifies, cover the vial’s mouth with a cheesecloth. Leave the tray containing the egg-laying vials at room temperature for 30–60 min.
When the fly media solidifies, remove the cheesecloth and place a fresh plug in the vial.
Using CO2, anesthetize adult parasitoid wasps (recommended age: 4–7-days-old) from the culture vials. Using a paintbrush, gently place three female Leptopilina or Trical parasitoids into the egg-laying vials and leave them on their side.
Anesthetize all age-(5–6 days) and genotype-matched Drosophila (Canton S) and transfer five female and two male flies to the egg-laying vials that contain parasitoids (wasp-exposed vials).
To prepare the mock vials (unexposed controls), follow the steps above, except for introducing the parasitoids.
When all flies recover from anesthetization, place the wasp-exposed and mock vials in different fly racks and leave them undisturbed in an incubator at 25 °C, 60% humidity, and 12:12 h light/dark cycles.
After 24 h, discard all the flies, including the wasps, to the fly morgue and manually document the total number of eggs laid in each vial using a stereomicroscope (Figure 5A and 5B). The Drosophila egg is approximately 0.5 mm long, ovoid in shape, with dorsal appendages on the anterior side (Figure 5C).
Compared to unexposed mock controls (Figure 5A), 24 h L. boulardi –exposed (Figure 5B) female flies show reduced mean egg laying (Figure 6) (Sadanandappa et al., 2021).
This behavioral assay can be easily adaptable to study the environmental—nutrition, temperature, daylight, infections, etc.—or genetic effects on germline development (Kurz et al., 2017). By transferring the experimental flies to a fresh vial, the continued egg-laying assay informs the prolonged influence of these factors on oviposition and reproductive behaviors (Pang et al., 2022).
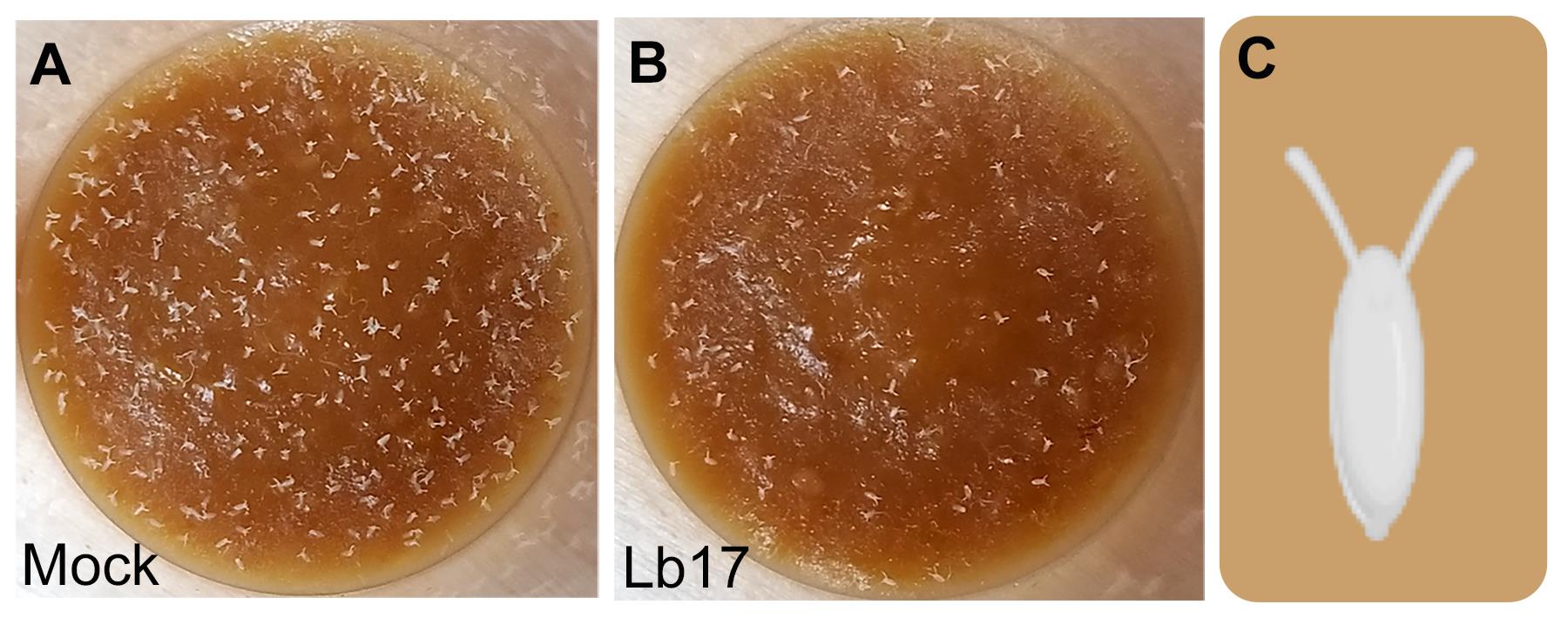
Figure 5. Egg-laying assay in Drosophila. Representative images showing 24 h of egg-laying in (A) mock and (B) L. boulardi (Lb17) parasitoid–exposed vials. Images are adapted from Sadanandappa et al. (2021, PLOS Genet) under CC BY 4.0. (C) Schematic of Drosophila egg.
Notes
While setting up the behavioral experiments, avoid exposing the mock flies to the wasp-exposed group and limit prolonged CO2 exposure as it influences the egg laying in Drosophila females.
Exclude egg-laying documentation from the vials with dead flies/wasps.
To avoid biases, we recommend double blinding the egg-laying vials before documentation and then decoding the egg count data after counting.
Parasitoids used for the exposure experiments were not given any hosts for infection and were never used again after the assay.
Previous studies demonstrated that Drosophila adults respond differently to female and male parasitoids. Therefore, we used only female parasitoids in this protocol.
Data analysis
Any subtle variation in environmental conditions—fly medium, temperature, humidity, nutrition, overcrowding—, infections, and genetic background may influence the egg-laying assay. We recommend at least 3–5 experimental sets along with unexposed genotype controls per replicate for reproducibility and accuracy.
Using the Prism or the OriginPro software, the average number of eggs laid by unexposed controls and parasitoid wasp–exposed females can be plotted as a column graph with the standard error mean calculated from at least three independent experiments (Figure 6).
Perform the two-tailed unpaired t-test with Welch’s correction to determine the statistical significance between the groups egg-laying means.
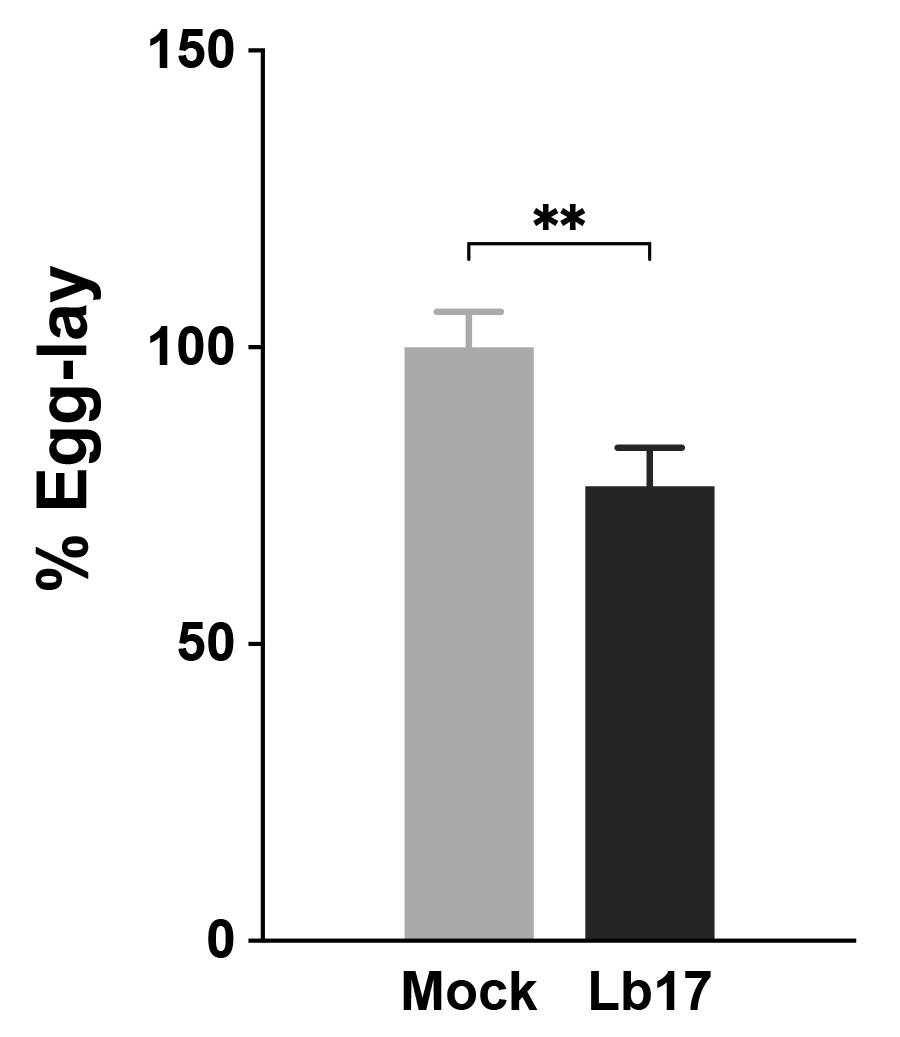
Figure 6. Larval parasitoid–induced egg laying reduction in Drosophila. Histogram showing the 24 h percentage of egg laying in unexposed (light grey) and Lb17 parasitoid–exposed (dark grey) wildtype Canton S flies. The egg laying responses are represented as a percentage of the mock egg lay, and error bars are ± standard error mean (N = 35, three independent experiments). ** p = 0.0098, calculated using a two-tailed unpaired t-test with Welch’s correction.
Recipes
Cornmeal molasses yeast medium
Reagent Final concentration Amount Agar 1% 10 g Cornmeal 7.6% 76 g Molasses 7.6% 76 g Yeast 5% 50 g ddH2O n/a 1 L Total n/a 1 L Cook the fly medium until it begins to boil and allow it to cool down to below 60 °C before adding NIPAGIN (0.4%) and propionic acid (0.2%).
Allow food in bottles or vials to dry overnight at room temperature, covered with a sterile cheesecloth before capping with clean plugs.
Fly food can be stored for up to one week in a cold room or fridge between 4 °C and 18 °C. Culture vials and bottles should be brought to room temperature and free of condensation before introducing the flies.
Acknowledgments
This project was supported by the Human Frontier Science Program Long-Term Fellowship to M.K.S and the National Institute of Health, Pioneer grant 1DP1MH110234 and the Defense Advanced Research Projects Agency grant HR0011-15-1-0002 to G.B. We thank Todd Schlenke (University of Arizona) for providing the wasp strains and Victoria L. Marlar, our lab technician, for all the help. The protocol is adapted from Small et al. (2012) and Sadanandappa et al. (2021). BioRender.com was used for making the graphic.
Competing interests
The authors of this work have no conflicts of interest to declare.
References
- Carton, Y. and Nappi, A. J. (1997). Drosophila cellular immunity against parasitoids. Parasitol Today 13(6): 218-227.
- Ebrahim, S. A. M., Dweck, H. K. M., Stökl, J., Hofferberth, J. E., Trona, F., Weniger, K., Rybak, J., Seki, Y., Stensmyr, M. C., Sachse, S., et al. (2015). Drosophila avoids parasitoids by sensing their semiochemicals via a dedicated olfactory circuit. PLOS Biol 13(12): e1002318.
- Ebrahim, S. A. M., Talross, G. J. S. and Carlson, J. R. (2021). Sight of parasitoid wasps accelerates sexual behavior and upregulates a micropeptide gene in Drosophila. Nat Commun 12(1): 2453.
- Fleury, F., Ris, N., Allemand, R., Fouillet, P., Carton, Y. and Boulétreau, M. (2004). Ecological and genetic interactions in Drosophila–parasitoids communities: A case study with D. melanogaster, D. simulans and their common Leptopilina parasitoids in Southe-astern France. Genetica 120(1-3): 181-194.
- Hwang, R. Y., Zhong, L., Xu, Y., Johnson, T., Zhang, F., Deisseroth, K. and Tracey, W. D. (2007). Nociceptive neurons protect Drosophila larvae from parasitoid wasps. Curr Biol 17(24): 2105-2116.
- Kacsoh, B. Z., Lynch, Z. R., Mortimer, N. T. and Schlenke, T. A. (2013). Fruit flies medicate offspring after seeing parasites. Science 339(6122): 947-950.
- Kacsoh, B. Z., Bozler, J., Ramaswami, M. and Bosco, G. (2015). Social communication of predator-induced changes in Drosophila behavior and germ line physiology. eLife 4: e07423.
- Kurz, C. L., Charroux, B., Chaduli, D., Viallat-Lieutaud, A. and Royet, J. (2017). Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition. eLife 6: e21937.
- Lynch, Z. R., Schlenke, T. A. and de Roode, J. C. (2016). Evolution of behavioural and cellular defences against parasitoid wasps in the Drosophila melanogaster subgroup. J Evol Biol 29(5): 1016-1029.
- Pang, L., Liu, Z., Chen, J., Dong, Z., Zhou, S., Zhang, Q., Lu, Y., Sheng, Y., Chen, X. and Huang, J. (2022). Search performance and octopamine neuronal signaling mediate parasitoid induced changes in Drosophila oviposition behavior. Nat Commun 13(1): 4476.
- Robertson, J. L., Tsubouchi, A. and Tracey, W. D. (2013). Larval defense against attack from parasitoid wasps requires nociceptive neurons. PLoS One 8(10): e78704.
- Sadanandappa, M. K., Sathyanarayana, S. H., Kondo, S. and Bosco, G. (2021). Neuropeptide F signaling regulates parasitoid-specific germline development and egg-laying in Drosophila. PLOS Genet 17(3): e1009456.
- Small, C., Paddibhatla, I., Rajwani, R. and Govind, S. (2012). An introduction to parasitic wasps of Drosophila and the antiparasite immune response. J Vis Exp (63): e3347.
- Wertheim, B. (2022). Adaptations and counter-adaptations in Drosophila host–parasitoid interactions: advances in the molecular mechanisms. Curr Opin Insect Sci 51: 100896.
Article Information
Copyright
© 2023 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sadanandappa, M. K., Sathyanarayana, S. H. and Bosco, G. (2023). Parasitoid Wasp Culturing and Assay to Study Parasitoid-induced Reproductive Modifications in Drosophila. Bio-protocol 13(1): e4582. DOI: 10.21769/BioProtoc.4582.
Category
Neuroscience > Behavioral neuroscience
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Tips for asking effective questions
+ Description
Write a detailed description. Include all information that will help others answer your question including experimental processes, conditions, and relevant images.
Share
Bluesky
X
Copy link