- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Preparation of Yeast tRNA Sample for NMR Spectroscopy
Published: Vol 10, Iss 12, Jun 20, 2020 DOI: 10.21769/BioProtoc.3646 Views: 5090
Reviewed by: Beatrice LiChristian RothLouise Jane Walport

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
An Aptamer-based mRNA Affinity Purification Procedure (RaPID) for the Identification of Associated RNAs (RaPID-seq) and Proteins (RaPID-MS) in Yeast
Rohini R. Nair [...] Jeffrey E. Gerst
Jan 5, 2022 5373 Views
Chromatin-RNA in situ Reverse Transcription Sequencing (CRIST-seq) Approach to Profile the Non-coding RNA Interaction Network
Shilin Zhang [...] Jiuwei Cui
Jul 20, 2023 2331 Views
Purification of Long Non-coding RNAs on Replication Forks Using iROND (Isolate RNAs on Nascent DNA)
Weidao Zhang [...] Bo Zhao
Nov 5, 2023 2048 Views
Abstract
Transfer RNAs (tRNAs) are heavily decorated with post-transcriptional modifications during their biosynthesis. To fulfil their functions within cells, tRNAs undergo a tightly controlled biogenesis process leading to the formation of mature tRNAs. In addition, functions of tRNAs are often modulated by their modifications. Although the biological importance of post-transcriptional RNA modifications is widely appreciated, methods to directly detect their introduction during RNA biosynthesis are rare and do not easily provide information on the temporal nature of events. To obtain information on the tRNA maturation process, we have developed a methodology, using NMR as a tool to monitor tRNA maturation in a non-disruptive and continuous fashion in cellular extracts. By following the maturation of a model yeast tRNA with time-resolved NMR, we showed that modifications are introduced in a defined sequential order, and that the chronology is controlled by cross-talk between modification events. The implementation of this method requires the production for NMR spectroscopy of tRNA samples with different modification status, in order to identify the NMR signature of individual modifications. The production of tRNA samples for the analysis of modification pathways with NMR spectroscopy will be presented here and examplified on the yeast tRNAPhe, but can be extended to any other tRNA by changing the sequence of the construct. The protocol describes the production of unmodified tRNA samples by in vitro transcription, and the production of modified tRNA samples by recombinant expression of tRNAs in E. coli.
Keywords: Transfer RNABackground
In all domains of life, the synthesis and maturation of RNAs involve post-transcriptional chemical modifications of their nucleotides at specific sites. Among the different RNA families, tRNAs display not only the highest variety of chemical modifications, but also the highest density of modification per transcript (~8-25% of modified nucleotides in tRNAs of various organisms) (Boccaletto et al., 2018). The biogenesis of tRNAs is tightly regulated, and in particular, the introduction of post-transcriptional modifications in tRNAs is controlled and influenced by multiple factors (El Yacoubi et al., 2012; Jackman and Alfonzo, 2013; Barraud and Tisné, 2019). Although all aspects of tRNA biology are controlled and modulated by modifications, methods to directly detect their introduction during tRNA biosynthesis are rare and do not easily provide information on the temporality of modification events. We have developed a methodology, using NMR as a tool to monitor tRNA maturation in a non-disruptive and continuous fashion (Barraud et al., 2019). Briefly, introducing isotope-labeled tRNAs into unlabeled cell extracts containing the cellular enzymatic activities combined with the use of isotope-filters in NMR experiments enables the detection of the tRNA of interest within the complex cell extract environment. This method requires the production of tRNA samples with different modification status, in order to identify the NMR signature of individual modifications (Barraud et al., 2019).
Here we describe the production and purification of two forms of the yeast tRNAPhe differing in their modification content. The first sample corresponds to a yeast tRNAPhe produced by in vitro transcription, which presents no post-transcriptional modifications. The second sample corresponds to a recombinant yeast tRNAPhe produced in an E. coli strain overproducing this tRNA. Since E. coli has a less prolific but related tRNA modification machinery than S. cerevisiae, the modification pattern of this sample resembles that of the fully modified yeast tRNAPhe, but overall exhibits fewer modifications (Barraud et al., 2019). The production and purification of tRNA samples with different modification content is essential to reveal the NMR signature of individual post-transcriptional modifications and correspond to the first steps towards the time-resolved NMR monitoring of tRNA maturation (Barraud et al., 2019). The reported protocol describes the production of unmodified tRNA samples by in vitro transcription, and the production of modified tRNA samples by recombinant expression of tRNAs in E. coli. These productions and purifications will be examplified on the yeast tRNAPhe. Part of the protocol has been published elsewhere (Barraud et al., 2019), but some parts are described here in more detail. Protocol for the expression and purification of active T7 RNA polymerase in E. coli are not detailed here, but have been described comprehensively elsewhere (Rio, 2013; Dégut et al., 2016).
Materials and Reagents
- Production of unmodified yeast tRNAPhe by in vitro transcription
For tRNA in vitro transcription- Commercial or in-house produced T7 RNA polymerase (stock stored at -20 °C at 1 mg/ml in 20 mM Na-phosphate pH 7.7, 100 mM NaCl, 10 mM DTT, 1 mM EDTA, 50% (v/v) glycerol).
- RNase-free water (double-distilled or MilliQ water 18.2 MΩ)
- Ready to use ATP, UTP, GTP, CTP at 100 mM in water at pH 8.0 (Jena Bioscience, catalog number: NU-1014L )
- Ready to use 15N-labeled UTP and GTP at 100 mM in water at pH 7.8 (Eurisotop, catalog numbers: NLM-4270-CA , NLM-4268-CA)
- DNA template
5′ TGGTGCGAATTCTGTGGATCGAACACAGGACCTCCAGATCTTCAGTCTGGCGCTCTCCCAACTGAGCTAAATCCGCTATAGTGAGTCGTATTA 3′ (Eurogentec, 1 μmol of synthesis scale, PAGE purification) - T7 promotor primer: 5′ TAATACGACTCACTATAG 3′ (Eurogentec, 0.2 μmol of synthesis scale, desalted)
- 280 mM MgCl2 (prepared in water) (CAS Number 7791-18-6)
- 80 mM GMP (prepared in water at pH 7.5, pH adjusted with concentrated HCl) (Sigma-Aldrich, catalog number: G8377-56 )
- 0.5 M Ethylenediaminetetraacetic acid (EDTA) (prepared in water at pH 8.0, pH adjusted with concentrated NaOH) (CAS Number 60-00-4)
- 0.1 M Spermidine (Sigma-Aldrich, catalog number: 0 5292 )
- 1 M 1,4-Dithiothreitol (DTT) (Sigma-Aldrich, catalog number: 43816 )
- TritonTM X-100 solution (Sigma-Aldrich, catalog number: T9284 )
- 20x T7 transcription buffer (TB) (see Recipes)
- Urea (Euromedex, catalog number: EU0014-B )
- 40% acrylamide/bis-acrylamide (19:1) solution (Euromedex, catalog number: EU0076-B )
- Ammonium persulfate (APS) (Euromedex, catalog number: EU0009-B ) prepared as 10% (w/v) solution in water
- N,N,N’,N’-Tetramethylethylenediamine (TEMED) (Euromedex, catalog number: 50406 )
- Bromophenol blue (Sigma-Aldrich, catalog number: B0126 )
- Xylene cyanol (Sigma-Aldrich, catalog number: X4126 )
- Toluidine blue (CAS Number 92-31-9, Sigma-Aldrich, catalog number: 198161 )
- Ethanol absolute (CAS Number 64-17-5, Millipore/Merck, catalog number: 1070172511 )
- 10x TBE buffer (Sigma-Aldrich, catalog number: T4415-1L )
- 2x RNA loading dye (see Recipes)
- Toluidine-blue staining solution (see Recipes)
For tRNA transcript purification and NMR sample preparation- Sterile syringe filters 0.22 μm (VWR, catalog number: 514-0073 )
- Dialysis sacks Spectra/Por 3 Dialysis Tubing MWCO < 3.5 kDa (Spectra/Por, catalog number: 132724)
- Standard Closures for Dialysis Tubing (Spectrum®, catalog number: 132735)
- Concentrators Amicon® Ultra-15 3,000 MWCO (Millipore/Merck, catalog number: UFC900308 )
- NaH2PO4 and Na2HPO4·12H2O (CAS Numbers 7558-80-7 and 10039-32-4, Sigma-Aldrich, catalog numbers: S3139 and 71649 )
- UltraPureTM 5 M NaCl (e.g., Thermo Fisher Scientific, catalog number: 24740011 )
- 1 M MgSO4 (prepared in water) (CAS Number 7487-88-9)
- 1 M MgCl2 (prepared in water) (CAS Number 7791-18-6)
- Purification buffer A (see Recipes)
- Purification buffer B (see Recipes)
- NMR buffer (see Recipes)
- Commercial or in-house produced T7 RNA polymerase (stock stored at -20 °C at 1 mg/ml in 20 mM Na-phosphate pH 7.7, 100 mM NaCl, 10 mM DTT, 1 mM EDTA, 50% (v/v) glycerol).
- Production of modified yeast tRNAPhe by in vivo recombinant expression in E. coli
For modified yeast tRNAPhe in vivo expression and extraction- Gene Pulser®/MicroPulserTM Electroporation Cuvettes 0.2 cm gap (Bio-Rad, catalog number: 1652082 )
- 50 ml conical tube (e.g., FalconTM, product number: 352070)
- Electrocompetent bacteria Escherichia coli JM101Tr strain (Mechulam et al., 1987)
Note: Alternatively, Escherichia coli JM101 or JM109 strains may also be used. - pBSTNAV plasmid (Addgene, catalog number: 45801 ) previously cloned with the yeast tRNAPhe DNA gene sequence (see Note 1 and Figure 3)
- Lysogeny Broth (LB) medium Gibco® (Thermo Fisher Scientific, catalog number: 10855021 )
- SPECTRA 9 medium (15N, 98%) (Eurisotop, catalog number: CGM-3030-N-1 )
- Celtone® Base Powder (15N, 98%+) (Eurisotop, catalog number: CGM-1030P-N-0.5 )
- Ampicillin, Ready Made Solution, 100 mg/ml, 0.2 μm filtered (Sigma-Aldrich, catalog number: A5354-10ML )
- Water-Saturated Phenol, pH 6.6 (Thermo Fisher Scientific, catalog number: AM9710 )
- Ethanol absolute (e.g., Merck, catalog number: 1070172511 )
- UltraPureTM 5 M NaCl (e.g., Thermo Fisher Scientific, catalog number: 24740011 )
- 1 M NaCl solution (e.g., FisherScientific, catalog number: S25877 )
- 2 M Tris-HCl pH 8.0
- 1 M Magnesium acetate (Sigma-Aldrich, catalog number: 63052 )
- RNase-free water (double-distilled or MilliQ water 18.2 MΩ)
- Extraction buffer (see Recipes)
For modified yeast tRNAPhe purification and NMR sample preparation- Microtubes 0.2 ml
- Dialysis sacks Spectra/Por 3 Dialysis Tubing MWCO < 3.5 kDa (Spectra/Por, product number: 132724)
- Standard Closures for Dialysis Tubing (Spectrum®, product number: 132735)
- Concentrators Amicon® Ultra-15 3,000 MWCO (Millipore/Merck, catalog number: UFC900308 )
- HiLoad 26/600 Superdex 75 pg column (GE Healthcare, catalog number: 28989334 )
- Mono Q 10/100 GL column (GE Healthcare, catalog number: 17516701 )
- Phenyl superose HR 10/10 column (GE Healthcare)
Note: Alternatively, a HiPrep 16/10 Phenyl HP column (GE Healthcare, catalog number: 29018184 ). - HiPrep 26/10 Desalting column (GE Healthcare, catalog number: 17508701 )
- 0.5 M NaOH solution (Fisher Scientific, catalog number: 15614900 )
- RNase-free water (double-distilled or MilliQ water 18.2 MΩ)
- Ammonium sulfate powder (Fisher Scientific, catalog number: 10325410 )
- 40% acrylamide/bis-acrylamide (19:1) solution (Euromedex, catalog number: EU0076-B )
- 1 M Tris-HCl pH 6.8
- 1 M Tris-HCl pH 8.8
- 20% (w/v) Sodium dodecyl sulfate solution (SDS) (Sigma-Aldrich, catalog number: 05030-1L-F )
- Ammonium persulfate (APS) (Euromedex, catalog number: EU0009-B ) prepared at 10% (w/v) solution in water
- N,N,N’,N’-Tetramethylethylenediamine (TEMED) (Euromedex, catalog number: 50406 )
- Tris-Glycine-SDS Buffer 10x Concentrate (TGS) (Sigma-Aldrich, catalog number: T7777 )
- 2-mercaptoethanol (CAS Number 60-24-2, Sigma-Aldrich, catalog number: M3148 )
- Glycerol (CAS Number 56-81-5, Sigma-Aldrich, catalog number: G5516 )
- Complementary DNA oligonucleotide at 100 µM in water 5′ TCGAACACAGGACCTCCAGATCTTCAGTCTGGCGCTCT 3′ (Eurogentec, 0.2 μmol of synthesis scale, desalted)
- 2x Laemmli loading buffer (see Recipes)
- Toluidine-blue staining solution (see Recipes)
- Purification buffer C (see Recipes)
- Purification buffer D (see Recipes)
- Purification buffer E (see Recipes)
- NMR buffer (see Recipes)
- Gene Pulser®/MicroPulserTM Electroporation Cuvettes 0.2 cm gap (Bio-Rad, catalog number: 1652082 )
Equipment
- Production of unmodified yeast tRNAPhe by in vitro transcription
For tRNA in vitro transcription- Water Bath
For transcription analysis by denaturing Urea-PAGE- Electrophoresis chamber, glass slides, spacers, clamps and combs for vertical PAGE (e.g., Bio-Rad Mini-PROTEAN), power supply (e.g., Bio-Rad PowerPac)
For tRNA transcript purification and NMR sample preparation- ÄKTA Purifier (GE Healthcare) or similar chromatographic system
- MonoQ 10/100 GL column (GE Healthcare, catalog number: 17516701 )
- Centrifuge (e.g., Beckman Coulter, model: Allegra X-30R )
- NanoDropTM spectrophotometer (Thermo Fisher Scientific, catalog number: ND-2000 )
- Production of modified yeast tRNAPhe by in vivo recombinant expression in E. coli
For modified yeast tRNAPhe in vivo expression and extraction- 2 L Erlenmeyer flasks (e.g., Fisher BrandTM, Fisher Scientific, catalog number: 15459103 )
- Incubation shaker (e.g., INFORS-HT Minitron)
- MicroPulserTM Electroporator (Bio-Rad, catalog number: 1652100 )
- Centrifuge for culture liters (e.g., Beckman Coulter, model: Avanti® J-E )
- Tube rotator (Thermo Fisher Scientific, catalog number: 88881002 )
- Vortex mixer (e.g., Scientific Industries SITM, model: Vortex-GenieTM 2, catalog number: SI-0236 )
For modified yeast tRNAPhe purification and NMR sample preparation- ÄKTA Purifier (GE Healthcare) or similar chromatographic system
- Sample loop 5 ml (GE Healthcare, catalog number: 18114053 )
- Superloop 10 ml and 50 ml (ÄKTA design) (GE Healthcare, catalog numbers: 18111381 and 18111382 )
- Electrophoresis chamber, glass slides, spacers, clamps and combs for vertical PAGE (e.g., Bio-Rad Mini-PROTEAN), power supply (e.g., Bio-Rad PowerPac)
- Dry bath incubator (Thermo Fisher Scientific, catalog number: 88870004 )
- Centrifuge (e.g., Beckman Coulter, model: Allegra X-30R )
- NanoDropTM spectrophotometer (Thermo Fisher Scientific, catalog number: ND-2000 )
- 2 L Erlenmeyer flasks (e.g., Fisher BrandTM, Fisher Scientific, catalog number: 15459103 )
Procedure
- Production of unmodified yeast tRNAPhe by in vitro transcription
- Small scale tRNA in vitro transcription
The optimal in vitro transcription conditions depend greatly on the MgCl2 and NTPs concentrations. The [MgCl2]/[NTP] ratio should be adjusted to optimize the yield of the RNA of interest (Figure 1). It is recommended to test small-scale reactions (e.g., 40 μl) before large-scale production of RNA (5-20 ml). In practice, the concentration of MgCl2 is varied in small-scale reactions as described in Table 1. The yield of tRNA transcript is then analyzed by Urea-PAGE (Step A2). The conditions of Table 1 are first starting points to determine best MgCl2 concentration. Concentrations of T7 RNA polymerase, DNA template and the addition of GMP at 2-6 mM can also be further optimized in a second step.- Prepare small scale reaction tests of 40 μl as described in Table 1 in PCR microtubes.
- Incubate the reactions in a water bath for 3 h at 37 °C. After ~1 h, the appearance of a white precipitate of magnesium pyrophosphate is the sign of a working transcription reaction.
- Stop the reaction by adding 5 μl of 0.5 M EDTA pH 8.0 to each small scale reaction.
- Store at -20 °C or analyze the reactions by Urea-PAGE (Step A2).
Table 1. Small scale reaction tests of 40 μl to determine best MgCl2 concentration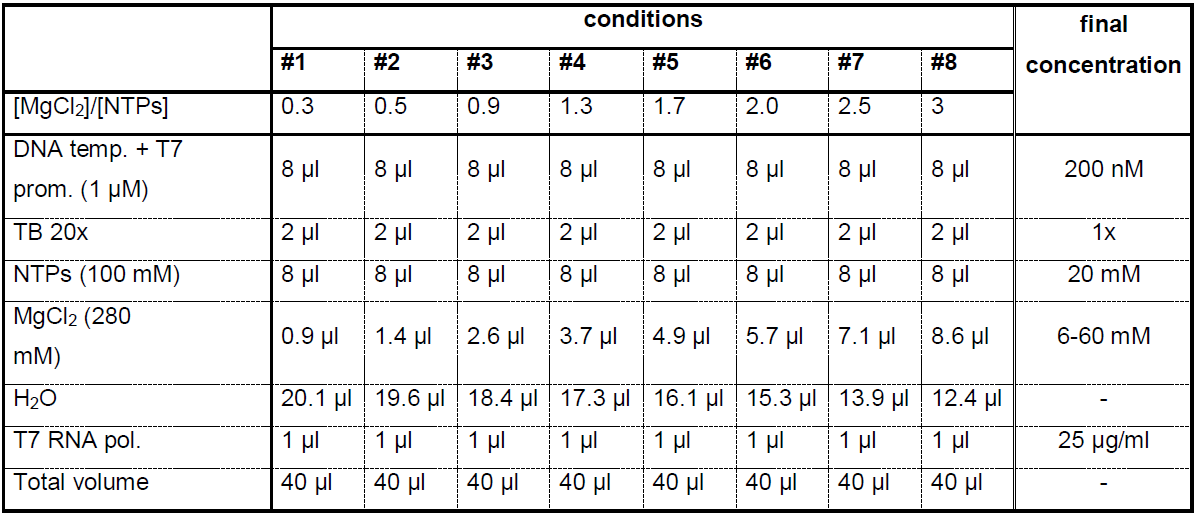
- Prepare small scale reaction tests of 40 μl as described in Table 1 in PCR microtubes.
- Transcription analysis by denaturing Urea-PAGE
- To analyze the products of transcription, prepare a Urea-PAGE gel using the same materials as those used for protein SDS-PAGE analysis. For tRNA of ~75 nts, prepare a gel mix with 7M Urea and 14% (w/v) acrylamide:bisacrylamide 19:1 by mixing 16.8 g of urea, 14 ml of 40% (w/v) acrylamide:bisacrylamide 19:1, 4 ml of 10x TBE and adjusting the volume to 40 ml final with H2O. (Note that this mix can be stored at room temperature for several months). Take 6 ml of this mix and add 60 μl of 10% APS and 6 μl of TEMED just before placing the gel between the glass plates and put the comb immediately.
- Take an aliquot of each small-scale reaction (typically 2.5 μl) and add 2.5 μl of 2x RNA loading dye (Recipe 2). Load your samples in the wells and run the gel for 1 h at 200 V in 1x TBE buffer.
- The visualization is then done by toluidine blue staining. Carefully remove the gel from the glass plates and let it stain in the toluidine-blue staining solution (Recipe 3) for 5-10 min. Pour the staining solution back into the stock bottle, and destain with water. Destain several times with fresh water until the background color of the gel is clear.
- Take a picture with a regular camera. The gel corresponding to the assays described in Table 1 and revealed by toluidine-blue staining is shown in Figure 1. The best yield for the yeast tRNAPhe is obtained for conditions #5 and #6 with [MgCl2]/[NTPs] = 1.7-2.0, i.e., [MgCl2] = 34-40 mM and [NTPs] = 20 mM. These concentrations are thus used for large-scale preparation of this tRNA.
The yield of RNA produced by in vitro transcription is sometimes very sensitive to the concentration of MgCl2. We recommend to perform these small-scale assays each time that a new DNA template is used and each time new NTPs are used, indeed, best MgCl2 concentration might be slightly different with different sources of NTP. Especially, best MgCl2 concentration might differ slightly for 15N-labeled nucleotides and must therefore be determined with the actual NTPs that will be used in large scale transcription reaction.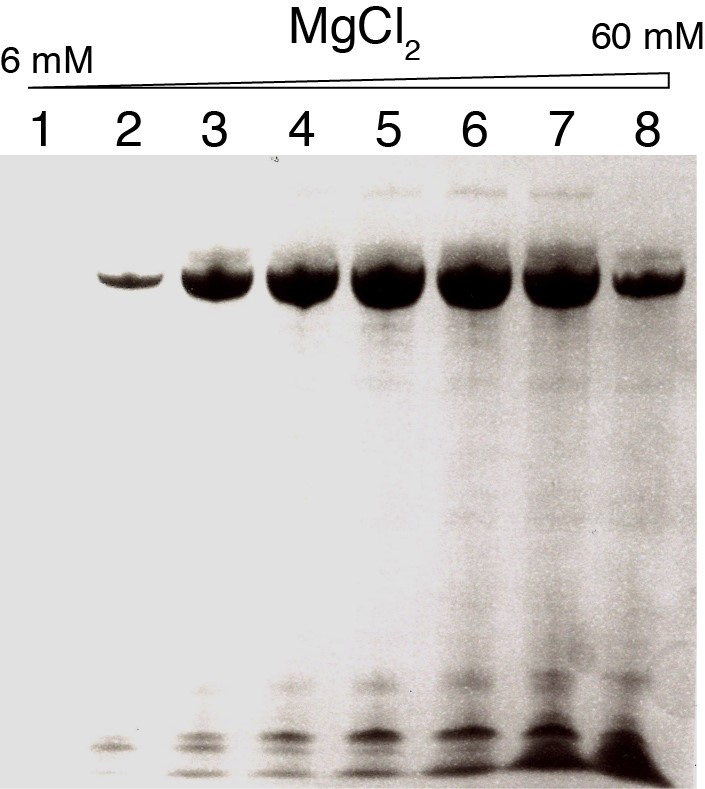
Figure 1. Sensitivity of T7 polymerase in vitro RNA production to the magnesium concentration. Urea-PAGE revealed by toluidine-blue staining showing the analysis of small-scale transcription assays of Table 1 corresponding to the production of unmodified yeast tRNAPhe. The MgCl2 concentration is varied from 6 mM to 60 mM. Numbers above the gel correspond to the conditions #1 to #8 in Table
- To analyze the products of transcription, prepare a Urea-PAGE gel using the same materials as those used for protein SDS-PAGE analysis. For tRNA of ~75 nts, prepare a gel mix with 7M Urea and 14% (w/v) acrylamide:bisacrylamide 19:1 by mixing 16.8 g of urea, 14 ml of 40% (w/v) acrylamide:bisacrylamide 19:1, 4 ml of 10x TBE and adjusting the volume to 40 ml final with H2O. (Note that this mix can be stored at room temperature for several months). Take 6 ml of this mix and add 60 μl of 10% APS and 6 μl of TEMED just before placing the gel between the glass plates and put the comb immediately.
- Large scale in vitro transcription of unmodified yeast tRNAPhe
For large scale in vitro transcription, up-scale the best conditions as determined in Step A1 with small scale reactions. In our hands, a concentrated NMR sample of unmodified yeast tRNAPhe 15N-labeled on Us and Gs can be obtained from a 10 ml transcription reaction prepared as described below.- Prepare the large scale transcription reaction of 10 ml in a 50 ml tube as follow:
40 μl of DNA template pre-mixed with the T7 promotor primer at a 1:1 ratio (stock at 50 μM)
500 μl of 20x T7 transcription buffer (TB).
500 μl of non-labeled ATP (stock at 100 mM)
500 μl of non-labeled CTP (stock at 100 mM)
500 μl of 15N-labeled UTP (stock at 100 mM)
500 μl of 15N-labeled GTP (stock at 100 mM)
1,300 μl of MgCl2 (stock at 280 mM)
500 μl of GMP (stock at 80 mM)
250 μl of T7 RNA polymerase (stock at 1 mg/ml)
RNase-free water to 10 ml - Incubate the reactions in a water bath for 3-4 h at 37 °C.
- Stop the reaction by adding 1-1.5 ml of 0.5 M EDTA pH 8.0.
- Store at -20°C until purification or purify readily as described in Step A4.
- Prepare the large scale transcription reaction of 10 ml in a 50 ml tube as follow:
- Purification and NMR sample preparation of unmodified yeast tRNAPhe
The purification is performed on a ÄKTA Purifier (or other FPLC purification system) at 4 °C. Transcribed tRNAs are sensitive to RNases, one should therefore be careful to operate the purification in RNase free conditions. For that, wash the purification system and the columns with 0.5 M NaOH and rinse them extensively with water before equilibrating the MonoQ HR 10/100 column in buffer A.- Filter the large scale transcription sample of Step A3 with 0.22 μm filters.
- Load 5-6 ml of the transcription sample on the MonoQ column previously equilibrated with buffer A.
Remark: It is not recommended to load larger quantities of tRNAs onto the column. The purification indeed becomes difficult due to a broadening of the elution peaks. Perform Steps A4b to A4d as many times as needed for large scale transcriptions of more than 5 ml. - The purification of the tRNA transcript is achieved using a gradient from 20% to 80% of buffer B with a length of 10 column volumes (CV) and a flow rate of 3 ml/min. Collect 1 ml fractions. Transcribed yeast tRNAPhe is usually eluted between 50% and 55% of buffer B.
- Analyse the content of the fractions with denaturing Urea-PAGE as described in Step A2, and pool the fractions containing the tRNA of interest (usually 5 fractions of 1 ml). Do not add fractions of tRNAPhe eluted after 55% of buffer B, since they usually contain longer RNA transcripts originating from non-templated nucleotide addition at the 3′-end of the transcript.
- Dialyze extensively the pooled fractions of yeast tRNAPhe in a dialysis sack against 1 mM Na-phosphate pH 6.5. For that one usually dialyzes the sample against a 2 L dialysis solution for at least 2 h and changes it with a fresh solution 3 times.
- Recover the dialyzed tRNAPhe in a 50 ml plastic tube and refold it by heating the sample at 95 °C for 5 min and letting it cool down slowly at room temperature.
- Add concentrated buffer stock solutions (e.g., 0.5 M Na-phosphate pH 6.5 and 1 M MgCl2) to place the refolded sample in the final NMR buffer (i.e., 10 mM Na-phosphate pH 6.5, 10 mM MgCl2).
- Concentrate the unmodified yeast tRNAPhe sample in the NMR buffer to ~1.5-2.0 mM using Amicon 3,000 MWCO. In our hands, with a 10 ml transcription with 15N-labeled on Us and Gs, one usually obtains a final NMR sample of 300 μl at ~1.8 mM (i.e., 0.5-0.6 µmol).
- Store the sample at -20 °C or use it directly for NMR spectra measurements (see Note 2).
- Filter the large scale transcription sample of Step A3 with 0.22 μm filters.
- Small scale tRNA in vitro transcription
- Production of modified yeast tRNAPhe by in vivo recombinant expression in E. coli
- Modified yeast tRNAPhe recombinant expression and extraction
- Transformation of E. coli with the tRNA expression plasmid and 15N-labeled expression
The volumes and quantities mentioned below refer to a 1 L culture. We recommend to grow several liters of culture (i.e., 2-4 L) to increase the quantity of modified yeast tRNAPhe purified following this protocol. We also recommend to grow 500 ml of culture in 2 L flasks to get enough oxygenation or ideally to use a fermenter to optimize certain parameters (e.g., pH of the medium, oxygenation…).- Use a 100 μl aliquot of electrocompetent E. coli JM101Tr strain. Add 2 μl of the plasmid (at 50-200 ng/μl).
- Put the mix in an electroporation cuvette. Electroporate the cells, with the machine set according to the electroporation cuvette and organism that is used (namely set the electroporator to “Ec2” meaning “E. coli 0.2 cm cuvette”). Then add 1 ml of LB medium and incubate for 30 min at 37 °C.
- Divide the 1.1 ml of transformation product in two and pour each half directly into a 2 L Erlenmeyer flasks containing 0.5 L of 15N-labeled rich growth media (i.e. Spectra 9) supplemented with 0.5 g of 15N-labeled celtone base powder and 50 µg/ml of ampicillin (total culture volume of 1 L).
- Grow the bacteria for ~20 h at 37 °C.
- Recover the bacteria by centrifugation at 5,000 x g for 20 min.
- Use a 100 μl aliquot of electrocompetent E. coli JM101Tr strain. Add 2 μl of the plasmid (at 50-200 ng/μl).
- Total small RNAs extraction
- Resuspend the pellets with 10 ml of extraction buffer in a 50 ml conical tube.
- Add 10 ml of water-saturated phenol. Mix gently for 1 h at room temperature on a tube rotator.
- Centrifuge at 8,000 x g for 30 min at 20 °C. Carefully recover the aqueous phase, which corresponds to the upper phase lying above the phenol phase.
- Do a counter-extraction with the addition of 1 ml of extraction buffer to the phenol phase. Mix gently for 15 min, centrifuge at 8,000 x g for 30 min at 20 °C, and carefully recover the aqueous phase.
- Add 0.1 volume of 5 M NaCl and 2 volumes of ethanol absolute to the pooled aqueous phases of Steps B1biii and B1biv.
- Vortex vigourously and then centrifuge at 8,000 x g for 20 min at 4 °C. Discard the supernatant.
- Resuspend the pellet in 5 ml of 1 M NaCl. Centrifuge at 8,000 x g for 30 min at 4 °C.
- Recover the supernatant, and add 2.5 volume of ethanol absolute.
- Centrifuge again 30 min at 8,000 x g at 4 °C. All the small RNAs are now precipitated. Discard the supernatant.
- Resuspend the pellet in 2 ml of 2 M Tris-HCl pH 8.0 and incubate 3 h at 37 °C to completely deacylate the 3′ extremity of the tRNAs.
- Precipitate the deacylated RNAs by adding 2 volumes of ethanol absolute, vortex vigourously and centrifuge at 8,000 x g for 30 min at 4 °C.
- Dissolve the pellet in 5 ml of purification buffer C. The sample is ready for the purification step (Step B2).
- Resuspend the pellets with 10 ml of extraction buffer in a 50 ml conical tube.
- Transformation of E. coli with the tRNA expression plasmid and 15N-labeled expression
- Modified yeast tRNAPhe purification and NMR sample preparation
- Before starting the purification, wash the purification system, the loops and the columns with 0.5 M NaOH and rinse them extensively with water in order to work in RNase free conditions.
- Gel-filtration chromatography
- Load the 5 ml sample (obtained at the end of Step B1) at 0.5 ml/min on a HiLoad 26/600 Superdex 75 pg column previously equilibrated with purification buffer C.
- Elute at 0.5 ml/min, collect 3.5 ml per fraction.
- Load the 5 ml sample (obtained at the end of Step B1) at 0.5 ml/min on a HiLoad 26/600 Superdex 75 pg column previously equilibrated with purification buffer C.
- Gel-shift assay to identify the fractions containing the yeast tRNAPhe
- Design a DNA oligonucleotide sequence that is specifically complementary to the overexpressed yeast tRNAPhe and not conserved in the other E. coli tRNAs. In our hands, it works well when the complementary DNA oligonucleotide includes the anticodon arm and parts of the D- and TΨC-arms (see Figure 2A).
- In 0.2 ml-microtubes, mix 5 μl of each fraction with 1 μl of the complementary DNA oligonucleotide (stock at 100 µM), or with 1 μl of water (negative control).
- Heat the samples at 95 °C for 2 min in a dry bath incubator, and cool them down on ice.
- Add 6 μl of the 2x Laemmli loading buffer to the samples.
- Prepare a 14% SDS polyacrylamide gel: For the separating gel, mix 1.75 ml of 40% acrylamide/bis-acrylamide (19:1) solution, 1.875 ml of 1 M Tris-HCl pH 8.8, 25 μl of 20% (w/v) SDS and adjust to 5 ml with water. Add 40 μl of 10% APS and 5 μl of TEMED just before pouring the gel between the glass plates at 2/3 of the total height. For the stacking gel, mix 500 μl of 40% acrylamide/bis-acrylamide (19:1) solution, 630 μl of 1 M Tris-HCl pH 6.8, 50 μl of 20% (w/v) SDS and adjust to 5 ml with water. Add 40 μl of 10% APS and 5 μl of TEMED just before pouring the gel on top of the polymerized separating gel. Put immediately the 10-well comb.
- Load the samples on the 14% SDS polyacrylamide gel. Migrate in 1x TGS buffer at 200 V for ~1 h 30 min.
- Visualize the RNAs in the fractions with the toluidine-blue staining (as described in Step A2c). Fractions containing the tRNA of interest can be easily identified with the appearance of an additionnal band at higher molecular weight in the presence of the complementary DNA oligonucleotide (Figure 2B).
- Design a DNA oligonucleotide sequence that is specifically complementary to the overexpressed yeast tRNAPhe and not conserved in the other E. coli tRNAs. In our hands, it works well when the complementary DNA oligonucleotide includes the anticodon arm and parts of the D- and TΨC-arms (see Figure 2A).
- Ion exchange chromatography
- Pool the fractions from Step B2b. that contain the yeast tRNAPhe as identified in Step B2c.
- Load the pool on a Mono Q 10/100 GL column previously equilibrated with purification buffer C.
- Apply a gradient from 45% to 60% of buffer D over 30 CV at 2 ml/min. Collect fractions of 1.5 ml.
- Identify the fractions containing the yeast tRNAPhe with a gel-shift assay as described in Step B2c and pool the fractions of interest.
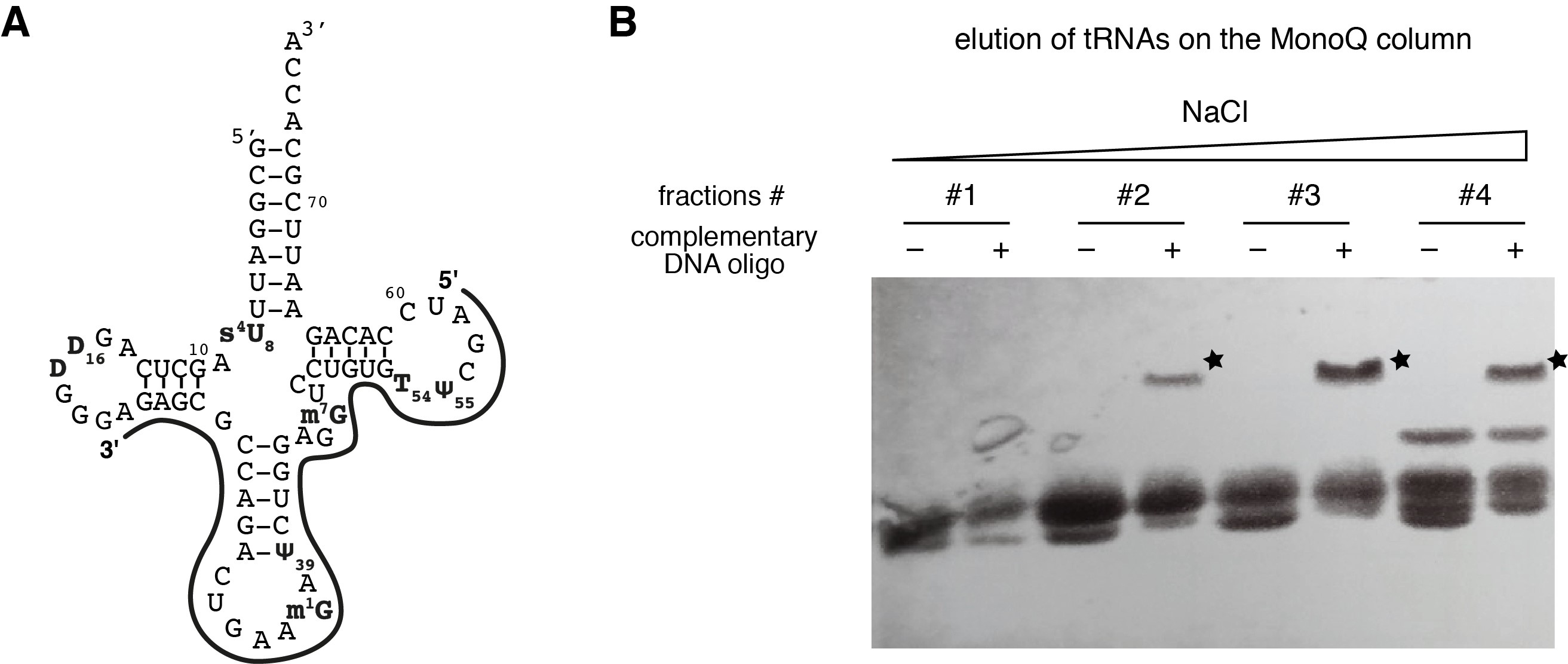
Figure 2. Gel-shift assays with a complementary DNA oligonucleotide. A. Schematic representation of the complementary DNA oligonucleotide used to identify the yeast tRNAPhe in purification fractions. The exact sequence is given in the list of Material and Reagents. B. Gel-shift assay with a 14% SDS-PAGE done with fractions (#1-#4) of the Mono Q column elution step revealed by toluidine-blue staining. Fractions containing the tRNA of interest (i.e., yeast tRNAPhe) can be easily identified with the appearance of an additionnal band at higher molecular weight in the presence of the complementary DNA oligonucleotide (labeled with a star), as compared with the control samples without the complementary DNA oligonucleotide.
- Pool the fractions from Step B2b. that contain the yeast tRNAPhe as identified in Step B2c.
- Hydrophobic interaction chromatography
- Add ammonium sulfate to the pooled fractions of Step B2d to reach a final concentration of 1.7 M. Dissolve the salt by vortexing.
- Load this sample at 0.5 ml/min on a Phenyl superose HR 10/10 column previously equilibrated with purification buffer E.
- Apply a gradient from 20% to 45% of buffer C over 15 CV at 0.5 ml/min. Collect fractions of 2.5 ml.
- Identify the fractions containing the yeast tRNAPhe with a gel-shift assay as described in Step B2c and pool the fractions of interest.
- Add ammonium sulfate to the pooled fractions of Step B2d to reach a final concentration of 1.7 M. Dissolve the salt by vortexing.
- Desalting the sample
Desalt the sample with a HiPrep 26/10 Desalting column previously equilibrated with the NMR buffer or dialyze the sample extensively against the NMR buffer. For that, one usually dialyzes the sample against a 2 L dialysis solution for at least 2 h and changes it with a fresh solution 3 times. - Sample concentration
Concentrate the modified yeast tRNAPhe sample in the NMR buffer to ~0.2-0.5 mM using Amicon 3,000 MWCO. In our hands, with a 2 L culture in 15N-labeled rich media, one usually obtains a final NMR sample of 250 μl at ~0.4 mM (i.e., 0.08-0.12 µmol). - Store the sample at -20 °C or use it directly for NMR spectra measurements (see Note 2).
- Before starting the purification, wash the purification system, the loops and the columns with 0.5 M NaOH and rinse them extensively with water in order to work in RNase free conditions.
- Modified yeast tRNAPhe recombinant expression and extraction
Notes
- The cloning step is not described in the present protocol since the approach and the protocol have been previously detailed in Dégut et al. (2016). Briefly, five DNA oligonucleotides are designed to create after a phosphorylation and a hybridization step a DNA insert which corresponds to the yeast tRNAPhe gene flanked by overhangs corresponding to EcoRI and PstI restriction sequences at the 5′- and 3′-ends, respectively (see Figure 3). The pBSTNAV vector is linearized with EcoRI and PstI and classical ligation protocol is performed. A counter-selection step with SmaI can be performed to increase the number of positive clones bearing the yeast tRNAPhe gene as described in Dégut et al. (2016). Positive clones are checked by sequencing.
Oligonucleotides for yeast tRNAPhe gene cloning in pBSTNAV:
oligo #1: 5′ AATTCGCGGATTTAGCTCAGTTGGGAGAG 3′
oligo #2: 5′ CGCCAGACTGAAGATCTGGAGGTCCTG 3′
oligo #3: 5′ TGTTCGATCCACAGAATTCGCACCACTGCA 3′
oligo #4: 5′ GTGGTGCGAATTCTGTGGATCGAACACAGGACCTCCAGA 3′
oligo #5: 5′ TCTTCAGTCTGGCGCTCTCCCAACTGAGCTAAATCCGCG 3′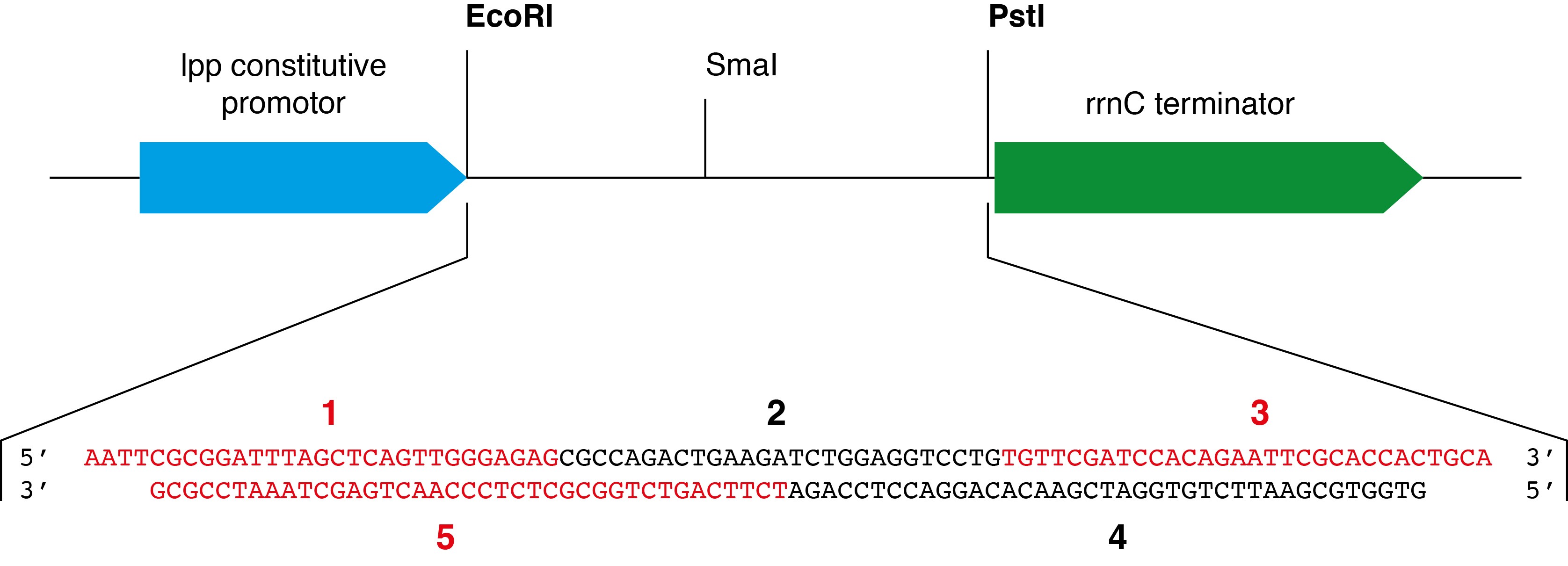
Figure 3. Schematic representation of the cloning region of pBSTNAV vector and sequence coding for the yeast tRNAPhe inserted between the EcoRI and PstI restriction sites. In pBSTNAV, the strong constitutive lpp promoter upstream of the tRNA gene leads to high levels of recombinant transcripts production. The five oligos used to generate the yeast tRNAPhe coding sequence (oligos #1-#5) are labeled on the figure and displayed with different colors for clarity. - For the yeast tRNAPhe samples produced as described in the current protocol, we measured NMR spectra on Bruker AVIII-HD 600 MHz and AVIII-HD 700 MHz (equipped with TCI 5-mm cryoprobes) with 5-mm Shigemi tubes. Measurements were conducted at 38 °C in the NMR buffer (10 mM Na-phosphate pH 6.5, 10 mM MgCl2) supplemented with 5% (v/v) D2O. Other types of NMR spectrometers and/or NMR tubes may obviously also be used.
Recipes
- For the production of unmodified yeast tRNAPhe by in vitro transcription
- 20x T7 transcription buffer (TB)
1 M Tris-HCl pH 8.04 ml
100 mM spermidine1 ml
1 M DTT0.5 ml
TritonTM X-10010 μl
H2O to10 ml
Store at -20 °C in 1 ml aliquots for up to 1 year - 2x RNA loading dye
8 M Urea
0.01% (w/v) bromophenol blue
0.01% (w/v) xylene cyanol
store at -20 °C in 1 ml aliquots - Toluidine-blue staining solution
Toluidine blue0.5 g
Ethanol200 ml
Concentrated acetic acid5 ml
H2O to500 ml
store at room temperature - Purification and NMR buffers
Buffer A
25 mM Na-phosphate pH 6.5
50 mM NaCl
5 mM MgSO4
0.22 μm filtered
Buffer B
25 mM Na-phosphate pH 6.5
1 M NaCl
0.22 μm filtered
NMR buffer
10 mM Na-phosphate pH 6.5
10 mM MgCl2
- 20x T7 transcription buffer (TB)
- For the production of modified yeast tRNAPhe by in vivo recombinant expression in E. coli
- Extraction buffer
1 mM Tris-HCl pH 7.4
10 mM Mg-acetate - 2x Laemmli loading buffer
125 mM Tris-HCl pH 6.8
20% (v/v) glycerol
4% (w/v) SDS
10% (v/v) 2-mercaptoethanol
0.004% (w/v) bromophenol blue - Purification and NMR buffers
Buffer C
25 mM K-phosphate pH 6.5
0.22 μm filtered
Buffer D
25 mM K-phosphate pH 6.5
1 M NaCl
0.22 μm filtered
Buffer E
25 mM K-phosphate pH 6.5
1.7 M (NH4)2SO4
0.22 μm filtered
NMR buffer
10 mM Na-phosphate pH 6.5
10 mM MgCl2
- Extraction buffer
Acknowledgments
This work was supported by the ANR NMR-VitAmin (ANR-14-CE09-0012), the CNRS, the Université de Paris, and the Labex DYNAMO (ANR-11-LABX-0011).
Competing interests
The authors declare no competing interest.
References
- Boccaletto, P., Machnicka, M. A., Purta, E., Piatkowski, P., Baginski, B., Wirecki, T. K., de Crecy-Lagard, V., Ross, R., Limbach, P. A., Kotter, A., Helm, M. and Bujnicki, J. M. (2018). MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 46(D1): D303-D307.
- El Yacoubi, B., Bailly, M. and de Crécy-Lagard, V. (2012). Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu Rev Genet 46: 69-95.
- Jackman, J. E. and Alfonzo, J. D. (2013). Transfer RNA modifications: nature's combinatorial chemistry playground. Wiley Interdiscip Rev RNA 4(1): 35-48.
- Barraud, P. and Tisné, C. (2019). To be or not to be modified: Miscellaneous aspects influencing nucleotide modifications in tRNAs. IUBMB Life 71(8): 1126-1140.
- Barraud, P., Gato, A., Heiss, M., Catala, M., Kellner, S. and Tisné, C. (2019). Time-resolved NMR monitoring of tRNA maturation. Nat Commun 10(1): 3373.
- Rio, D. C. (2013). Expression and purification of active recombinant T7 RNA polymerase from E. coli. Cold Spring Harb Protoc 2013(11).
- Dégut, C., Monod, A., Brachet, F., Crépin, T. and Tisné, C. (2016). In vitro/in vivo production of tRNA for X-Ray studies. Methods Mol Biol 1320: 37-57.
- Mechulam, Y., Blanquet, S. and Fayat, G. (1987). Dual level control of the Escherichia coli pheST-himA operon expression. tRNA(Phe)-dependent attenuation and transcriptional operator-repressor control by himA and the SOS network. J Mol Biol 197(3): 453-470.
Article Information
Copyright
© 2020 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Catala, M., Gato, A., Tisné, C. and Barraud, P. (2020). Preparation of Yeast tRNA Sample for NMR Spectroscopy. Bio-protocol 10(12): e3646. DOI: 10.21769/BioProtoc.3646.
Category
Biophysics > NMR spectroscopy > in vivo NMR spectroscopy
Biochemistry > RNA > RNA structure
Molecular Biology > RNA > RNA purification > Affinity purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









