- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of a Single Cell Suspension from the Murine Iridocorneal Angle
Published: Vol 12, Iss 10, May 20, 2022 DOI: 10.21769/BioProtoc.4426 Views: 2750
Reviewed by: Pilar Villacampa AlcubierreCarolina TecuatlAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Automated Layer Analysis (ALAn): An Image Analysis Tool for the Unbiased Characterization of Mammalian Epithelial Architecture in Culture
Christian Cammarota [...] Tara M. Finegan
Apr 20, 2024 4151 Views
Optimizing Confocal Imaging Protocols for Muscle Fiber Typing in the Mouse Masseter Muscle
Catalina Matias [...] Jeffrey J. Brault
Apr 5, 2025 2797 Views
A Novel Optimized Silver Nitrate Staining Method for Visualizing and Quantifying the Osteocyte Lacuno-Canalicular System (LCS)
Jinlian Wu [...] Libo Wang
Apr 20, 2025 1433 Views
Abstract
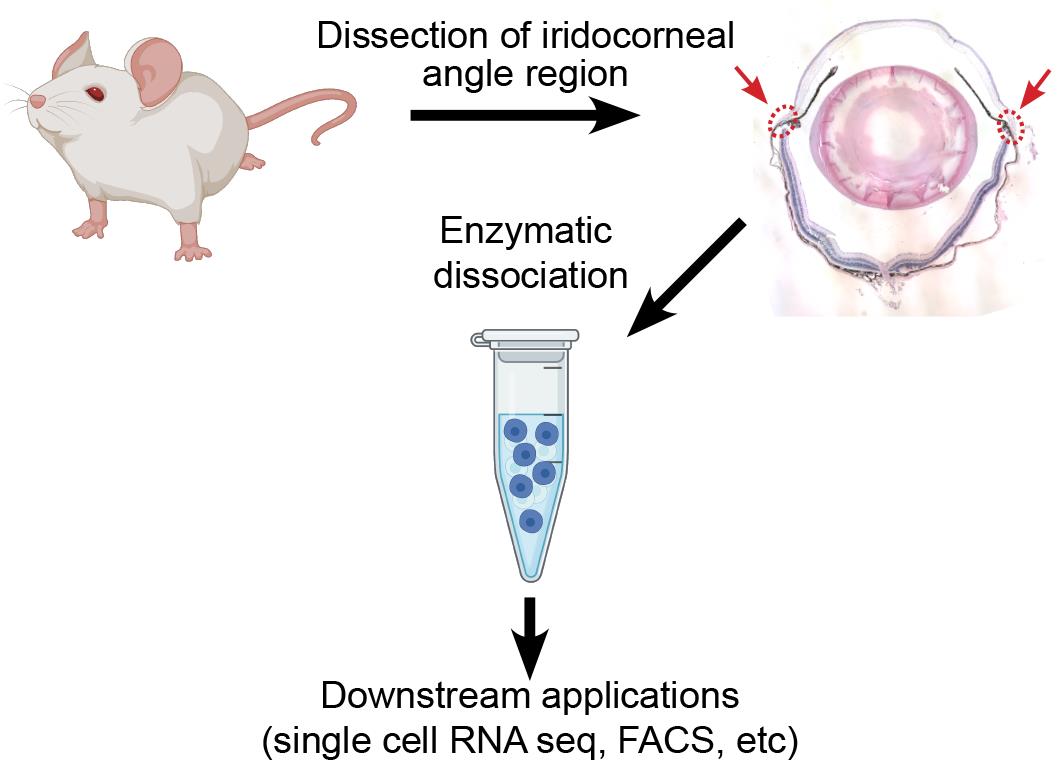
Single cell RNA sequencing is a powerful tool that can be used to identify distinct cell types and transcriptomic differences within complex tissues. It has proven to be especially useful in tissues of the eye, where investigators have identified novel cell types within the retina, anterior chamber, and iridocorneal angle and explored transcriptomic contribution to disease phenotypes in age-related macular degeneration. However, to obtain high quality results, the technique requires isolation of healthy single cells from the tissue of interest, seeking complete tissue digestion while minimizing stress and transcriptomic changes in the isolated cells prior to library preparation. Here, we present a protocol developed in our laboratory for isolation of live single cells from the murine iridocorneal angle, which includes Schlemm’s canal and the trabecular meshwork, suitable for single cell RNA sequencing, flow cytometry, or other downstream analysis.
Graphical abstract:
Background
Glaucoma is a progressive neurodegenerative disease with no cure, affecting over 60 million individuals worldwide and leaving 8 million blind (Quigley and Broman, 2006). Current therapy is focused on reduction of intraocular pressure (IOP), the only treatable risk factor for disease progression. IOP is determined by the ratio between aqueous humor secretion by the ciliary body and drainage through tissues in the iridocorneal angle, including the trabecular meshwork and Schlemm’s canal, which comprise the conventional outflow pathway. Patients with glaucoma, including primary congenital glaucoma, exhibit defects in tissues of the conventional outflow pathway leading to decreased aqueous humor outflow and elevated IOP (Tektas and Lütjen-Drecoll, 2009; Braunger et al., 2015; Zagora et al., 2015). The importance of these tissues to glaucoma pathogenesis, and consequently as therapeutic targets, has focused intense research interest in understanding their physiology, molecular phenotypes, and the cell-cell interactions governing their function. However, due to the small sizes of the tissues involved and their location within the iridocorneal angle, relatively little is known about the transcriptomes or overall phenotypes of Schlemm’s canal or drainage channel endothelial cells, potential differences between inner and outer wall endothelial cells of Schlemm’s canal or cells of the trabecular meshwork.
Recently, single cell RNA sequencing has been used to identify cell types in the iridocorneal angle region of mice, humans, and other model species (van Zyl et al., 2020; Patel et al., 2020; Thomson et al., 2021). These studies have provided exciting hints about the cells making up the angle and identified potential new therapeutic targets, but the extreme rarity of Schlemm’s canal endothelial cells within published datasets [van Zyl et al. (2020) identified just 25 Schlemm’s canal endothelial cells after sequencing 13,833 cells (0.18%), while our group has identified 109 in a dataset of 19,232 (0.57%)] has emphasized the need for sequencing of more cells, in healthy and disease conditions, to better understand the full complexity of this important tissue.
In the present protocol, we optimized a straightforward method for dissecting and dissociating the murine iridocorneal angle to obtain a single cell suspension of healthy cells to be used for downstream applications such as single cell RNA sequencing or flow cytometry. Previous studies utilizing the full anterior segment have reported that corneal and ocular surface epithelial cells represent over 50% of total cells in the dataset (van Zyl et al., 2020). While glaucoma-relevant iridocorneal angle cell populations remain rare, by removing the majority of the cornea during dissection, our method substantially increases their yield, resulting in a ~200% increase in proportion of Schlemm’s canal endothelial cells and ~80% increase in trabecular meshwork cells within the sample. Depending on the tissue(s) of interest, this protocol could be combined with flow cytometry or antibody-conjugated bead-based methods to enrich the sample in relevant cell types prior to library preparation. We have also found that this procedure can be readily adapted to dissociate cells of the cornea or posterior eye with similar results and cell viability.
Materials and Reagents
Pipet tip micro 40 μm cell strainers (Bel-Art Flomi, catalog number: 136800040)
Standard 10 cm Petri dishes (one per sample)
Standard 20 mm tissue culture dishes (one per sample)
Standard 1.5 mL microcentrifuge tubes
Standard 1 mL pipet tips (ThermoFisher ART 2079 or similar)
Wide orifice 1 mL pipet tips (ThermoFisher ART 2079GPK or similar)
DMEM, dye free (ThermoFisher, Gibco, catalog number: 21063029)
Fetal bovine serum (ThermoFisher, Gibco, catalog number: 26140079 or similar)
0.25% Trypsin EDTA (ThermoFisher, Gibco, catalog number: 25200056)
10 μm Rock inhibitor Y-27632 (ATCC, catalog number: ACS-3030)
DNase 1 (Roche, catalog number: 04716728001)
Collagenase A (Roche, catalog number: 10103578001)
HBSS (ThermoFisher, Gibco, catalog number: 14175095)
Bovine Serum Albumen (Sigma-Aldrich, catalog number: 03117332001)
Acridine orange/propidium iodide live/dead staining kit compatible with your automated cell counter (i.e., Nexcelom CS2-0106)
Compatible disposable slides if needed for your automated cell counter (i.e., Nexcelom CHT4-PD100)
Digestion mix (see Recipes)
Resuspension solution (see Recipes)
25 mg/mL Collagenase A stock solution (see Recipes)
10 mM Y27632 stock solution (see Recipes)
Note: All reagents stored per manufacturer’s recommendations and used before labeled expiry dates.
Equipment
Shaking hot block (e.g., Eppendorf Thermomixer 5384000020)
Dissecting Microscope (we prefer a microscope with 6.3×–40× variable zoom optics for this method)
Refrigerated microcentrifuge capable of 350 × g (e.g., Eppendorf 5425R)
Automated cell counter (Nexcelom Cellometer Auto2000 or similar with compatible fluorescent live/dead staining kit)
Vannas scissors, curved (Fine Science Tools, catalog number: 15019-10 or similar)
Fine Bonn Scissors, straight or straight bladed Vannas scissors (Fine Science Tools, catalog number: 14084-08 or similar).
Fine forceps (Dumont #1, #5 or similar)
Procedure
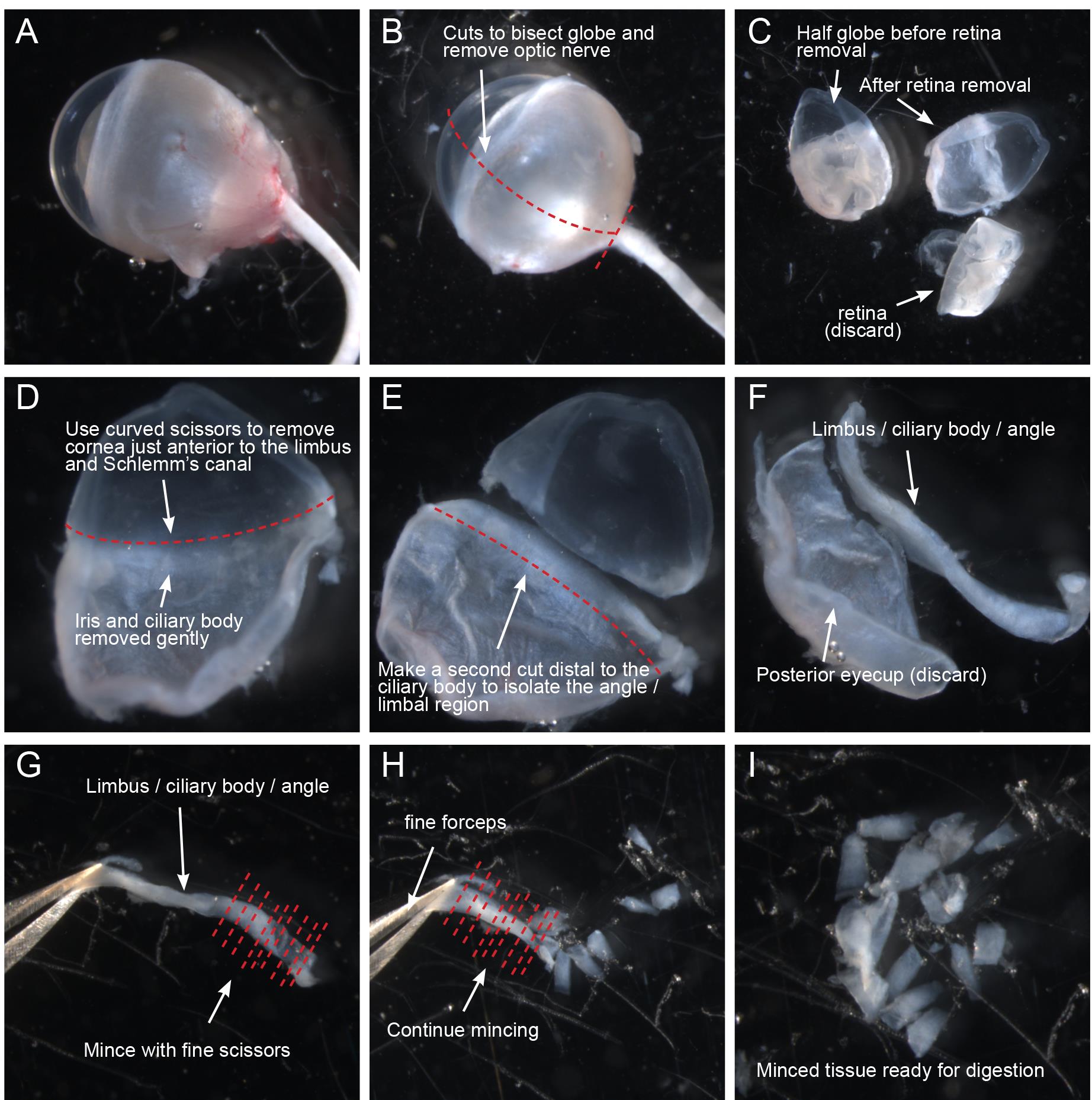
Figure 1. Dissection of corneal rim/iridocorneal angle/limbal region for dissociation. (A–D) Working in a 10 cm dish containing ice-cold HBSS, freshly harvested eyes are cleaned of connective tissue, bisected, and the lens, retina, iris, and ciliary body are removed. (E) Using curved Vannas scissors, the cornea is removed immediately anterior to the limbus. (F) A second cut is then made posterior to the ciliary body to isolate the corneal rim/iridocorneal angle/limbal region. (G–H) Isolated corneal rim/iridocorneal angle/limbal region samples are immediately transferred to ice-cold dye-free DMEM and minced using fine scissors. (I) Minced tissue is transferred to a microcentrifuge tube for subsequent dissociation.
Dissection of iridocorneal angle region
Once begun, dissection should be performed as quickly as practical to limit cell mortalities. In our experience, the full dissection procedure takes a practiced surgeon about 5 min per eye, with a few minutes added if the same person is performing both euthanasia/enucleation and microdissection.
Sacrifice mice according to the protocol approved by your local animal care and use committee. In our laboratory, mice are first anesthetized by intraperitoneal injection of ketamine/xylazine cocktail followed by cervical dislocation for euthanasia.
Immediately after euthanasia, enucleate eye globes by cutting on the lateral side of the eye socket using scissors to cut through the conjunctiva and surrounding tissue. Take care not to puncture the eye at this stage. After making this incision, enucleate the eye by pulling back the tissue surrounding the eye socket and firmly grasping the optic nerve with forceps. The eye can then be cleanly pulled away and placed in ice-cold HBSS.
To dissect the iridocorneal angle/limbal region, first use straight scissors to cleanly trim as much conjunctival and connective tissue from globe as possible (Figure 1A). Take care not to puncture the globe itself, as deflation of the eye can make this process more difficult. Next, cut the globe in half in either the sagittal or transverse direction so that the limbus and ciliary body bisect each section (Figure 1B–1C). Remove the lens and discard. Remove the retina using forceps and then, using curved Vannas scissors, carefully trim away the iris and ciliary body (Figure 1D). Take care not to damage the angle region, and err on the side of leaving some ciliary body or iris tissue. Next, while grasping the sclera near the hole left by the optic nerve, use the curved scissors to remove the cornea, cutting as close to the limbus as possible without damaging the angle region (Figure 1D–1E). Remove the cornea, and then make a second smooth cut immediately below the root of the ciliary body to free the limbal/iridocorneal angle region (Figure 1E–1F). Working in albino mice, this piece of tissue will look similar to a soft fingernail clipping. Immediately transfer this tissue to a 20 mm cell culture dish containing ice-cold DMEM with 10% FBS and continue isolating the limbal regions from the other half of the eye and the opposite globe. After dissection, you will have 4 slivers of limbal/iridocorneal angle tissue from each mouse.
The tissues are then minced before digestion. Working submerged in the small dish of ice-cold DMEM + 10% FBS to prevent small tissue fragments from sticking to pipet tips, grasp each sliver of tissue by one end and, working from the other end with the fine scissors, reduce the tissue to fine slices by making a series of cuts perpendicular to the length of the tissue (Figure 1G–1I). Repeat until all tissues have been minced into the ice-cold media and proceed immediately with tissue digestion.
Tissue dissociation
Using a 1 mL pipet equipped with a wide bore tip, collect the minced tissues and transfer them into 2 mL microcentrifuge tubes for tissue dissociation (Figure 2). If necessary, allow tissues to settle (1–3 min), or centrifuge 350 × g for 30 s and remove supernatant to make space for additional volume until all tissue is collected.

Figure 2. Flow chart showing an overview of the dissociation procedure.Allow samples to settle on ice or centrifuge 350 × g for 30 s before carefully aspirating media without disturbing tissues.
Add 1 mL of pre-warmed digestion mix (see Recipes) containing 10% FBS, 1 mg/mL Collagenase A and 1 μM of Y27362. Rotate gently to mix and then place on a pre-warmed 37°C hot block shaking at 450 rpm.
Incubate tissues for 2 h. At 30 min intervals, gently mix by inverting the tube 3–4 times to ensure tissue remains in suspension.
After 2 h incubation, collect tissue fragments and dissociated cells by centrifugation at 350 × g for 5 min at 20°C. Aspirate supernatant and resuspend gently in 1 mL of DPBS to wash.
Repeat centrifugation step, and then resuspend tissues in 1mL of pre-warmed 0.25% Trypsin EDTA containing 100 units of RNAse free DNase 1.
Return samples to shaking 37°C block for 10 min.
After 10 min, triturate samples using a 1 mL pipet equipped with a wide bore tip. Gently resuspend tissues by slowly aspirating 1 mL and returning it to the tube. Make 10 strokes, taking care not to introduce bubbles or damage cells by aggressive mixing.
Return to 37°C block for an additional 10 min, then use a normal bore P1000 tip to make 10 additional mixing strokes. Incubate for 5 additional mins and then mix using 10 additional strokes with the standard bore tip. On the final stroke, transfer the cell suspension from the 2 mL to a 1.5 mL microcentrifuge tube.
Collect dissociated cells by centrifugation at 350 × g for 5 min at 20°C. Gently aspirate supernatant and resuspend cell pellet in 1 mL of DMEM + 10% FBS to neutralize the Trypsin EDTA.
Gently aspirate the full sample using a 1 mL pipet equipped with a standard bore P1000 tip, place a Flowmi micro 40 μm filter over the aperture of the pipet tip and slowly filter the cells into a new 1.5 mL microcentrifuge tube to remove debris. Take care not to damage the cells by subjecting them to high pressure during filtration.
Repeat centrifugation step and resuspend in 1 mL of HBSS containing 1% BSA.
Repeat centrifugation step and aspirate supernatant. Gently resuspend cells in 200 μL (see Notes) of additional HBSS containing 1% BSA.
Filter cells through an additional 40 μm Flowmi filter, transferring to a new 1.5 mL microcentrifuge tube.
Place cells on ice and use immediately for downstream applications.
Data analysis
Prior to library preparation for single cell RNA sequencing or other applications, assess cell concentration and viability using an automated cell counter equipped with live/dead cell detection (see note). Typically, we obtain 75,000–100,000 cells from dissociation of six eyes and 150–200,000 cells from dissociation of eight eyes with viability of between 80–95%. Smaller samples can be prepared using a similar protocol, but will result in disproportionally fewer cells as per-sample losses due to filtration and sample handling appear similar regardless of final cell number. Final sample concentration should be optimized based on downstream application and abundance of target cell population.
In one typical experiment, following sequencing of 19,232 cells from 12 wild-type mouse eyes in two independent preps, cells were clustered using Seurat, and distributions of cell types were estimated (Figure 3). While the majority of cells obtained mapped to uveal, scleral, corneal tissues, and melanocytes, Schlemm’s canal endothelial cells made up <1% of total cells present in the sample (109/19232), and trabecular meshwork cells made up almost 4% (701/19232). The extreme rarity of these cell types within the tissue is a critical consideration during experimental design and optimization.
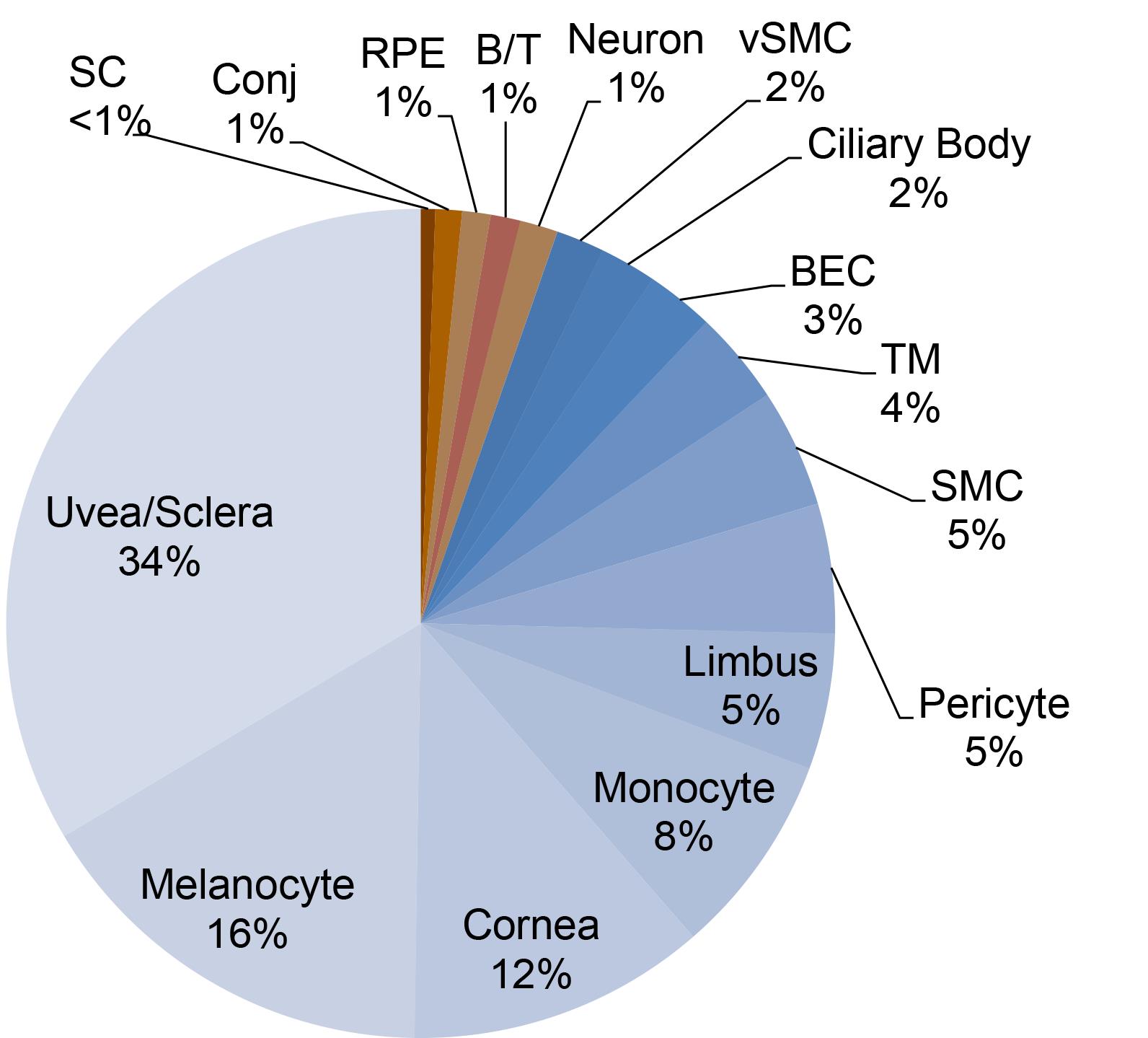
Figure 3. Distribution of cell types obtained after single cell RNA sequencing on the 10x genomics platform. Following sequencing of 19,232 cells from 12 wild-type mouse eyes in two independent preps, cells were clustered using Seurat, and distributions of cell types were estimated. SC: Schlemm’s canal endothelial cells, Conj: Conjunctival epithelial cells, RPE: Retina pigment epithelia, B/T: B and T cells, vSMC: Vascular smooth muscle cells, BEC: Blood vascular endothelial cells, TM: Trabecular meshwork, SMC: Smooth muscle cells.
Notes
Mouse strain selection: We have found that dissociation of pigmented eyes leads to aggregation of pigment material that is difficult to remove through washing and filtration and makes subsequent viability testing or single cell RNA sequencing library preparation difficult. Using eyes from albino mice eliminates this issue, but limits model selection and necessitates extensive backcrossing for use with genetic models present on a pigmented background. When using pigmented eyes, additional flow cytometry or filtration steps may be required to eliminate pigment aggregate from the sample prior to use in downstream applications.
Dissection: We typically perform this stage of the protocol using two people, so that one person can be responsible for humane euthanasia of mice and enucleation of the globes while their colleague works under the dissecting microscope on dissection of the iridocorneal angle region. It is possible for a single investigator to handle both tasks, but in that case, we typically collect all eyes in ice-cold HBSS before beginning the fine dissections.
Tissue digestion: Each sample requires 1 mL of pre-warmed DMEM + 10% FBS, and before beginning dissections, we prepare 1 mL aliquots in 1.5 mL microcentrifuge tubes. These can be placed on the 37°C block to pre-warm prior to beginning the protocol.
Final suspension volume: For a prep using six adult eyes, we typically perform final resuspension in a volume of 200 μL. Following filtration, this results in a final sample volume of ~150 μL and a cell concentration of ~650 cells per μL. For preparations using fewer eyes, a smaller volume should be used as needed to optimize sample concentration for downstream applications.
Cell counting and live-dead staining: In our laboratory, cell counting and live-dead staining is performed using a Nexcelom Cellometer Auto2000 automatic cell counter and compatible Nexcelom-branded Acridine orange/propidium iodide live/dead staining reagents.
Recipes
Digestion mix
Make 1 mL per sample immediately before beginning the digestion step of the protocol.
Typically, we pre-warm 1 mL aliquots of DMEM to 37°C and add other reagents immediately (i.e., <5 min) before use.
Reagent Final concentration Amount DMEM containing 10% FBS - 1 mL Collagenase A, 25 mg/mL stock 1 mg/mL 40 μL Y27362, 10 mM stock 10 μM 1 μL n/a Resuspension solution
Filter sterilize before use and store at 4°C
Reagent Final concentration Amount HBSS 10 mL BSA 1% W/V 0.1 g Total 10 mL 25 mg/mL Collagenase A stock solution
Store at -20°C in 50 μL of aliquots
Reagent Final concentration Amount Collagenase A (Lyophilized) 25 mg/mL 100 mg ddH2O n/a 4 mL Total n/a 4 mL 10 mM Y27632 stock solution
Store at -20°C in 50 μL of aliquots. Once thawed, aliquots can be kept at 4°C for two weeks.
Reagent Final concentration Amount Y27632 10 mM 10 mg ddH2O n/a 3 mL Total n/a 3 mL
Acknowledgments
We are grateful to Drs. Robert Lavker and Nihal Kaplan (Northwestern University Feinberg School of Medicine) for helpful discussions and for sharing their protocol for cell dissociation (described in Kaplan et al., 2019), upon which this protocol was based. Automated cell counting and quality control was performed by Dr. Jennifer Chin Man Wai of the Center for Genetic Medicine of the Feinberg School of Medicine. Work described here was funded by R01 EY025799 (to SEQ.), R01 EY032609 (to BRT) and a Brightfocus foundation new investigator grant in macular degeneration research to BRT.
Competing interests
Benjamin Thomson receives research funding from Bayer. Susan Quaggin owns stock in Mannin Research Inc. and receives consulting fees from AstraZeneca, Janssen, the Lowy Medical Research Foundation, Novartis, Pfizer, Janssen, and Roche/Genentech.
Ethics
Animal experiments were approved by the Animal Care and Use Committee at Northwestern University (Evanston IL, USA, animal protocol number IS000150015, validity period 2020/4/30-2023/4/29) and comply with ARVO guidelines for care and use of vertebrate research subjects in Ophthalmology research.
References
- Braunger, B. M., Fuchshofer, R. and Tamm, E. R. (2015). The aqueous humor outflow pathways in glaucoma: A unifying concept of disease mechanisms and causative treatment. Eur J Pharm Biopharm 95(Pt B): 173-181.
- Kaplan, N., Wang, J., Wray, B., Patel, P., Yang, W., Peng, H. and Lavker, R. M. (2019). Single-Cell RNA Transcriptome Helps Define the Limbal/Corneal Epithelial Stem/Early Transit Amplifying Cells and How Autophagy Affects This Population. Invest Ophthalmol Vis Sci 60(10): 3570-3583.
- Patel, G., Fury, W., Yang, H., Gomez-Caraballo, M., Bai, Y., Yang, T., Adler, C., Wei, Y., Ni, M., Schmitt, H., et al. (2020). Molecular taxonomy of human ocular outflow tissues defined by single-cell transcriptomics. Proc Natl Acad Sci U S A 117(23): 12856-12867.
- Quigley, H. A. and Broman, A. T. (2006). The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90(3): 262-267.
- Tektas, O. Y. and Lutjen-Drecoll, E. (2009). Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res 88(4): 769-775.
- Thomson, B., Liu, P., Onay, T., Du, J., Tompson, S., Misener, S., Purohit, R., Young, T., Jin, J. and Quaggin, S. (2021). Cellular crosstalk regulates the aqueous humor outflow pathway and provides new targets for glaucoma therapies. Nat Commun 12(1): 6072.
- van Zyl, T., Yan, W., McAdams, A., Peng, Y. R., Shekhar, K., Regev, A., Juric, D. and Sanes, J. R. (2020). Cell atlas of aqueous humor outflow pathways in eyes of humans and four model species provides insight into glaucoma pathogenesis. Proc Natl Acad Sci U S A 117(19): 10339-10349.
- Zagora, S. L., Funnell, C. L., Martin, F. J., Smith, J. E., Hing, S., Billson, F. A., Veillard, A. S., Jamieson, R. V. and Grigg, J. R. (2015). Primary congenital glaucoma outcomes: lessons from 23 years of follow-up. Am J Ophthalmol 159(4): 788-796.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Thomson, B. R. and Quaggin, S. E. (2022). Preparation of a Single Cell Suspension from the Murine Iridocorneal Angle. Bio-protocol 12(10): e4426. DOI: 10.21769/BioProtoc.4426.
Category
Developmental Biology > Cell signaling
Cell Biology > Tissue analysis > Tissue imaging
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








