- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Age, Wound Size and Position of Injury – Dependent Vascular Regeneration Assay in Growing Leaves
(*contributed equally to this work) Published: Vol 11, Iss 9, May 5, 2021 DOI: 10.21769/BioProtoc.4010 Views: 5344
Reviewed by: Charles MelnykRekha R. WarrierAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Isolation and Quantification of Mandelonitrile from Arabidopsis thaliana Using Gas Chromatography/Mass Spectrometry
Ana Arnaiz [...] Isabel Diaz
Jun 20, 2023 1574 Views

Mobilization of Plasmids from Bacteria into Diatoms by Conjugation Technique
Federico Berdun [...] Eduardo Zabaleta
Mar 5, 2024 1921 Views

A Step-by-step Protocol for Crossing and Marker-Assisted Breeding of Asian and African Rice Varieties
Yugander Arra [...] Wolf B. Frommer
Sep 20, 2024 2349 Views
Abstract
Recurring damage to the aerial organs of plants necessitates their prompt repair, particularly their vasculature. While vascular regeneration assays for aerial plant parts such as the stem and inflorescence stalk are well established, those for leaf vasculature remain unexplored. Recently, we established a new vascular regeneration assay in growing leaves and discovered the underlying molecular mechanism. Here, we describe the detailed stepwise method for the incision and regeneration assay used to study leaf vascular regeneration. By using a combination of micro-surgical perturbations, brightfield microscopy, and other experimental approaches, we further show that the age of the leaf as well as the position and size of the injury determine the overall success rate of regeneration. This easy-to-master vascular regeneration assay is an efficient and rapid method to study the mechanism of vascular regeneration in growing leaves. The assay can be readily combined with cellular and molecular biology techniques.
Keywords: Arabidopsis regenerationBackground
Due to their sessile nature, plants are frequently subjected to injuries caused by biotic and abiotic factors. These injuries, when left unattended, can compromise plant immunity, growth, and even survival (Hwang et al., 2017; Radhakrishnan et al., 2020). To overcome the adversities of wounding, plants have evolved a remarkable repertoire of regenerative responses ranging from wound healing in the form of local cell proliferation to complete replacement of amputated organs, such as root tip regeneration (Ikeuchi et al., 2016; Shanmukhan et al., 2020). Although numerous studies have probed the mechanisms underlying several regenerative responses in plants, investigations regarding the regeneration potential of aerial organs are limited (Iwase et al., 2011; Kareem et al., 2015; Durgaprasad et al., 2019). Thus, despite their higher susceptibility to injury than underground organs, there is a dearth of information on regeneration in the aerial organs of plants, particularly, the leaves. Although leaves play a crucial role in plant physiology, their regeneration potential has hardly been investigated (Kuchen et al., 2012; Radhakrishnan et al., 2020).
Leaves possess an elaborate network of vascular tissue with a central midvein that transports substances back-and-forth between the main plant body. Damage to the midvein calls for prompt repair, failing which the transport of substances, and consequently the growth of the leaf and its adjacent branches, are impaired (Sachs and Hassidim, 1996; Radhakrishnan et al., 2020). Recently, a new vascular incision assay in leaves has been developed to study wound repair and tissue restoration in response to injury. The assay revealed that the mechanically disconnected parental stands are reunited by regenerating vascular tissue that bypasses the site of injury. The assay was instrumental in understanding the molecular mechanism underlying vascular regeneration in aerial organs growing in the normal developmental context. Upon injury, a coherent feed-forward loop comprising cell fate determinants, PLETHORA (PLT) and CUP-SHAPED COTYLEDON2 (CUC2), activates local auxin biosynthesis, leading to vascular regeneration in growing aerial organs (Radhakrishnan et al., 2020). Although vascular regeneration necessitates the PLT-CUC2 regulatory axis, the extent of injury acted as a limiting factor (Radhakrishnan et al., 2020). Here, we show that the regenerative ability of leaf vasculature is determined by the size of the injury, age of the leaf explant, and position of the injury along the proximodistal axis of the leaf blade.
This easy-to-master, reproducible assay can be performed using readily available laboratory supplies. The convenience of performing real-time confocal imaging and other molecular techniques, such as quantitative real-time PCR, using the injured leaves makes the assay valuable for studying the molecular players and mechanisms regulating wound-induced response and regeneration in the normal developmental context. The method will also be useful for studying the interplay between mechanisms of vein patterning during development and that of vein regeneration.
Materials and Reagents
Reagents for seed sterilization
Seeds of wildtype (Columbia) Arabidopsis thaliana
Note: For the purpose of standardization, we used wildtype plants. The assay can be performed without any modifications in Arabidopsis mutant plants to study vascular regeneration efficiency (Radhakrishnan et al., 2020).
20% sodium hypochlorite
70% ethanol
Sterile autoclaved water
35 mm (diameter) round Petri dish (Himedia, catalog number: PW050)
15% ethanol
50% ethanol
70% ethanol
96% ethanol
100% ethanol
Glycerol (Sigma-Aldrich, catalog number: G5516)
Chloral hydrate (Sigma-Aldrich, catalog number: 23100)
Sterile autoclaved water
Murashige and Skoog (MS) salt (Sigma-Aldrich, catalog number: M5524)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Plant agar (Sigma-Aldrich, catalog number: A7921)
Clearing solution (see Recipes)
Half-strength Murashige and Skoog (MS) medium (see Recipes)
Equipment
Equipment for in vitro culture
Laminar air flow chamber (LAF)
Sterile pipette tips (200 µl and 1 ml)
Micropipettes
1.5 ml microcentrifuge tubes
Sterile disposable square Petri dishes, size: 120 mm × 120 mm (Himedia, model: PW050-1)
Plastic wrap (Himedia Phytawrap)
Plant growth chamber (Percival, model: AR-100L3)
Fine-point tweezers (Dumont tweezer, model: Style 5)
Sterile razor blade
Forceps
Micro-Vannas scissors, straight (Ted Pella, model: 1340)
Gloves
Face mask
Stereozoom microscope (Zeissstemi , model: 2000) for incision and sample collection
Confocal laser-scanning microscope (Leica, model: TCS SP5 II) for brightfield imaging
Microscope slides (Labtech)
Microscope cover glass, 22 × 22mm (Corning, model: 2850-22)
Watercolor brush (with small bristles)
Software
R software
Procedure
Seed sterilization
Seed sterilization should to be performed within the LAF under sterile conditions. The workspace and tools (micropipette, tip boxes, reagent bottles) required for the procedure must be thoroughly wiped with 70% ethanol and UV irradiated. Prior to commencing in vitro culture, hands should be washed using soap and wiped with 70% ethanol. A liquid surface sterilization protocol for seeds is described here.
Aliquot the required number of wildtype seeds in a 1.5-ml microcentrifuge tube.
Note: For efficient sterilization, do not take more than 300 seeds per tube and remove any debris such as parts of siliques left over from seed collection.
Add 1 ml 70% ethanol. Agitate the contents by inverting the tube for 2-3 min.
Note: Prior to centrifugation, ensure that the centrifuge and rotor surfaces are clean. Avoid touching the inside of the lid of the microcentrifuge tube while opening and closing to minimize contamination.
Briefly spin the tube at 4,226 × g (Rcf) and carefully discard the ethanol without losing any seeds.
Add 1 ml 20% sodium hypochlorite and shake the contents for 2-3 min. Repeat Step A3.
Wash the seeds 5-7 times using 1 ml sterile autoclaved water.
Stratify the seeds in 1 ml sterile autoclaved water for two days at 4°C.
Pipette approximately 25 seeds using a 1-ml pipette and place on the surface of half-strength MS medium. Space the seeds out in a row using the pipette tip, leaving at least a 0.5-cm gap between each seed.
Note: For ease of incision, avoid placing the seeds very close to each other.
Incubate the Petri dishes vertically in a growth chamber under 45 μmol/m2/s continuous white light (24 h) at 22°C and 70% relative humidity.
Note: The assay can also be performed under long day and short day conditions using 5 dpg plants.
Leaf incision
Leaf incision can be performed on the work bench after adopting the necessary measures to minimize contamination. Wear gloves and a face mask during the procedure. Prior to incision, wipe the surface of the dissection microscope (Zeiss stemi 2000) and gloved hands with 70% ethanol. The tweezers used for incision should be dipped in 70% ethanol and allowed to dry for a few minutes before incision. Opening the plate on multiple days for incision will increase the incidence of contamination.
Note: Due to the fragile nature of the tweezer tips, sterilization techniques that may damage or blunt are not recommended.
The plate containing the seedlings is opened under a dissection microscope to confirm the age of the seedlings and to perform an incision in 5 dpg (days post-germination) seedlings.
Note: Due to the asynchronous nature of seed germination, the seedlings may not all be of the same age. The age of the seedlings is determined by counting the number of days post-germination (dpg). The first day of radicle emergence is counted as 0 dpg. To maintain consistency, only injure the plants that are at the desirable developmental stage. Move aside the uninjured plants to distinguish them from injured ones. The age of the seedlings is important as older seedlings display reduced regeneration efficiency, while very young seedlings are extensively damaged during the procedure. The appropriate age of incision is 4-6 dpg (Figure 1).
Of the two leaves belonging to the first pair (true leaves), the leaf that faces the lid of the Petri dish is chosen for incision due to ease of access. Using the sharp tip of the tweezers, an incision is made on the lower abaxial surface of the leaf belonging to the first pair. The incision is carefully performed at the junction between the petiole and the basal end of the lamina (Figure 1a, Supplementary Figure 1, Video 1). This ensures that the injury occurs just above the first lateral vein (counted from the base of the leaf), where the regeneration efficiency is highest in comparison with other positions along the proximodistal axis of the midvein (Figure 2). The incision should be performed with just enough force so that it punctures the vascular tissue located close to the abaxial surface of the leaf without piercing through the adaxial surface. This is important as extensive damage that creates a gap exceeding 400 µm between the parental vascular strands is not repaired (Radhakrishnan et al., 2020) (Figure 1p).
Note: Care should be taken not to inflict multiple damages on the leaves; therefore, it is not advisable to perform incision in both leaves of the first pair. During incision, a sterile 200 μl microtip or forceps may be used to restrict the movement of the plant by supporting the cotyledon or hypocotyl during incision. This will prevent the plant from submerging into the media due to the force of incision; however, avoid touching the damaged leaves as this can inflict further damage.
Video 1. Demonstration of performing leaf incision in 5 dpg plantsAfter incision, the plates are closed and incubated vertically under continuous light at 22°C in a growth chamber.
Four days post-incision (dpi), the injured leaf is carefully cut at the petiole using Vannas scissors (Video 2) and placed in 15% ethanol in a small round Petri dish (35 mm).
Note: Around 20-30 leaves can be treated using 2-3 ml 15% ethanol in a 35 mm Petri dish during the decolorizing procedure. Alternatively, 6-well plates can be used when handling multiple types of samples.
Video 2. Demonstration of sample collection 4 days post-leaf incision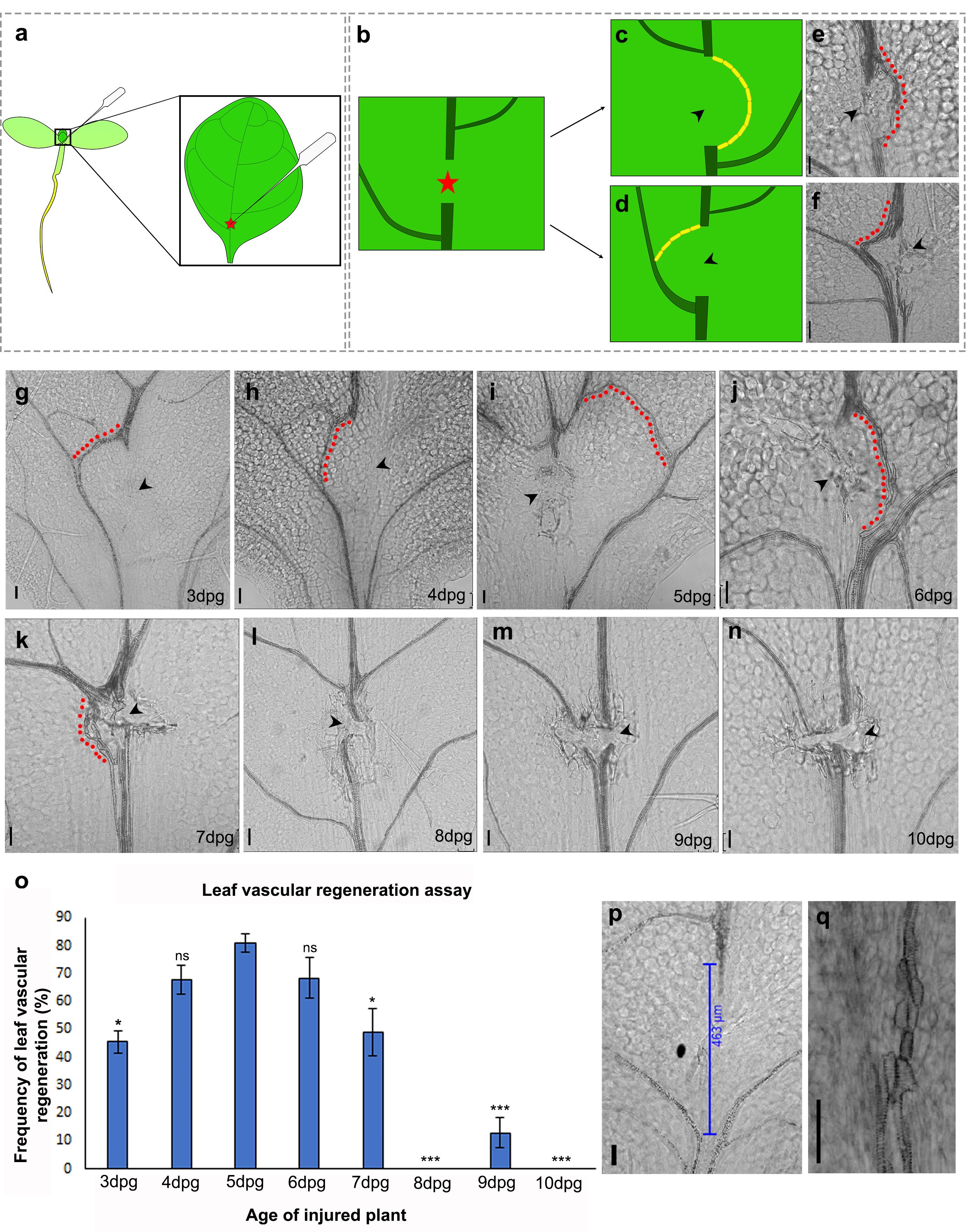
Figure 1. Leaf vascular regeneration upon midvein injury depends on the age of the injured leaf. (a-b) Illustration depicting the location of the incision for effective vascular regeneration. The red star represents the site of injury. (c, e) Schematic and brightfield image showing the regenerating vasculature reuniting the disconnected parental stands to form a D-loop bypassing the site of injury. (d, f) Illustration and brightfield image showing the regenerating vasculature connecting the apical cut end to the nearest lateral vein. Yellow blocks in (c) and (d) represent end-to-end connected xylem elements. (g-n) Regeneration response in leaves injured at 3 dpg (g) (*P = 0.016, n = 24), 4 dpg (h) (P = 0.426, not significant (ns), n = 45), 5 dpg (i) (n = 20), 6 dpg (j) (P = 0.605, not significant (ns), n = 40), 7 dpg (k) (*P = 0.03, n = 34), 8 dpg (l) (***P = 1.6 × 10-12, n = 43), 9 dpg (m) (***P = 2.405 × 10-9, n = 52), and 10 dpg (n) (***P = 4.8 × 10-8, n = 21). Statistical analysis using Pearson’s χ2 test. Note that the 3-7 dpg leaves are capable of reconnecting their disconnected vasculature but the regeneration efficiency declines with progressive aging of the leaves. The regenerating vasculature is indicated by the red dots. The black arrowheads indicate the site of injury. (o) Graph depicting the frequency of vascular regeneration in leaves injured at different ages. (p) Extensive damage creates a gap exceeding 400 µm between the parental vascular strands; as a result, no vascular regeneration is observed. (q) End-to-end attached xylem elements of a regenerated vascular strand. Scale bar: 50 µm.
Sample decolorization and clearing
After a 15-min incubation in 15% ethanol, the ethanol is drained using a micropipette with care being taken not to damage the samples.
Note: Initially, the leaves float on the surface of the solvent. Gently submerge the leaves using a paint brush with small bristles.
The tissue is gradually dehydrated by subsequent treatment with 50%, 70%, and 96% ethanol consecutively for 15 min each. After discarding 96% ethanol, the leaves are incubated for 12 h in 100% ethanol for tissue dehydration and removal of the chlorophyll pigmentation.
After discarding the ethanol, the samples are consecutively incubated for 15 min each in 96%, 70%, 50%, and 15% ethanol for rehydration.
After discarding the ethanol, freshly prepared clearing solution (see Recipes) containing chloral hydrate is added to the sample. The samples are incubated in the clearing solution for at least 3 h prior to mounting the slides for brightfield imaging.
Note: Increasing the duration of clearing can enhance the contrast during brightfield imaging to some extent.
Slide preparation
Using a small paint brush, each cleared leaf is picked up from the clearing solution and placed on a clean slide with the adaxial surface of the leaf facing upward. The brush can be used to gently tease open any curled leaves without inflicting any further damage. The coverslip is mounted over the sample, taking care not to create any bubbles. Multiple leaves (6-8) can be placed under a single coverslip. Using a 200 µl pipette tip, approximately 80 µl clearing solution is added from the corner of the mounted coverslip, thereby filling the gaps between the mounted leaves.
Brightfield imaging
The regeneration of vascular strands in the cleared samples can be assessed by imaging the site of incision using the brightfield mode of a fluorescent or confocal microscope. A snapshot of the regenerating vascular strand captured by a confocal microscope is recommended to acquire high-resolution images of the regenerating xylem elements. The settings described here are for a Leica TCS SP5 II inverted microscope. An argon laser or DPSS 561 can be used to capture a snapshot at a laser power of 30%, scan speed of 200 Hz, line average of 2, and a pixel format of 1024 × 1024. Newly formed vascular strands display distinct morphology characterized by end-to-end connected xylem elements (Figure 1e, f, j, k, q). When the regenerating vein reunited the cut ends of the midvein forming a D-loop (Figure 1c, e) or connected either of the cut ends to a lateral vein (Figure 1d, f), the outcomes were scored as successful regeneration. To maintain consistency in the methodology, incisions made in locations other than the junction of the first lateral vein were not scored while studying the age dependency of regeneration. Additionally, only incisions creating a gap less than 400 µm between the detached parental strands were scored.
Data analysis
Statistical analysis was performed using the R software. The collected data were statistically analysed using Pearson’s χ2 test.
Results and Discussion
Although the regeneration ability of plants has been widely investigated, leaves have been seldom studied for their regeneration or local wound repair abilities (Kuchen et al., 2012; Radhakrishnan et al., 2020). We describe a detailed stepwise method for a novel leaf vascular regeneration assay that can be used to study regeneration of the midvein in response to local injury. Our previous studies have shown that a mechanical disconnection of the midvein, creating a gap of under 400 µm (measured after sample clearing), can be bridged by regenerating vascular strands (Radhakrishnan et al., 2020). While the injured vascular tissue degenerates, the newly synthesized vasculature can either reunite the disconnected strands or connect the cut end to the nearest lateral vein (Figure 1b-f). Either way, the reconnection ensures restoration of the leaf vascular network and transport between the leaf and the rest of the plant body. However, extensive damage generating a gap larger than 400 µm cannot be repaired, thereby denying functional restoration of the leaf vascular tissue (Radhakrishnan et al., 2020) (Figure 1p). Here, the wound-size dependency of vascular regeneration was recapitulated in silico by implementing a computational model based on the canalization hypothesis of vein formation in leaves (Rolland-Lagan and Prusinkiewicz, 2005). According to the canalization hypothesis (Sachs, 1991), positive feedback between auxin flux and auxin conductivity leads to channelized auxin flow, which in turn, promotes the differentiation of vascular tissue. Consistent with our experimental observations (Figure 1p), the computational model demonstrates that the formation of a new vascular strand is indeed dependent on the size of the opening (mimicking a wound-induced gap) created in a matrix of cells (resembling a leaf blade). The model also predicts that the failure of vascular regeneration upon extensive damage can be attributed to the disruption of auxin flux and concentration as a result of the larger wound (Video 5). Such a disruption would hinder the proper channelization of auxin and prevent the efficient differentiation of the regenerating vascular strands. Equations governing the mathematical model and other relevant details are presented in the Supplementary Information (Supplementary information 1, Videos 3-5). Our results indicate that in addition to animal cells and unicellular Dictyostelium, wound size sensitivity of the repair process is also conserved in plants (Pervin et al., 2018).
Having substantiated the wound-size dependency of vascular regeneration, we next investigated whether the regeneration response is dependent on the age of the wounded plant. In many higher animals, progressive aging is associated with reduced regeneration ability (Yun, 2015). To probe how age regulates the regeneration response in leaves, we performed the incision in plants aged 3-10 dpg. Performing incisions on the miniscule leaves of 3 dpg plants was tedious and often damaged the leaves excessively. Upon comparison, leaves of 3 dpg plants showed lower regeneration efficiency than those of 5 dpg plants (Figure 1g, 1o). Leaves of plants aged 4-6 dpg displayed the highest regeneration efficiency, making this the optimal age to study vascular regeneration in leaves (Figure 1h-j, 1o). Although it is easier to perform incisions in older and larger leaves, the regeneration efficiency declined steeply, with leaves of 10 dpg plants completely failing to regenerate (Figure 1k-o). It is important to note that even when the injury-induced gap was less than 400 µm, vascular regeneration was impeded in these older leaves (Figure 1l-n); thus, our data suggest that vascular regeneration efficiency reduces with age in injured plants.
Regeneration studies in plants and animals have demonstrated that the competence to regenerate in response to injury can vary even within a specific organ (Durgaprasad et al., 2019; Morgan, 1902); therefore, we next examined how the position of incision on the growing leaf influenced the vein regeneration efficiency. To analyze this, we made incisions at different positions along the leaf blade, namely the petiole of the leaf, the basal end (proximal to the plant body axis) of the midvein, the apical end (distal to the plant body axis) of the midvein, and the lateral veins (Figure 2a, 2c-f). The highest regeneration frequency was recorded at the basal end of the midvein, particularly between the first and second lateral vein (Figure 2b, 2d). The petiole also showed a similar regeneration efficiency upon incision and often led to the formation of multiple strands in response to injury (Figure 2b, 2c). However, since the leaf is excised at the petiole during sample collection, the incision site and regenerated vascular strand are occasionally damaged, leading to loss of valuable samples. Additionally, since incisions performed in the petiole lead to multiple stand formation instead of single-stand regeneration, it is more appropriate to make injuries in the leaf blade, as the study involves following a single regenerating strand in real-time to study recognition, communication, and reunion of vascular strands. Upon injuring other positions, we observed that the regeneration efficiency drastically declined toward the apical regions of the midvein and in the lateral veins (Figure 2b, 2e-g).
Collectively, our data demonstrate that leaf vascular regeneration is sensitive to the size of the wound, the age of the injured leaf, and the position of incision on the leaf.
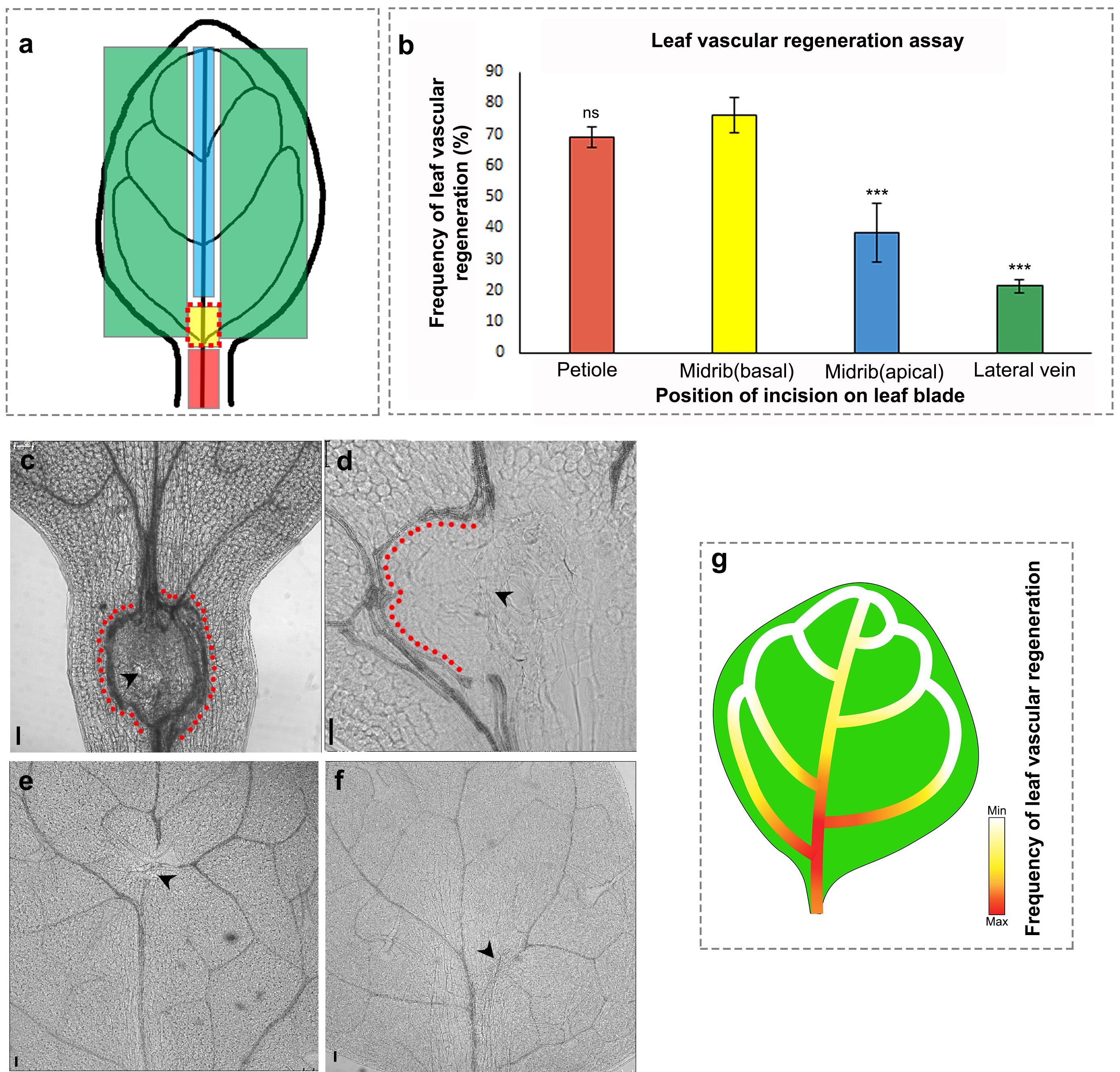
Figure 2. Vascular regeneration depends on the position of injury in the leaf. (a) Schematic depicting the positions of incision on the leaf blade and petiole. (b) The frequency of vascular regeneration at different positions (represented by colored boxes in (a)) in the leaf is represented by the same color bars in the graph (b) Petiole (n = 21, P = 0.95, not significant [ns]), basal correct position (n = 45), midvein upper end (n = 42, ***P = 0.0009), lateral vein (n = 21, ***P = 0.0002). (c-f) Images showing the incision to the vasculature in the petiole (c), base of the midvein (d), apical region of the midvein (e), and lateral vein (f). Note the multiple strand formation upon injury in the petiole (c). The red dots indicate regenerated vascular strands and the black arrowheads represent the site of incision. Scale bar: 50 µm. (g) Gradient represents the efficiency of vascular regeneration along the leaf blade with maximum regeneration (represented by red) at the base of midvein. Lateral veins and the distal end of the midvein exhibit reduced regeneration frequency.
Conclusion
Our study reveals that 4-6 dpg leaves respond most efficiently to smaller wounds (400 µm or less in size) that are inflicted at the junction of the first lateral vein at the proximal end of the leaf blade. While adopting this assay to study regeneration in other plant species, we recommend standardization of the method with respect to the above-mentioned criteria.
The assay will be helpful in exploring the mechanisms underlying the regeneration of vascular tissue in growing leaves. To begin with, the assay may prove tedious; however, with repeated practice, this method can be performed deftly and rapidly in a large number of samples. The short duration of the experiment (experimental data can be collected 5 days post-injury) and the dispensability of specialized equipment makes it amenable to the larger scientific community. As demonstrated previously (Radhakrishnan et al., 2020), this method can be used in combination with other cellular and molecular biology techniques with little-to-no standardization, thereby adding to its utility.
Recipes
Clearing solution
Dissolve 8 g chloral hydrate in 3 ml water
Vortex the solution until chloral hydrate is completely dissolved
Add 1 ml glycerol to the solution
Note: The solution has to be freshly prepared for clearing the leaf samples. It is also used for mounting samples for brightfield confocal microscopy.
Half-strength MS medium
Add 2.165 g MS salt and 10 g sucrose to about 850 ml Milli-Q water
Adjust pH to 5.7 with 1 N KOH and make up the volume to 1 L
Add 8 g plant agar
Autoclave the medium (121°C for 20 min) and cool to about 45-50°C.
Add 1 ml 100 mg/ml filter-sterilized ampicillin (final concentration in medium: 100 µg/ml) to 1 L medium and pour 50 ml into each sterile square Petri dish within the LAF
Allow to cool and solidify
Acknowledgments
K.P. acknowledges grants from the Department of Biotechnology (DBT), Government of India [grant BT/PR12394/AGIII/103/891/2014] and the Department of Science and Technology, Science and Engineering Research Board (DST-SERB), Government of India [grant EMR/2017/002503/PS], and also acknowledges the Indian Institute of Science Education and Research-Thiruvananthapuram (IISER-TVM) for infrastructure and financial support. D.R. and M.M.M acknowledge University Grants Commission (UGC) fellowships. A.K. was supported by an Indian Institute of Science Education and Research-Thiruvananthapuram fellowship. A.P.S. and V.V. are recipients of Council of Scientific and Industrial Research (CSIR) fellowships. M.A. acknowledges the Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India for granting the DBT-Post Doctoral Fellowship (DBT-RA Program). K.R.M. and R.K.R acknowledge funding by the Department of Biotechnology (DBT). A.S. acknowledges support from the Science and Engineering Research Board, Government of India through EMR grant No. EMR/2016/007221. We are thankful to Aswathy Syam, Aleesha Jaleel, and Kaustuv Ghosh for assistance with preliminary experiments. We are also grateful to Bejoy Manoj for technical help with video preparation.
Competing interests
The authors declare no competing or financial interests.
References
- Durgaprasad, K., Roy, M. V., Venugopal, M. A., Kareem, A., Raj, K., Willemsen, V., Mahonen, A. P., Scheres, B. and Prasad, K. (2019). Gradient Expression of Transcription Factor Imposes a Boundary on Organ Regeneration Potential in Plants. Cell Rep 29(2): 453-463 e453.
- Hwang, H. H., Yu, M. and Lai, E. M. (2017). Agrobacterium-mediated plant transformation: biology and applications. Arabidopsis Book 15: e0186.
- Ikeuchi, M., Ogawa, Y., Iwase, A. and Sugimoto, K. (2016). Plant regeneration: cellular origins and molecular mechanisms. Development 143(9): 1442-1451.
- Iwase, A., Mitsuda, N., Koyama, T., Hiratsu, K., Kojima, M., Arai, T., Inoue, Y., Seki, M., Sakakibara, H., Sugimoto, K. and Ohme-Takagi, M. (2011). The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr Biol 21(6): 508-514.
- Kareem, A., Durgaprasad, K., Sugimoto, K., Du, Y., Pulianmackal, A. J., Trivedi, Z. B., Abhayadev, P. V., Pinon, V., Meyerowitz, E. M., Scheres, B. and Prasad, K. (2015). PLETHORA Genes Control Regeneration by a Two-Step Mechanism. Curr Biol 25(8): 1017-1030.
- Kuchen, E. E., Fox, S., de Reuille, P. B., Kennaway, R., Bensmihen, S., Avondo, J., Calder, G. M., Southam, P., Robinson, S., Bangham, A. and Coen, E. (2012). Generation of leaf shape through early patterns of growth and tissue polarity. Science 335(6072): 1092-1096.
- Morgan, T. H. (1902). Further Experiments on the Regeneration of the Tail of Fishes. Archiv für Entwicklungsmechanik der Organismen. 14: 539-561.
- Pervin, M. S., Itoh, G., Talukder, M. S. U., Fujimoto, K., Morimoto, Y. V., Tanaka, M., Ueda, M. and Yumura, S. (2018). A study of wound repair in Dictyostelium cells by using novel laserporation. Sci Rep 8(1): 7969.
- Radhakrishnan, D., Shanmukhan, A. P., Kareem, A., Aiyaz, M., Varapparambathu, V., Toms, A., Kerstens, M., Valsakumar, D., Landge, A. N., Shaji, A., Mathew, M. K., Sawchuk, M. G., Scarpella, E., Krizek, B. A., Efroni, I., Mahonen, A. P., Willemsen, V., Scheres, B. and Prasad, K. (2020). A coherent feed-forward loop drives vascular regeneration in damaged aerial organs of plants growing in a normal developmental context. Development 147(6): dev185710. doi: 10.1242/dev.185710.
- Rolland-Lagan, A. G. and Prusinkiewicz, P. (2005). Reviewing models of auxin canalization in the context of leaf vein pattern formation in Arabidopsis.Plant J 44(5): 854-865.
- Sachs, T., and Hassidim, M. (1996). Mutual Support and Selection between Branches of Damaged Plants. Vegetatio 127: 25-30.
- Sachs, T. (1991). Cell Polarity and Tissue Patterning in Plants. Development (Supplement 1) 113: 83-93.
- Shanmukhan, A. P., Mathew, M. M., Radhakrishnan, D., Aiyaz, M. and Prasad, K. (2020). Regrowing the damaged or lost body parts. Curr Opin Plant Biol 53: 117-127.
- Yun, M. H. (2015). Changes in Regenerative Capacity through Lifespan. Int J Mol Sci 16(10): 25392-25432.
Article Information
Copyright
© 2021 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Radhakrishnan, D., Shanmukhan, A. P., Kareem, A., Mathew, M. M., Varaparambathu, V., Aiyaz, M., Radha, R. K., Mekala, K. R., Shaji, A. and Prasad, K. (2021). Age, Wound Size and Position of Injury – Dependent Vascular Regeneration Assay in Growing Leaves. Bio-protocol 11(9): e4010. DOI: 10.21769/BioProtoc.4010.
Category
Plant Science > Plant developmental biology
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










