- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluating Human Natural Killer Cells Antibody-dependent Cellular Cytotoxicity (ADCC) Using Plate-bound Anti-CD16 Antibodies
Published: Vol 12, Iss 1, Jan 5, 2022 DOI: 10.21769/BioProtoc.4285 Views: 4587
Reviewed by: Gal HaimovichPorkodi PanneerselvamChris Tibbitt

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detection of Autophagy in Human Peripheral Blood Mononuclear Cells Using Guava® Autophagy and Flow Cytometry
Melanie Scherer [...] Jörg Bergemann
Sep 20, 2025 1418 Views

Protocol for the Isolation and Analysis of Extracellular Vesicles From Peripheral Blood: Red Cell, Endothelial, and Platelet-Derived Extracellular Vesicles
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
Nov 5, 2025 1448 Views

In Vitro Model of Cytokine-Induced Inflammatory 3T3-L1 Adipocytes Mimicking Obesity
Lucille Cartier [...] Stéphane Potteaux
Feb 20, 2026 53 Views
Abstract
Natural killer (NK) cells are large granular lymphocytes that keep in check the health of neighboring cells through a large array of intrinsically expressed germline-coded receptors. Most importantly, CD16 is a low affinity Fc receptor for IgG that mediates the antibody-dependent cellular cytotoxicity (ADCC) of NK cells, bridging the innate and adaptive immunities. There has been a significant interest in genetically engineering NK cells to enhance its ADCC, with the ultimate goal to produce off-the-shelf NK cell therapy products that can be combined with target-specific monoclonal antibodies to improve clinical outcomes. Previous protocols of ADCC assays use complex cell-based antigen-antibody models, which are both costly and time-consuming. This current protocol is devoid of target cells and uses plate-bound immobilized anti-CD16 antibodies as the trigger. It greatly shortens the experimental time, while faithfully evaluating NK cells ADCC.
Graphic abstract:
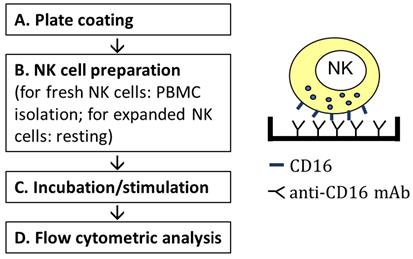
Workflow of stimulating NK cells via CD16 by plate-bound anti-CD16 mAb.
Background
Immunotherapies for cancer treatment are rapidly emerging, and many studies aim at increasing the safety and efficacy of anti-tumor immunotherapies. More research is being conducted on natural killer (NK) cells, as they have minimal risk of causing neurotoxicity in autologous settings (Gong et al., 2021), graft-versus-host disease in allogenic settings (Du et al., 2021), or cytokine release syndrome in general (Du et al., 2021). Other than being relatively safe in treatment, NK cells are effective in direct killing of target cells and regulating the immune system (Vivier et al., 2011).
Antibody-dependent cellular cytotoxicity (ADCC) is an adaptive immune response largely mediated by NK cells through their CD16 (FCγRIII) receptor, which binds the Fc portion of IgG antibodies (Alderson and Sondel, 2011). However, this important aspect of NK cells remains underexplored. This can be ascribed to difficulties in establishing assay platforms. Previous protocols of ADCC assays use complex cell-based antigen-antibody models, such as the combination of SCC-4 cells and cetuximab (Kohrt et al., 2014), which is costly due to the low productivity of clinically relevant monoclonal antibodies, such as cetuximab. These target cell-based experiments also take a minimum of three days due to the need of culturing and maintaining target cells. The use of anti-CD16 antibody-opsonized P815 cells in reverse ADCC assays also evaluates NK cells ADCC (Euchner et al., 2021), but likewise requires time to culture and maintain the target cells.This current protocol is devoid of target cells, and uses plate-bound immobilized anti-CD16 antibodies as the trigger. Major steps include coating the plate with CD16 antibodies, preparing, and seeding the cells. It greatly shortens the experimental time to about 10 h, while faithfully evaluating NK cells ADCC. It can be used to quickly evaluate the ADCC aspect of any NK cell-based therapy product in vitro before subsequent in vivo testing, to facilitate the research and development (R&D) processes.
Materials and Reagents
1.5 mL Eppendorf (EP) tube (Eppendorf, catalog number: 022363204)
50 mL reagent reservoir (Corning, catalog number: CLS4870)
200 μL multi-channel pipette (Gilson, catalog number: FA10012)
Wide Mouthed Polypropylene Standard Spout Wash Bottle (SP SCIENCEWARE, catalog number: 46C798)
Paper towel (Skilcraft, catalog number: 5LA76)
Costar 96 well flat-bottom EIA plate (Bio-Rad, catalog number: 2240096)
12 × 75 mm FACS tube (BD Biosciences, catalog number: 342065)
DPBS, without calcium chloride and magnesium chloride (Sigma-Aldrich, catalog number: D8537)
RPMI medium 1640 (Sigma-Aldrich, catalog number: R8758-24X500ML)
Fetal bovine serum (Fisher Scientific, catalog number: 16140089)
Penicillin-Streptomycin (Gibco, catalog number: 15-140-122)
L-glutamine (Thermo Scientific, catalog number: 25030081)
Antibodies:
Ultra-LEAFTM Purified anti-human CD16 (Bioledgend, catalog number: 302050)
Anti-CD3-AF700 (BD Biosciences, catalog number: 557943)
Anti-CD14-APC/Cyanine7 (BioLegend, catalog number: 301820)
Anti-CD19-APC/Cyanine7 (BioLegend, catalog number: 302218)
Anti-CD56-PE/Cyanine7 (Beckman Coulter, catalog number: A51078)
Anti-CD107a-BV786 (BD Biosciences, catalog number: 563869)
Anti-IFN-gamma-APC (BioLegend, catalog number: 502512)
Anti-TNF-alpha-Pacific Blue (BioLegend, catalog number: 502920)
LIVE/DEADTM Fixable Far Red Dead Cell Stain Kit, for 633 or 635 nm excitation (Invitrogen, catalog number: L34973)
Brefeldin A Solution (1,000×) (BioLegend, catalog number: 420601)
BSA (Sigma-Aldrich, catalog number: 12133C)
Sodium Azide (Sigma-Aldrich, catalog number: 71289)
Formaldehyde (Sigma-Aldrich, catalog number: F8775)
Saponin (Sigma-Aldrich, catalog number: 47036-250G-F)
R10 medium (see Recipes)
FACS Buffer (see Recipes)
Fixation buffer (see Recipes)
Perm buffer (see Recipes)
Equipment
37°C 5% CO2 humidified incubator (Being Instrument)
Countess 3 Automated Cell Counter (Thermo Fisher Scientific)
LSR Fortessa flow cytometer (BD Biosciences)
Software
FlowJo Software (version 10.6.1)
Procedure
Plate coating
Dilute anti-CD16 antibodies with DPBS, to achieve a final concentration of 2 µg/mL. Use 100 μL of DPBS for each sample. (The antibody is bottled at the concentration indicated on the vial, typically between 2 mg/mL and 3 mg/mL. Older lots may have also been bottled at 1 mg/mL. Please contact Biolegend technical support for concentration and total µg amount, or use Biolegend Lookup tool (https://www.biolegend.com/en-us/concentration-expiration-lookup) with the lot number.)
Invert/briefly vortex the diluted anti-CD16 antibodies to mix well and transfer them into a reagent reservoir.
Use a 200 μL multi-chanel pipette to distribute 100 μL of diluted anti-CD16 antibodies into each well of a 96-well EIA plate (the number of wells to be filled depends on the number of NK cell samples to be stimulated). Fill an equal number of wells with DPBS as the no stimulation controls.
Cover the plate with a lid and incubate the plate in a 37°C 5% CO2 humidified incubator for 90 min.
Wash off unbound antibodies. Wash the plate twice by first filling the wells with DPBS using a spout wash bottle and then dumping the liquid from the plate. To dump the liquid, grab the plate from the bottom with the thumb in the middle of one side and the fingers on the other side. Holding the plate over the sink, turn the plate upside down and rapidly accelerate arm and hand downward. Immediately place the plate onto paper towels to blot the plate.
If NK cells are ready to be seeded (done with Procedure B), continue to Procedure C immediately. Make sure that wells coated with antibodies are not dried for more than 10 s at any given step. If NK cells are not ready yet, fill wells with 100 μL DPBS, cover the plate and store it in the 37°C 5% CO2 humidified incubator until cells are ready.
NK cell preparation
NK cells for stimulation can be either amidst freshly prepared PBMCs (fresh NK cells) or isolated and expanded in advance (expanded NK cells).
For fresh NK cells:
Obtain whole blood upon Institutional Review Board (IRB) approval.
Based on the total number of samples, prepare a master mix containing R10 medium, anti-CD107a-BV786 antibodies and BFA (v:v:v = 100:2:0.1). The total volume needed for each sample is 200 μL + 4 μL + 0.2 μL = 204.2 μL.
Follow any PBMC isolation protocol to isolate PBMC samples, e.g., EasySepTM Direct PBMC isolation by STEMCELL Technologies (https://www.stemcell.com/isolating-mononuclear-cells-from-whole-blood-by-density-gradient-centrifugation.html).
Count the number of cells in each PBMC sample using the Countess 3 Automated Cell Counter.
From each PBMC sample, transfer 0.4 × 106 cells into an EP tube.
Pellet PBMCs using a benchtop microcentrifuge by centrifuging at 150 × g for 5min.
Resuspend the pelleted 0.4 × 106 cells in each EP tube with 204.2 μL of master mix. (BFA is cytotoxic, so conduct step 7 only close to the end of the 90 min incubation period in Procedure A step 4. Do not collect more than 0.4 × 106 cells/EP tube. The cells will be seeded into a well coated with CD16 antibodies. Too many cells will prohibit cell-antibody interaction.)
For expanded NK cells (an example of FcRγ KO cells are shown, Figure 1):
Count the number of cells in each sample using the Countess 3 Automated Cell Counter.
Based on the total number of samples, prepare a master mix containing R10 medium, anti-CD107a-BV786 antibodies and BFA (v:v:v = 100:2:0.1). The total volume needed for each sample is 200 μL + 4 μL + 0.2 μL = 204.2 μL.
From each sample, transfer 0.02 × 106 cells into an EP tube.
Pellet cells using a benchtop microcentrifuge at 800 × g for 5min.
Resuspend cells using an appropriate amount of R10 medium (This step washes off stimulatory components in NK cell culture and will rest the cells).
Pellet cells using a benchtop microcentrifuge at 150 × g for 5min.
Resuspend the pelleted 0.02 × 106 cells in each EP tube with 204.2 μL master mix. (BFA is cytotoxic, so conduct step 7 only close to the end of the 90 min incubation period in Procedure A step 4).
NK cell stimulation
For each sample, seed 100 μL of cells into one well coated with anti-CD16 antibodies and another 100 μL of cells into one well coated with no anti-CD16 antibody.
Cover the plate and incubate the cells in a 37°C 5% CO2 humidified incubator for 6-9 h.
Flow cytometric analysis
Before collecting the cells, prepare the surface staining cocktail (needs to be protected from light). Prepare a master mix using the following guideline: For each sample to be stained, use 50 μL of FACS buffer containing 0.2 μL of LIVE/DEADTM Fixable Far Red Dead Cell Stain, 1 μL of Anti-CD3-AF700, 1 μL of Anti-CD14-APC/Cyanine7, 1 μL of Anti-CD19-APC/Cyanine7, and 1 μL of Anti-CD56-PE/Cyanine7 antibodies. (If staining with a different volume, use manufacturers’ suggested concentrations.)
Place the plate on ice to slow down cellular activities.
Add 100 μL of ice-cold FACS buffer into each well that has cells seeded.
Transfer cells into FACS tubes. Pipet up and down for multiple times to make sure that all cells are off the plate.
Pellet the cells by centrifuging at 350 × g for 5 min.
Briefly vortex to dislodge the cells.
Add 54.2 μL surface antibody cocktail into each cell suspension, and stain on ice for 30 min.
Note: Protect cells from light from this step onwards.
Wash cells twice with 3 mL of ice-cold FACS buffer at 350 × g for 5 min and discard supernatant.
Briefly vortex to dislodge the cells.
Fix cells with fixation buffer (working concentration: 1% v/v formaldehyde) on ice for 30 min.
Permeabilize cells by adding 3× volume of ice-cold Perm buffer to fixed cells.
Keep cells on ice for 30 min.
Prepare intracellular staining cocktail. Prepare a master mix using the following guideline: For each sample to be stained, use 50 μL of Perm buffer containing 2.5 μL of Anti-IFN-gamma-APC and 2.5 μL of Anti-TNF-alpha-Pacific Blue antibodies. (If staining with a different volume, use manufacturers’ suggested concentrations.)
Wash cells twice with 3 mL of ice-cold Perm buffer at 600 × g for 5 min and discard supernatant.
Briefly vortex to dislodge the cells.
Add 55 μL of intracellular antibody cocktail into each cell suspension and stain at RT for 30 min.
Wash cells twice with 3 mL of ice-cold Perm buffer at 600 × g for 5 min and discard supernatant.
Wash cells once with 3 mL of ice-cold FACS buffer at 600 × g for 5 min and discard supernatant.
Acquire cells by LSR Fortessa flow cytometer.
Analyze the data using FlowJo Software.
Data analysis
An example of stimulating expanded NK cells is shown here (Figure 1). NK cells from different blood donors are first expanded and then electroporated with FCER1G-targeting CRISPR-Cas9 ribonucleoprotein (FCER1G encodes for FcRγ). The resultant cells are a mixture of FcRγ+ and FcRγ– cells. During stimulation with plate-bound anti-CD16 antibodies, the cells are stained for surface CD107a (a degranulating marker). After stimulation, the cells are first stained for surface markers and then stained for intracellular cytokines (IFN-γ and TNF-α). In analysing data using FlowJo Software, cells are gated by FSC-A/SSC-A to exclude debris and by FSC-H/FSC-A to select for single cells. Dead cells, CD3+ T cells, CD19+ B cells, CD14+ monocytes are excluded, while CD56+ NK cells are selected for intracellular cytokines and degranulation marker analysis.
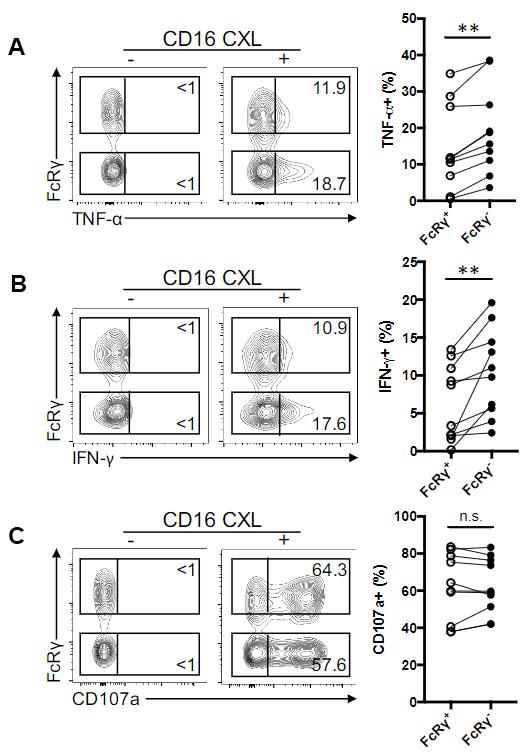
Figure 1. Deletion of FCER1G (encoding FcRγ) in expanded NK cells leads to enhanced ADCC (exemplified by cytotoxic cytokine release). (A-C) Contourplots show flow cytometric analysis of TNF-α (A), IFN-γ production (B) and cell surface CD107a expression (C) in FcRγ+ and FcRγ– NK subsets from a representative donor without (-) or with (+) CD16 crosslinking (CXL). Numbers represent the relative percentages of FcRγ+ or FcRγ– subset that produced the indicated cytokine or displayed CD107a. Line graphs show the relative percentages of FcRγ+ or FcRγ– subset that produced the indicated cytokine or displayed CD107a from several donors in response to CD16 CXL (n = 10). Circles connected by a line designate data obtained from the same donor sample.
Recipes
R10 medium
RPMI medium 1640 supplemented with 10% fetal bovine serum.
100 U/mL Penicillin-Streptomycin and 2 mM L-glutamine.
FACS Buffer
PBS supplemented with 1% BSA and 0.1% Sodium Azide.
Fixation buffer
PBS supplemented with 4% formaldehyde.
Perm buffer
PBS supplemented with 1% BSA and 0.1% saponin.
Acknowledgments
This protocol is adapted from Liu et al. (2020). The work of Liu et al. (2020) was supported by the NIH grant ( AI110894).
Competing interests
The authors declare no competing interests.
Ethics
Studies exemplified in this protocol using human samples were approved by the Institutional Review Board of the University of California, Davis.
References
- Alderson, K. L. and Sondel, P. M. (2011). Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J Biomed Biotechnol 2011: 379123.
- Du, N., Guo, F., Wang, Y. and Cui, J. (2021). NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers (Basel) 13(16).
- Euchner, J., Sprissler, J., Cathomen, T., Furst, D., Schrezenmeier, H., Debatin, K. M., Schwarz, K. and Felgentreff, K. (2021). Natural Killer Cells Generated From Human Induced Pluripotent Stem Cells Mature to CD56bright CD16+ NKp80+/- In-Vitro and Express KIR2DL2/DL3 and KIR3DL1. Front Immunol 12: 640672.
- Gong, Y., Klein Wolterink, R. G. J., Wang, J., Bos, G. M. J. and Germeraad, W. T. V. (2021). Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol 14(1): 73.
- Kohrt, H. E., Colevas, A. D., Houot, R., Weiskopf, K., Goldstein, M. J., Lund, P., Mueller, A., Sagiv-Barfi, I., Marabelle, A., Lira, R., et al. (2014). Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest 124(6): 2668-2682.
- Liu, W., Scott, J. M., Langguth, E., Chang, H., Park, P. H. and Kim, S. (2020). FcRgamma Gene Editing Reprograms Conventional NK Cells to Display Key Features of Adaptive Human NK Cells. iScience 23(11): 101709.
- Vivier, E., Raulet, D. H., Moretta, A., Caligiuri, M. A., Zitvogel, L., Lanier, L. L., Yokoyama, W. M. and Ugolini, S. (2011). Innate or adaptive immunity? The example of natural killer cells. Science 331(6013): 44-49.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Liu, W. (2022). Evaluating Human Natural Killer Cells Antibody-dependent Cellular Cytotoxicity (ADCC) Using Plate-bound Anti-CD16 Antibodies. Bio-protocol 12(1): e4285. DOI: 10.21769/BioProtoc.4285.
Category
Immunology > Immune cell function > Lymphocyte
Cell Biology > Cell-based analysis > Flow cytometry
Cell Biology > Cell-based analysis > Inflammatory response
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









