- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation of iMyoblasts from Human Induced Pluripotent Stem Cells
Published: Vol 12, Iss 17, Sep 5, 2022 DOI: 10.21769/BioProtoc.4500 Views: 3904
Reviewed by: Gal HaimovichXiaokang WuAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Culture of Ferret Airway Stem Cells
Ziying Yan [...] Feng Yuan
Jul 20, 2025 2413 Views

Whole-Mount Immunostaining for the Visual Separation of A- and C-Fibers in the Study of the Sciatic Nerve
Valeriia Ustymenko [...] Nana Voitenko
Dec 5, 2025 1434 Views

Optimization of Adipogenic Differentiation Protocol for Murine and Human Cell Culture Models
Junwan Fan [...] Wenyan He
Jan 20, 2026 230 Views
Abstract
Skeletal muscle stem cells differentiated from human-induced pluripotent stem cells (hiPSCs) serve as a uniquely promising model system for investigating human myogenesis and disease pathogenesis, and for the development of gene editing and regenerative stem cell therapies. Here, we present an effective and reproducible transgene-free protocol for derivation of human skeletal muscle stem cells, iMyoblasts, from hiPSCs. Our two-step protocol consists of 1) small molecule-based differentiation of hiPSCs into myocytes, and 2) stimulation of differentiated myocytes with growth factor-rich medium to activate the proliferation of undifferentiated reserve cells, for expansion and cell line establishment. iMyoblasts are PAX3+/MyoD1+ myogenic stem cells with dual potential to undergo muscle differentiation and to self-renew as a regenerative cell population for muscle regeneration both ex vivo and in vivo. The simplicity and robustness of iMyoblast generation and expansion have enabled their application to model the molecular pathogenesis of Facioscapulohumeral Muscular Dystrophy and Limb-Girdle Muscular Dystrophies, to both ex vivo and in vivo muscle xenografts, and to respond efficiently to gene editing, enabling the co-development of gene correction and stem cell regenerative therapeutic technologies for the treatment of muscular dystrophies and muscle injury.
Graphical abstract:
Background
Human-induced pluripotent stem cells (hiPSCs) (Takahashi et al., 2007) reprogrammed from somatic cells retain the genetic background of donor cells and have the ability to self-renew and differentiate into cell types of the three germ layers, providing a powerful tool for investigating human myogenesis, muscular dystrophies, and therapeutic development. Over the past decades, several strategies have been developed to differentiate hiPSCs into skeletal muscle, including regulating growth factors and signal pathways that control muscle development during embryogenesis (Chal et al., 2016; Xi et al., 2017), overexpressing transcription factors (Dekel et al., 1992; Darabi et al., 2012; Abujarour et al., 2014), generating myogenic gene reporter iPSC cell lines (Wu et al., 2018; Al Tanoury et al., 2020), or fluorescence-activated cell sorting (FACS) for myogenic cell surface markers (Uezumi et al., 2016).
Here, we report the development of a transgene-free protocol to isolate and expand PAX3+ human skeletal muscle stem cells, iMyoblasts, from patient and unaffected control hiPSCs using a two-step gene-free myogenic induction protocol. The first step utilizes commercially available reagents to generate cultures of differentiated iMyoctyes (Caron et al., 2016). The second step utilizes growth factor-rich medium to stimulate the proliferation of quiescent undifferentiated reserve cells embedded in differentiated iMyocyte cultures, to maintain their growth as PAX3+/MYOD1+/CD82+ iMyoblasts, and to establish low passage frozen cell stocks (Guo et al., 2022). Myogenic reserve cells have been previously identified in mouse and human satellite cell cultures (Yoshida et al., 1998; Laumonier et al., 2017). By contrast, previous gene-free myogenesis protocols have primarily utilized human embryonic stem cell lines (hESCs) and FACS isolation methods to recover cells expressing PAX7+ (Shelton et al., 2014; van der Wal et al., 2018).
iMyoblasts isolated as reserve cells from differentiated hiPSC cultures using our protocol have been extensively characterized as myogenic progenitors. PAX3+/MYOD1+/CD82+ iMyoblasts can be maintained in culture for more than 12 passages and greater than 30 population doublings, while retaining their potential to differentiate into myotubes and to self-renew as reserve cells. Single-cell RNA Seq reveals that iMyoblasts have embryonic/fetal transcriptomes. Following xenoengraftment into the mouse tibialis anterior (TA) fast twitch muscle, iMyoblasts undergo muscle fiber differentiation and myosin isoform switching for adult muscle myosin isoform expression, revealing their plasticity to undergo adult muscle differentiation in response to cues in the in vivo muscle environment. iMyoblasts also populate muscle xenografts and can regenerate muscle in response to injury, providing evidence that iMyoblasts behave as myogenic stem cells with dual potential to undergo muscle differentiation and to self-renew as a regenerative cell population. When generated from iPSCs of Facioscapulohumeral Muscular Dystrophy (FSHD) patient fibroblasts or myoblasts, iMyoblasts express the FSHD disease gene, DUX4, and its downstream target genes, at levels comparable to primary FSHD biopsy myoblasts after differentiation. iMyoblasts from Limb-Girdle Muscular Dystrophy (LGMD) R7 and R9 (formerly LGMD2G and 2I) and Walker Warburg Syndrome (WWS) patients modeled their molecular disease pathologies and were responsive to small molecule and gene editing therapeutics (Iyer et al., 2019; Guo et al., 2022). These findings establish the utility of iMyoblasts for investigating human myogenesis and disease pathogenesis and for the co-development of gene editing and muscle stem cell therapies.
Materials and Reagents
Materials
Cell culture 6-well plate, flat bottom (Falcon, catalog number: 353046)
BioCoat collagen I 6-well clear plate (Corning, catalog number: 356400)
15 cm dishes (Nunc, catalog number: 168381)
100 mm TC-treated culture dish (Corning, catalog number: 430167)
1,000 μL graduated filter tips (USA Scientific, catalog number: 1122-1830)
200 μL graduated filter tips (USA Scientific, catalog number: 1120-8810)
20 μL graduated filter tips (USA Scientific, catalog number: 1123-1810)
10 μL graduated filter tips (USA Scientific, catalog number: 1120-3810)
1.5 mL microcentrifuge tube (Axygen, catalog number: MCT-150-C-S)
15 mL PP centrifuge tubes (Corning, catalog number: 430791)
50 mL PP centrifuge tubes (Corning, catalog number: 430829)
0.2 μm Nalgene Rapid-Flow sterile single use vacuum filter units (Thermo Scientific, catalog number: 566-0020)
Cell lifter (Bio Basic, catalog number: SP91151)
Bench coat or diapers
Sharp-pointed surgical dissecting scissors (Fisherbrand, catalog number: 08-940)
Two autoclaved 500 mL (Corning, catalog number: 1060-500) or 1 L beakers (Corning, catalog number: 6480-1L)
500 mL graduated cylinder (Fisherbrand, catalog number: 08-550G)
Magnetic stir bar, sterilized (Fisherbrand, catalog number: 14-512-129)
50 mL syringe (no needle), sterile (Fisherbrand, catalog number:14-955-455)
Cryovials (Corning, catalog number: 430488)
0.2 mL PCR 8-tube strips (USA Scientific, catalog number: 1402-2900)
Hard-shell PCR plate 384-well, thin-wall (Bio-Rad, catalog number: HSP3805)
Microseal seals (Bio-Rad, catalog number: MSB1001)
Reagents
Dulbecco’s phosphate-buffered saline, 1× (Corning, catalog number: 21-031-CV)
DMEM and Ham's F-12, 50/50 Mix (Corning, catalog number: MT10090CV)
Matrigel hESC-qualified matrix (Corning, catalog number: 354277)
ROCK inhibitor Y-27632 (STEMCELL Technologies, catalog number: 72307)
StemMACS iPS-Brew XF, human (Miltenyi Biotec, catalog number: 130-104-368)
StemMACS passaging solution XF (Miltenyi Biotec, catalog number:130-104-688)
Skeletal muscle differentiation kit (Amsbio, catalog number: SKM-KITM)
Fetal bovine serum (Hyclone, catalog number: SH3007103)
Dimethyl sulfoxide (Fisher, catalog number: BP231-100)
TrypLE express enzyme (Gibco, catalog number: 12605010)
Cell culture grade water (Corning, catalog number: 25-055-CM)
Gelatin from bovine skin (Sigma, catalog number: G9391)
Calcium chloride dihydrate (Sigma, catalog number: 233506)
Ethanol, 200 proof (Fisher Scientific, catalog number: 04-355-223)
Day 12 SPF premium fertilized white leghorn chicken eggs (Charles River, North Franklin, CT)
HBSS without calcium and magnesium (Corning, catalog number: 21-022-CV)
RNeasy Plus mini kit (Qiagen, catalog number: 74136)
SuperScript III first-strand synthesis system (Invitrogen, catalog number: 18080-051)
iQ SYBR® green supermix (Bio-Rad, catalog number: 170-8826)
Distilled water, DNAse, RNAse free (Invitrogen, catalog number: 10977-015)
Paraformaldehyde solution 4% in PBS (Santa Cruz, catalog number: sc-281692)
DAPI (Sigma, catalog number: 9542)
Triton X-100 (Thermo Scientific, catalog number: A16046)
Bovine serum albumin (Sigma, catalog number: A9647)
Goat serum (Gibco, catalog number: 16210064)
Horse serum (Gibco, catalog number: 16050130)
N-2 Supplement (100×) (Gibco, catalog number:17502048)
Insulin-transferrin-selenium (ITS-G) (100×) (Gibco, catalog number: 41400045)
CHIR 99021 (Stemcell, catalog number: 72052)
SB431542 (Selleck chem, catalog number: S1067)
Prednisolone (Sigma, catalog number: P6004)
Antibodies used for IF (Table 1)
Table 1. Antibodies used for IFAntibody Vendor Catalog number Dilution Primary antibody MyoD1 (Clone: 5.8A) Dako M3512 1:50 MF20 DSHB MF 20 1:100 MEF2C Sigma HPA005533 1:100 Secondary antibody Goat anti-mouse IgG Alexa Fluor 488 Invitrogen A11001 1:500 Goat anti-mouse IgG2b Alexa Fluor 488 Invitrogen A21141 1:500 Donkey anti-rabbit IgG Alexa Fluor 488 Invitrogen A21206 1:500 Antibodies used for FACS assay (Table 2)
Table 2. Antibodies used for FACS assayAntibody Vender Catalog number APC mouse anti-human CD56 BD 555518 PE anti-human CD82 BioLegend 342103 FITC anti-human CD318 BioLegend 324004 APC anti-human ERBB3 BioLegend 324708 FITC anti-human NGFR BioLegend 345104 PE anti-human CD18 BioLegend 373407 0.1% gelatin (see Recipes)
HMP medium (see Recipes)
2× HMP freeze medium (see Recipes)
N2 medium for differentiation (Chal et al., 2016) (see Recipes)
Prednisolone medium for differentiation, modified from Al Tanoury et al. (2021) (see Recipes)
Equipment
Pipettes
4 °C Fridge, -20 °C freezer and -80 °C freezer
Biological safety cabinet (Thermo Scientific, catalog number: 1323TS)
Forma series II water-jacketed CO2 incubator (Thermo Scientific, catalog number: 3110)
In vitro hypoxic cabinet (Coy Laboratory, catalog number: O2 Control Cabinet Model 4)
Eclipse TS100 inverted routine microscope (Nikon, catalog number: Eclipse TS100)
DC300F digital microscope camera (Leica)
IEC CL30R centrifuge (Thermo)
Thermo Scientific Sorvall Legend XTR Centrifuge (Thermo)
Countess II automated cell counter (Invitrogen, catalog number: AMQAX1000)
Countess cell counting chamber slides (Invitrogen, catalog number: C10312)
Oil-free vacuum pump OFP-400 (Thermo)
Refrigerated vapor traps (Thermo)
Digital series SpeedVacTM systems (Thermo, mode SPD111V)
NanoDrop 1000 spectrophotometer (Thermo scientific)
C1000 touch thermal cycler (Bio-Rad)
CFX Opus 384 real-time PCR system (Bio-Rad)
Procedure
Chick Embryo Extract Preparation
Spread out bench coat and/or diapers to contain the mess.
Fill three 15 cm Petri dishes with ice-cold HBSS and put the dishes on ice.
Stab a hole into each chick egg using the pointed end of a pair of scissors, and then cut a window out of each eggshell.
Remove the embryo and place it in 15 cm Petri dish with ice-cold HBSS.
Decapitate embryo using sterile scissors, leaving as much of the neck as possible.
Rinse embryos two times in another two 15 cm Petri dishes with ice-cold HBSS to remove blood. Refresh HBSS as necessary.
Store embryos in a sterile beaker with HBSS on ice until all embryos have been collected.
Transfer the embryos (no HBSS) into a 50 mL syringe (no needle) and macerate embryos by pushing through the 50 mL syringe into an ice-cold, sterile graduated cylinder.
Add an equal volume of ice-cold HBSS, transfer to another sterile beaker with a stir bar, cover with aluminum foil, and stir gently at 4 °C for 1 h.
Transfer 45 mL to sterile 50 mL centrifuge tubes and spin at 4 °C for 1 h at 10,000 rpm.
Collect supernatant (Chick Embryo Extract) and store in aliquots at -80 °C. Five dozen eggs make approximately 200–250 mL final Chick Embryo Extract.
Maintenance of Human iPSCs (hiPSCs)
Maintain the hiPSCs in matrigel-coated 6-well plate in StemMACS iPS-Brew X medium at 5% CO2/37 °C /5% CO2 incubator and change the medium every day.
Passage of hiPSC
Check the hiPSCs confluence. When the hiPSCs grow to 95% confluence, it is time to passage the cells. We usually passage the cells every four to five days.
6-well plate matrigel coating: aliquot 250 μL of matrigel per tube and store at -80 °C. When coating plates, thaw aliquoted matrigel on ice and then dilute matrigel with 25 mL of cold DMEM/F12 (1:100). Add 1mL of diluted matrigel to each well of a 6-well plate and keep the plate in the incubator for more than 30 min before use; and then aspirate the matrigel and add 2 mL of StemMACS iPS-Brew XF supplemented with 10 μM of ROCK inhibitor Y-27632.
Aspirate the medium supernatant and wash the cells with 2 mL of DMEM/F12.
Add 1 mL of StemMACS passaging solution XF per well and incubate the plate in the incubator until the cells detach from the plate. It takes approximately 1.5–2 min for the cells at colony edges to start to lift off.
Aspirate the StemMACS passaging solution XF and add 3 mL of StemMACS iPS-Brew XF supplemented with ROCK inhibitor Y-27632.
Gently detach the colonies using a cell lifter and gently pipette up and down two to three times (1 mL pipette setting 900 μL) to break up the colonies into smaller cell clusters.
Transfer the cell clusters into a fresh coated 6-well plate with a splitting ratio between 1:5 and 1:20. Transfer the plate into the incubator and gently shake the plate to evenly distribute the cell clusters.
After 48 h, replace medium with fresh StemMACS iPS-Brew XF without ROCK inhibitor and continue with daily medium changes.
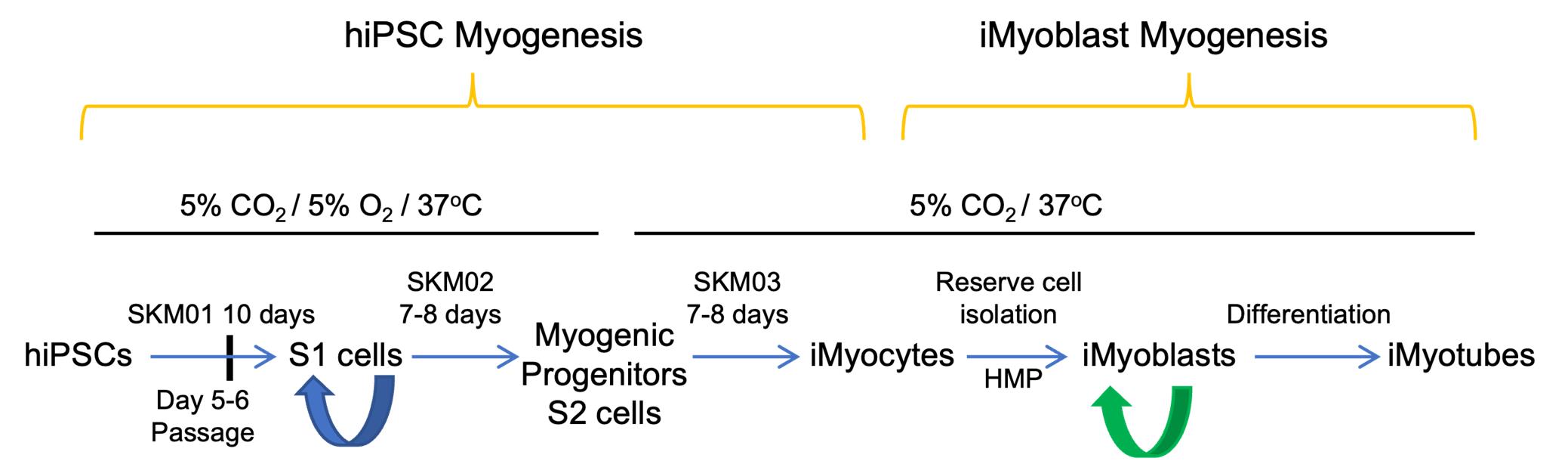
hiPSC Myogenic Differentiation
A commercial skeletal muscle differentiation kit, including SKM01 Skeletal Muscle Induction Medium, SKM02 Myoblast Medium, and SKM03 Myotube Medium, was selected for hiPSCs myogenic differentiation. The myogenic differentiation was completed based on the kit instructions with modifications.
hiPSCs myogenic progenitor cell induction (S1 cells)
Dissociate hiPSCs into single cells after the cells have reached 60%–80% confluence, aspirate the medium, and wash the cells with DMEM/F12. Add 1 mL of TrypLE:0.5 mM EDTA (3:1) per well and incubate in a 37 °C incubator for approximately 5 min.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium and collect the cells in a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 1 mL of warm Skeletal Muscle Induction Medium (SKM01).
Count the number of viable cells.
Plate 25,000 cells onto collagen I-coated 6-well plate with the density of 2,500 cells/cm2 in SKM01 medium.
Place the cells in 5% O2/5% CO2/37 °C incubator and gently shake the plate to evenly distribute the cell clusters.
Change fresh SKM01 medium every other day.
Passage the cells at day 6
Aspirate the medium and wash with PBS.
Add 1 mL of TrypLE per well and incubate the plate in a 37 °C incubator for 3–5 min until all of the cells detach from the plate.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium and collect the cells in a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 1 mL of warm Skeletal Muscle Induction Medium (SKM01).
Replate the cells onto collagen I-coated 6-well plate with a ratio of 1:4 in SKM01 medium.
Change SKM01 medium every other day until day 10 during maintenance of cultures in 5% O2/5% CO2/37 °C incubator.
Commitment of S1 cells to myogenic progenitors (S2 cells)
After 10 days in SKM01 Skeletal Muscle Induction Medium, dissociate S1 cells and replate into Skeletal Myoblast Medium (SKM02) for S2 myogenic progenitor induction.
Aspirate the medium and wash with PBS.
Add 1 mL of TrypLE per well and incubate the plate at 37 °C incubator for 5 min until all of the cells detach from the plate.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium and collect the cells in a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 1 mL of warm HMP medium.
Count the number of viable cells.
Take 25,000 cells per well of a 6-well plate with the density of 2,500 cells/cm2 to a 1.5 mL tube and centrifuge at 168 × g for 3–5 min. The remaining cells can be frozen in aliquots for future use (see note 1 “S1 cell cryopreservation and thawing” for more detail).
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 1 mL of warm SKM02 medium.
Replate the resuspended cells to collagen I-coated 6-well plate (Corning, catalog number: 356400) and add another 1 mL of SKM02 medium.
Place the cells in 5% O2/5% CO2/37 °C incubator and gently shake the plate to evenly distribute the cell clusters.
Change fresh SKM02 medium every other day until the cells reach confluence. It usually takes 7–8 days.
Skeletal Muscle Differentiation
After 7–8 days in SKM02 medium, aspirate the SKM02 medium and add 2 mL of warm SKM03 Myotube Medium.
Move the plate to 5% CO2/37 °C incubator and change SKM03 medium every other day until day 7 (Figure 1A).
Reserve Cell Isolation and iMyoblast Expansion
After 7 days in SKM03 medium, dissociate S3 cells and replate directly onto gelatin-coated dishes in HMP medium to stimulate growth of iMyoblasts and isolate iMyoblast lines. HMP medium includes chick embryo extract enriched with FGF and growth factors (Seed et al., 1988) required to activate reserve cells and then maintain iMyoblast growth and expansion. Representative images of proliferating S3 iMyocytes and iMyoblasts are shown in Figure 1.
Aspirate the medium and wash with PBS.
Add 0.5–1 mL of TrypLE per well and incubate the plate in a 37 °C incubator. Approximately 5 min later, most of the cells detach from the plate, and there are still some single cells attached to the plate.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium and gently detach the cells using a cell lifter.
Collect the cells into a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 5 mL of warm HMP medium.
Replate 0.5–1 mL of cell suspension onto 0.1% gelatin-coated 10 cm dishes in 10 mL of HMP medium.
Culture plates in 5% CO2/37 °C incubator after gently shaking plates to evenly distribute the cells.
The next day iMyoblasts should attach to the plates; change fresh HMP medium daily.
After 4–6 days in HMP medium, dissociate the cells from plate for expansion.
Aspirate the medium and wash with PBS.
Add 1.5 mL of TrypLE per 10 cm dish and incubate the plate at 37 °C for approximately 5 min until all of the cells detach from the plates.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium.
Collect the cells into a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 2 mL of warm HMP medium.
Count the number of viable cells.
Plate 150,000–200,000 (0.15–0.2 M) cells onto 0.1% gelatin-coated 10 cm dishes in HMP medium.
Move the plate into 5% CO2/37 °C incubator and shake the plate gently to evenly distribute the cell clusters.
Change fresh HMP medium daily until day 4.
Passage the cells again to expand the reserve cells.
After initial S3 cell dissociation and replating, cultures include iMyoblasts and residual fiber-like cells. These fiber-like cells do not survive several passages, providing enriched cultures of iMyoblasts (Figure 1B) that can either be further expanded or frozen in aliquots for future use.
Aspirate the medium and wash with PBS.
Add 1.5 mL of TrypLE per 10 cm dish and incubate the plate at 37 °C for approximately 5 min until all the cells detach from the plates.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium.
Collect the cells into a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 2 mL of warm HMP medium.
Count the number of viable cells.
Add an equal volume of 2xHMP freeze medium and mix well.
Aliquot to cryovials according to 1 mL per cryovial.
Transfer the cryovials to freezing container and put the freezing container into a -80 °C freezer.
Transfer the cryovials to a liquid nitrogen tank for long time storage the next day.

Figure 1. Representative image of differentiated S3 iMyocytes and proliferating iMyoblasts. Representative phase images of (A) S3 iMyocytes after 7 days of differentiation (17U); (B) proliferating iMyoblasts (17U). Scale bar = 100 μm. 17U iMyocytes and 17U iMyoblasts were isolated from iPSCs of a healthy control human subject.iMyoblast Characterization
iMyoblasts were characterized using qPCR for gene expression, immunofluorescence (IF), FACS assay, and differentiation (Figure 2).
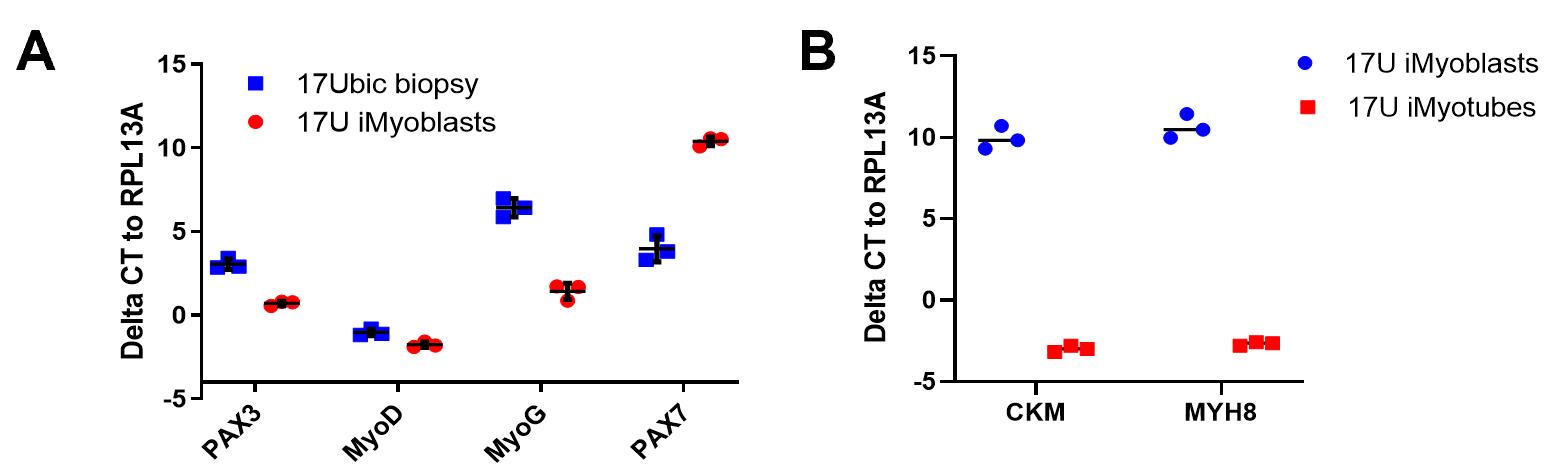
Figure 2. qPCR assays of iMyoblast and iMyotube muscle regulatory and differentiation gene expression. (A) qPCR assays of MyoD, PAX3, PAX7, and MYF5 in cultures of 17U proliferating iMyoblasts, which express MYOD and PAX3, compared to 17Ubic adult bicep biopsy myoblasts, which express MYOD, PAX3, PAX7, and MYF5. (B) qPCR assay of CKM and MYH8 muscle protein RNAs in undifferentiated 17U iMyoblast cultures and differentiated iMyotube cultures. ΔCT relative to housekeeping gene RPL13A is shown as mean ± SD. Each dot corresponds to data from individual biological replicates. 17U iMyoblasts and 17Ubic were isolated from iPSC and muscle of a healthy control human subject.qPCR assay for gene expression
RNA isolation from cell pellets
Collect cell pellets (iMyoblasts or differentiated iMyotubes) and isolate RNA using the RNA mini plus kit following the protocol.
Measure RNA concentration using Nanodrop; if needed, concentrate the RNA using Speedvac concentrator plus.
cDNA Synthesis
Prepare RNA/primer mix on ice in a 0.2 mL PCR tube. Mix thoroughly and centrifuge briefly.
Component Amount Up to 2 μg n μL 50 μM Oligo (dT)20 1 μL Random hexamers 1 μL 10 mM dNTP mix 1 μL RNase-free water To 10 μL Incubate the PCR tube at 65 °C for 5 min, then place on ice for at least 1 min.
Prepare the following cDNA synthesis mix and add each component in the indicated order. Mix thoroughly and centrifuge briefly.
Component Amount for 1 reaction 10× RT buffer 2 μL 25 mM MgCl2 4 μL 0.1 M DTT 2 μL RNaseOUT (40 U/μL) 1 μL SuperScript III RT (200 U/μL) 1 μL Add 10 μL of cDNA synthesis mix to each RNA/primer mixture. Mix thoroughly and centrifuge briefly. Proceed with the following incubation protocol.
Temperature Run Time 25 °C 10 min 50 °C 50 min 85 °C 5 min 4 °C hold Collect reactions by brief centrifugation and add 1 μL of RNase H to each tube. Centrifuge briefly.
Incubate for 20 min at 37 °C.
Dilute cDNA synthesis product to 10 ng/μL for qPCR or store at -20 °C freezer.
qPCR using iQ SYBR Green
Thaw iQ SYBR green supermix and cDNA, if stored at -20 °C freezer, on ice or cold room.
Make 20× primer stock at 6 mM stock. Final concentration of primer stock in reaction will be 300 nM: add 6 μL of forward primer (100 mM stock), 6 μL of reverse primer (100 mM stock), and 88 μL of dH2O. Mix thoroughly and centrifuge briefly. The primers used are listed in Table 3.
Table 3. Primers used for qPCR assaysGene name NCBI Gene ID Sequence (5'-3') RPL13A 23521 For: AACCTCCTCCTTTTCCAAGC Rev: GCAGTACCTGTTTAGCCACGA CKM 1158 For: ATGCCATTCGGTAACACCCAC Rev: GCTTCTTGTAGAGTTCAAGGGTC MYH8 4626 For: AATGCAAGTGCTATTCCAGAGG Rev: ACAGACAGCTTGTGTTCTTGTT MYOD1 4654 For: GCGGAACTGCTACGAAGG Rev: AGGGCTCTCGGTGGAGAT PAX3 5077 For: CACCTTCACAGCAGAACAGC Rev: CAGCTTGCTTCCTCCATCTT PAX7 5081 For: GGGAAGAAAGAGGAGGAGGA Rev: TTCAGTGGGAGGTCAGGTTC MYF5 4617 For: CCACCTCCAACTGCTCTGAT Rev: TGATCCGGTCCACTATGTTG Prepare the master mix on ice by adding the components below, except cDNA. Mix extra 10% for more reactions. Mix thoroughly and centrifuge briefly.
Component 10 μL total volume iQ SYBR Green Supermix (2×) 5 μL 6 mM for/rev primer stock (20×) 0.5 μL Water 3.5 μL Dispense 9 μL of aliquots into each well of 384-well PCR plate.
Add 1 μL of cDNA samples to PCR tubes/well.
Alternative: cDNA could also be diluted into 5 ng/μL and add 2 μL of cDNA to the master mix below:
Component 10 μL total volume iQ SYBR Green Supermix (2×) 5 μL 6 mM for/rev primer stock (20×) 0.5 μL Water 2.5 μL Seal the plate and centrifuge briefly. Proceed to the incubation below:
Step Temperature Reaction time 1 95 °C 2 min 2 95 °C 10 s 3 60 °C 30 s + Plate read 4 Go to step 2, 39 more times 5 Melt Curve, 65 °C to 95 °C, increment 0.5 °C 5 s + Plate read End Perform data analysis according to instrument-specific instructions.
IF staining for MF20, MYOD1
iMyoblasts are plated or differentiated in 24-well plate or 4-well chamber slide for IF staining.
Aspirate the medium and wash the cells with 500 μL of PBS.
Add room-temperature 2% PFA and incubate for 30 min at 37 °C.
Wash the plates three times with PBS.
Add permeabilizing solution (PBS containing 2% bovine albumin, 2% goat serum, 2% horse serum, and 0.2% Triton X-100) for 30 min at room temperature.
Incubate with primary antibody/antibodies listed in Table 1 in PBS at 4 °C overnight.
Wash three times with PBS.
Incubate with secondary antibodies listed in Table 1 for 1 h at room temperature covered with tin foil to block the light and wash three times with PBS.
Incubate with DAPI for 3–5 min covered with tin foil.
Wash three times with PBS.
Take images using a Nikon Eclipse TS 100 inverted microscope.
iMyoblast differentiation
iMyoblasts were maintained on gelatin-coated 10 cm plates in HMP growth medium and passaged at 70%–90% confluence.
Dissociate the iMyoblasts and replate onto gelatin-coated plates. We usually plate 120,000 cells per well in 6-well plates for qPCR gene expression and plate 30,000 cells per well in 24-well plates or 4-well chamber slides for IF staining assay.
Feed the cells with HMP medium every day until more than 90% confluence.
Aspirate HMP medium and wash with PBS.
Add 2 mL of N2 medium or N2 + Prednisolone medium per well for 6-well plate or 0.5 mL per well for 24-well plate or 4-well chamber slide.
Note: N2 + Prednisolone medium increases iMyoblast differentiation.
Incubate the plates in 37 °C/5% CO2 incubator for 2–7 days depending on the experimental plan. Representative images of iMyotube cultures of iMyoblasts after differentiation in N2 medium and in N2 + Prednisolone medium and then immunostained with MF20 myosin antibody and DAPI, are shown in Figure 3.
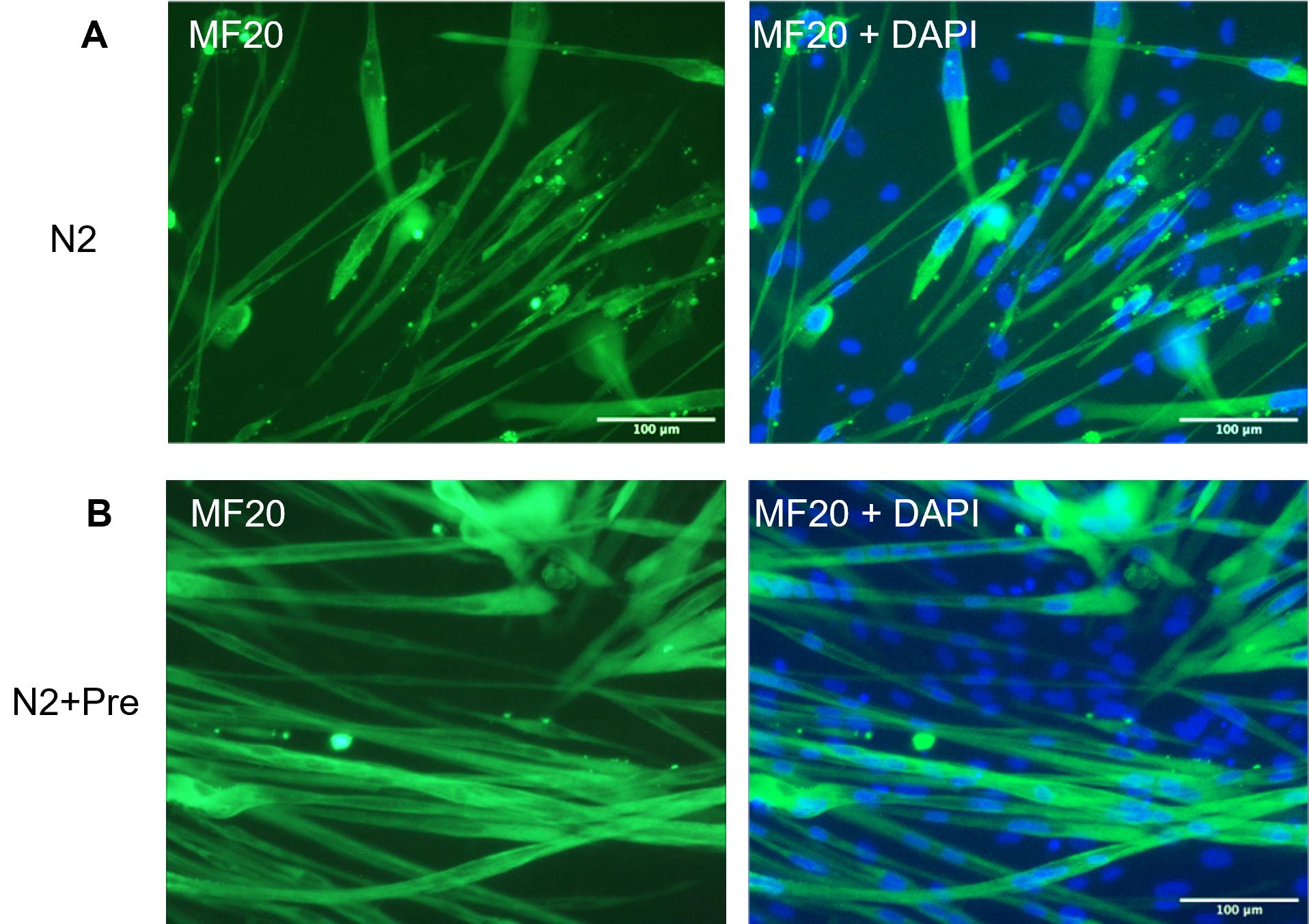
Figure 3. MF20 staining of iMyotubes. 17U iMyoblasts were differentiated in (A) N2 defined differentiation medium and (B) N2 medium supplemented with Prednisolone, as described. Cultures were maintained in these differentiation media for 7 days and then fixed and immunostained with myosin MF20 antibody. 17U iMyoblasts were isolated from iPSCs of a healthy control human subject. Scale bars = 100 μm.
FACS analysis
Culture iMyoblasts on 10 cm dishes coated with 0.1% gelatin, feeding daily until cells reach 50%–70% confluence.
Aspirate medium and wash cells with PBS.
Add 1.5 mL of TrypLE per 10 cm dish, and incubate the plate at 37 °C for approximately 5 min until all of the cells detach from the plates.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium.
Collect the cells into a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 3–5 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 1 mL of PBS.
Count the number of viable cells.
Prepare two 1.5 mL tubes, label one with sample ID and isotype control and the other with sample ID and antibody (CD56, CD82).
Transfer 100,000 cells to the tube labeled with sample ID and isotype control and other cells to the tube labeled with sample ID and antibody.
Centrifuge at 120 × g for 5 min, aspirate supernatant without disturbing the cell pellet, and resuspend cells in 100 μL of PBS.
Add 4 μL of APC-mouse IgG1 isotype control to the tube labeled with sample ID and isotype control, and add APC-CD56 antibody to the tube labeled with sample ID and antibody according to 10 μL of antibody per 106 cells. Antibodies used for FACS assays are listed in Table 2.
Incubate on ice for 30–60 min in the dark.
Centrifuge at 120 × g for 5 mins.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 100 μL of PBS including 0.2% FBS.
Filter the samples with 40 μm filter.
Run FACS assay using BD FACS Aria.
Analyze the FACS data using Flowjo software. A representative FACS assay for 17U iMyoblasts is shown in Figure 4.
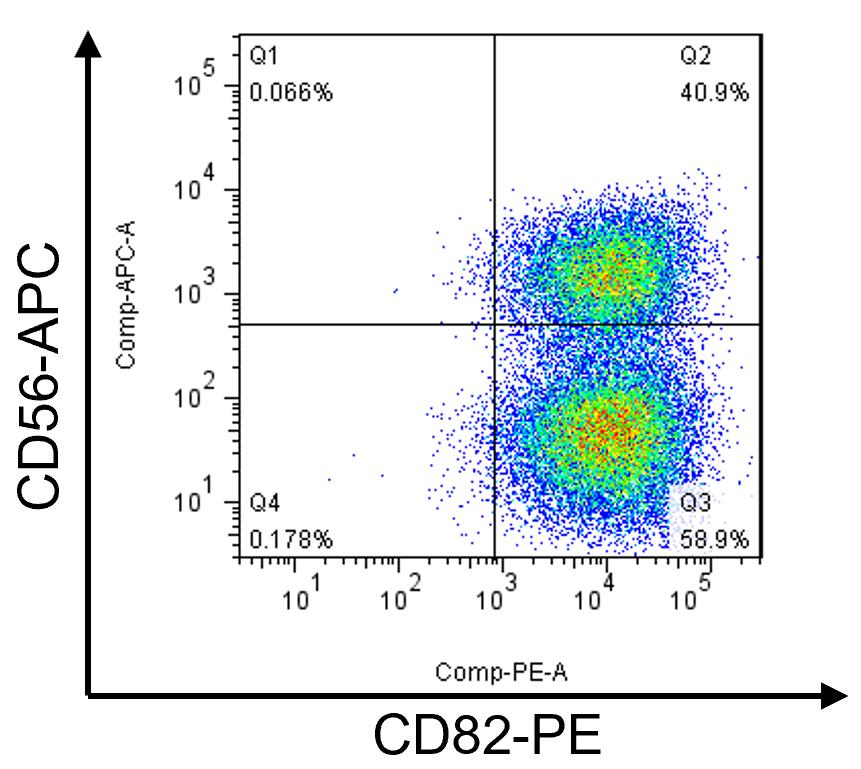
Figure 4. FACS assay of CD56 and CD82 of proliferating 17U iMyoblasts. iMyoblast cultures include CD56+/CD82+ and CD56-/CD82+ cells. CD82 is expressed in >90% of iMyoblast cultures, and the percentage of CD56+ varies from 15% to 50% for different lines. 17U iMyoblasts were isolated from iPSCs of a healthy control human subject.
Notes
S1 cell cryopreservation and thawing
After 10 days of induction in SKM01 medium, the S1 cells can be frozen and thawed again for subsequent differentiation.
Aspirate the medium and wash with PBS.
Add 1 mL of TrypLE per well and incubate the plate at 37 °C incubator for 5 min until all of the cells detach from the plate.
Neutralize the enzymatic reaction by adding 2 mL of HMP medium, collect the cells in a 15 mL PP centrifuge tube, and then centrifuge the cells at 168 × g for 4 min.
Aspirate supernatant without disturbing the cell pellet and resuspend cells in 1 mL of warm HMP medium.
Count the number of viable cells.
Adjust the S1 cell density to 300,000–400,000/mL with HMP medium.
Add an equal volume of 2× HMP Freeze medium and mix well.
Aliquot to cryovials according to 0.15–0.2 million per cryovial.
Transfer the cryovials to a freezing container and put the freezing container into a -80 °C freezer.
Transfer the cryovials to a liquid nitrogen tank for long term storage.
Gelatin coating
In a biosafety cabinet, add sufficient 0.1% gelatin to coat the first dish or well. Aspirate the solution using a sterile pipette and use it to coat the second dish. Repeat until all the dishes have been coated.
Airdry the plates or dishes in the biological safety cabinet.
Store in original sleeve at room temperature.
Recipes
0.1% gelatin
Reagent Final concentration Amount Gelatin power 0.1% 0.5g H2O n/a 500 mL Total n/a 500 mL Dissolve 1 g gelatin in 1 L tissue-culture grade water, mix well, and autoclave. Keep the 0.1% gelatin at room temperature.
HMP medium
Filter, sterilize, and store at 4 °C for up to one week.
Reagent Final concentration Amount Ham's F10 Medium N/A 390 mL FBS 20% 100 mL CaCl2 1.2 mM 2.4 mL of 250 mM stock Antibiotic antimycotic 1% 5 mL Chick embryo extract 1% 5 mL Add F10, FBS, CaCl2, and antibiotic antimycotic to Nalgene Rapid-Flow sterile single use vacuum filter units and connect to the vacuum in a tissue culture hood. Add chick embryo extract when 95% of reagents have passed through the filter to avoid blocking the filter. Store HMP at 4 °C for up to one week.
2× HMP freeze medium
Reagent Final concentration Amount of 50 mL FBS 50% 25 mL DMSO 20% 20 mL HMP 30% 15 mL N2 medium for differentiation (Chal et al., 2016)
Reagent Final concentration Amount DMEM/F12 N/A 48.5 mL N2 1% 500 μL Insulin-transferrin-selenium 1% 500 μL L-glutamine 1% 500 μL Prednisolone medium for differentiation, modified from Al Tanoury et al. (2021)
Reagent Final concentration Amount of 50 mL DMEM/F12 N/A 48.5 mL N2 1% 500 μL Insulin-transferrin-selenium 1% 500 μL L-glutamine 1% 500 μL CHIR99021 1 μM 50 μL of 1 mM stock SB431542 10 μM 50 μL of 10 mM stock Prednisolone 10 μM 50 μL of 10 mM stock
Acknowledgments
This work was funded by grants to CPE from the Muscular Dystrophy Association (480265) and NICHD Wellstone Muscular Dystrophy Cooperative Research Center P50 (HD060848). This protocol was derived from Guo et al. (2022).
Competing interests
Dongsheng Guo, Katelyn Daman, Jing Yan, and Charles P. Emerson Jr. are co-inventors on a patent application entitled "Methods And Compositions For Treatment Of Muscle Disease With iPSC-Induced Human Skeletal Muscle Stem Cells.” Dongsheng Guo and Charles P. Emerson Jr. are co-inventors of a patent "Microhomology Mediated Repair Of Microduplication Gene Mutations" (17/051,632).
Ethics
Human subjects: informed consent was obtained from patients who donated tissue for production of cell lines used in these studies. IRB protocols approved by UMass Medical School IRB: H00006581-10 and H00006581-11; IRB protocol approved by Kennedy Krieger Institute IRB: B0410080117; IRB protocol approved by the University of Iowa IRB: 200510769.
References
- Abujarour, R., Bennett, M., Valamehr, B., Lee, T. T., Robinson, M., Robbins, D., Le, T., Lai, K. and Flynn, P. (2014). Myogenic differentiation of muscular dystrophy-specific induced pluripotent stem cells for use in drug discovery. Stem Cells Transl Med 3(2): 149-160.
- Al Tanoury, Z., Rao, J., Tassy, O., Gobert, B., Gapon, S., Garnier, J. M., Wagner, E., Hick, A., Hall, A., Gussoni, E., et al. (2020). Differentiation of the human PAX7-positive myogenic precursors/satellite cell lineage in vitro. Development 147(12): dev187344.
- Al Tanoury, Z., Zimmerman, J. F., Rao, J., Sieiro, D., McNamara, H. M., Cherrier, T., Rodriguez-delaRosa, A., Hick-Colin, A., Bousson, F., Fugier-Schmucker, C., et al. (2021). Prednisolone rescues Duchenne muscular dystrophy phenotypes in human pluripotent stem cell-derived skeletal muscle in vitro. Proc Natl Acad Sci U S A 118(28): e2022960118.
- Caron, L., Kher, D., Lee, K. L., McKernan, R., Dumevska, B., Hidalgo, A., Li, J., Yang, H., Main, H., Ferri, G., et al. (2016). A Human Pluripotent Stem Cell Model of Facioscapulohumeral Muscular Dystrophy-Affected Skeletal Muscles. Stem Cells Transl Med 5(9): 1145-1161.
- Chal, J., Al Tanoury, Z., Hestin, M., Gobert, B., Aivio, S., Hick, A., Cherrier, T., Nesmith, A. P., Parker, K. K. and Pourquie, O. (2016). Generation of human muscle fibers and satellite-like cells from human pluripotent stem cells in vitro. Nat Protoc 11(10): 1833-1850.
- Darabi, R., Arpke, R. W., Irion, S., Dimos, J. T., Grskovic, M., Kyba, M. and Perlingeiro, R. C. (2012). Human ES- and iPS-derived myogenic progenitors restore DYSTROPHIN and improve contractility upon transplantation in dystrophic mice. Cell Stem Cell 10(5): 610-619.
- Dekel, I., Magal, Y., Pearson-White, S., Emerson, C. P. and Shani, M. (1992). Conditional conversion of ES cells to skeletal muscle by an exogenous MyoD1 gene. New Biol 4(3): 217-224.
- Guo, D., Daman, K., Chen, J. J., Shi, M. J., Yan, J., Matijasevic, Z., Rickard, A. M., Bennett, M. H., Kiselyov, A., Zhou, H., et al. (2022). iMyoblasts for ex vivo and in vivo investigations of human myogenesis and disease modeling. Elife 11: e70341.
- Iyer, S., Suresh, S., Guo, D., Daman, K., Chen, J. C. J., Liu, P., Zieger, M., Luk, K., Roscoe, B. P., Mueller, C., et al. (2019). Precise therapeutic gene correction by a simple nuclease-induced double-stranded break. Nature 568(7753): 561-565.
- Laumonier, T., Bermont, F., Hoffmeyer, P., Kindler, V. and Menetrey, J. (2017). Human myogenic reserve cells are quiescent stem cells that contribute to muscle regeneration after intramuscular transplantation in immunodeficient mice. Sci Rep 7: 3462.
- Seed, J., Olwin, B. B. and Hauschka, S. D. (1988). Fibroblast growth factor levels in the whole embryo and limb bud during chick development. Dev Biol 128(1): 50-57.
- Shelton, M., Metz, J., Liu, J., Carpenedo, R. L., Demers, S. P., Stanford, W. L. and Skerjanc, I. S. (2014). Derivation and expansion of PAX7-positive muscle progenitors from human and mouse embryonic stem cells. Stem Cell Reports 3(3): 516-529.
- Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K. and Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5): 861-872.
- Uezumi, A., Nakatani, M., Ikemoto-Uezumi, M., Yamamoto, N., Morita, M., Yamaguchi, A., Yamada, H., Kasai, T., Masuda, S., Narita, A., et al. (2016). Cell-Surface Protein Profiling Identifies Distinctive Markers of Progenitor Cells in Human Skeletal Muscle. Stem Cell Reports 7(2): 263-278.
- van der Wal, E., Herrero-Hernandez, P., Wan, R., Broeders, M., In 't Groen, S. L. M., van Gestel, T. J. M., van, I. W. F. J., Cheung, T. H., van der Ploeg, A. T., Schaaf, G. J., et al. (2018). Large-Scale Expansion of Human iPSC-Derived Skeletal Muscle Cells for Disease Modeling and Cell-Based Therapeutic Strategies. Stem Cell Reports 10(6): 1975-1990.
- Wu, J., Matthias, N., Lo, J., Ortiz-Vitali, J. L., Shieh, A. W., Wang, S. H. and Darabi, R. (2018). A Myogenic Double-Reporter Human Pluripotent Stem Cell Line Allows Prospective Isolation of Skeletal Muscle Progenitors. Cell Rep 25(7): 1966-1981 e1964.
- Xi, H., Fujiwara, W., Gonzalez, K., Jan, M., Liebscher, S., Van Handel, B., Schenke-Layland, K. and Pyle, A. D. (2017). In Vivo Human Somitogenesis Guides Somite Development from hPSCs. Cell Rep 18(6): 1573-1585.
- Yoshida, N., Yoshida, S., Koishi, K., Masuda, K., and Nabeshima, Y.-i. (1998). Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. JCell Sci 111: 769-779.
Article Information
Copyright
Guo et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Guo, D., Daman, K., Durso, D. F., Yan, J. and Emerson, C. P. (2022). Generation of iMyoblasts from Human Induced Pluripotent Stem Cells. Bio-protocol 12(17): e4500. DOI: 10.21769/BioProtoc.4500.
- Guo, D., Daman, K., Chen, J. J., Shi, M. J., Yan, J., Matijasevic, Z., Rickard, A. M., Bennett, M. H., Kiselyov, A., Zhou, H., et al. (2022). iMyoblasts for ex vivo and in vivo investigations of human myogenesis and disease modeling. Elife 11: e70341.
Category
Stem Cell > Pluripotent stem cell > Cell differentiation
Developmental Biology > Cell growth and fate > Regeneration
Cell Biology > Cell isolation and culture > Cell differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








