- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Construction of NSG-CTL Mice
Published: Vol 3, Iss 4, Feb 20, 2013 DOI: 10.21769/BioProtoc.329 Views: 14614

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

PBMC-Humanized Mouse Model for Multiple Sclerosis: Studying Immune Changes and CNS Involvement
Anastasia Dagkonaki [...] Lesley Probert
May 20, 2025 3991 Views

Isolation and Imaging of Microvessels From Brain Tissue
Josephine K. Buff [...] Sophia M. Shi
Aug 5, 2025 2636 Views

Novel Experimental Approach to Investigate Immune Control of Vascular Function: Co-culture of Murine Aortas With T Lymphocytes or Macrophages
Taylor C. Kress [...] Eric J. Belin de Chantemèle
Sep 5, 2025 3550 Views
Abstract
The NSG-CTL mouse model is a humanized mouse model that allows the generation of peripheral human immune responses, particularly CD8+ Cytotoxic T lymphocyte (CTL) responses, and serves as an effective model for studying gene-based therapies. Natural antigen-specific T cell responses in humanized mice are relatively weak and this model was developed to boost antigen specific responses, in this case to HIV, to more closely assess these responses in vivo. We have engineered human T cells that develop in these mice to express a molecularly cloned T cell receptor (TCR) specific to HIV. Cloned TCRs to any antigen can theoretically be used to study specific responses in vivo, as long as the tissue that is manipulated is of the same human leukocyte antigen (HLA) type. The modification of hematopoietic stem cells with antigen specific TCRs allows the development of mature, functional T cells in the periphery of these mice that are specific to that antigen following normal developmental processes. This model has recently been published (Kitchen et al., 2012) and this was a major modification of the Humanized Mouse BLT model published by Melkus et al. (2006). We use Non-obese diabetic (NOD)-Severe Combined Immunodeficient (SCID), common Gamma chain knockout (γc-/-)—or NSG—mice, and implant fetal thymus pieces along with genetically modified CD34+ hematopoietic stem cells (HSCs), isolated from fetal liver, under the kidney capsule to develop into a functional thymic implant. At the same time, we deplete the mouse’s bone marrow by total body irradiation and inject more modified HSCs intravenously for hematopoietic engraftment in the mouse bone marrow. This protocol outlines the procedures to process the fetal tissue, genetically transduce the HSCs using lentiviral vectors expressing a molecularly cloned T cell receptor, and perform the subcapsular kidney implant surgery.
Processing Fetal Thymus and Isolating CD34+ Cells from Fetal Liver
Materials and Reagents
- Fetal thymus/liver pair from 18 – 22 week gestation specimen
- Dulbecco's phosphate buffered saline (PBS) (Life Technologies, catalog number: 14190 )
- 1x Iscove's Modified Dulbecco's Medium (IMDM) (Life Technologies, catalog number: 12440 )
- RPMI Medium 1640 – L-Glutamine (Life Technologies, catalog number: 11875 )
- Number 11 scalpels
- Collagenase type IV, (Life Technologies, InvitrogenTM, catalog number: 17104-019 ) 50,000 U/ml in PBS diluted 1:10 in ddH2O
- Hyaluronidase (240,000 U/ml in 1:10 PBS) (Sigma-Aldrich, catalog number: H6254 )
- DNase I (30,000 U/ml in 1:10 PBS) (Worthington, catalog number: 2006)
- Pip/Tazo (Zosyn) – 45 mg/ml is 100x stock
- Ficoll-Paque PLUS (GE Healthcare, catalog number: 17-1440 )
- Fetal bovine serum (FBS) (Omega Scientific, catalog number: FB-12 )
- CD34 MicroBead Kit, human (Miltenyi Biotec, catalog number: 130-046-702/3 )
Equipment
- Class II biosafety cabinet
- 37 °C incubator
- Shaker
- Centrifuge
- 100 mm Petri dishes
- 100 μm cell strainer
- 10 ml syringes
- 16 gauge blunt end needle
- 0.22 μm filter
- 50 ml conical tubes
- 15 ml conical tubes
- T25 and T150 flask
Procedure
- An overview of the procedure is noted in Figure 1.
Note: all steps to be performed in a Class II biosafety cabinet or in a sterile, HEPA-filtered environment.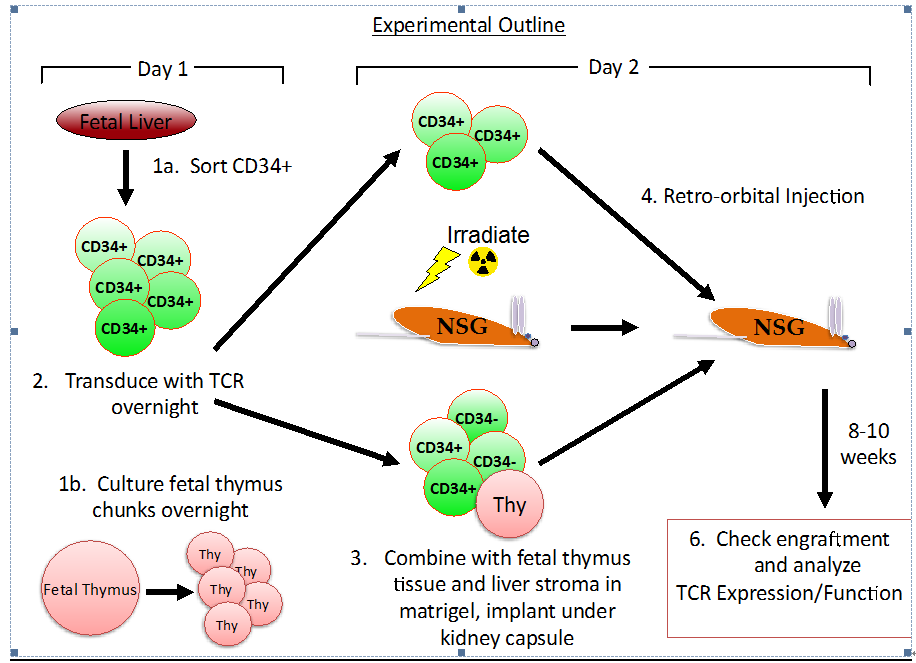
Figure 1.
- Wash the thymus in a 15 ml conical tube: Pour off the medium into a beaker of bleach, using a scalpel to prevent the thymus from sliding into the bleach. Fill the tube to 15 ml with PBS. Cap and invert several times to wash the thymus. Decant supernatant into the bleach. Repeat 3-4 times.
- Add 7 ml RPMI + 10% FBS and decant everything into a 100 mm Petri dish.
- With two scalpels, cut the thymus into small (~1 mm2) pieces. If you need to phenotype/ genotype your tissue, such as for HLA type*, remove some media containing free-floating thymocytes at this point for analysis. *HLA typing can be performed at low resolution by flow cytometry [we typically identify HLA-A*02+ Tissue), and genotyping for high resolution identification (i.e. HLA-A*0201) is performed by a clinical immunogenetics laboratory or by a PCR based analysis kit (Life Technologies, InvitrogenTM, catalog number: 470214D)].
- Pipette media and thymus pieces into a T25 flask. Supplement media with 450 μg/ml Pip/Tazo and gently rock the flask back and forth to mix. Culture overnight at 37 °C. This step is important in preventing bacterial contamination of tissue to be implanted into mice.
- Wash the liver in a 50 ml conical tube: Pour off the medium into a beaker of bleach, using a scalpel to prevent the liver from sliding into the bleach. Fill the tube with PBS. Cap and invert several times to wash the liver. Decant supernatant into bleach. Repeat 3-4 times.
- Add 10 ml IMDM and decant everything into a 100 mm Petri dish.
- With two scalpels, cut the liver into small (~3 mm2) pieces. If you find white connective tissue, scrape the red liver tissue from it and discard it in the bleach.
- Homogenize the tissue: suck the liver pieces and media into a 10 ml syringe fitted with a 16 gauge blunt needle, and transfer to a 50 ml conical tube. Suck up into syringe and expel one more time to fully homogenize tissue.
- Prepare enzymes and Pip/Tazo: Add 200 μl each of the Collagenase, Hyaluronidase, DNase, and Pip/Tazo to 10 ml IMDM in a new tube.
- Draw enzyme/media mixture into a 10 ml syringe, fit it with a 0.22 μm filter, and filter it directly into the cell suspension.
- Cap the suspension tube and seal with Parafilm on the outside to prevent leaks. Rotate or gently shake by machine at 37 °C for 90 min.
- Pipet the digested cell suspension through a 100 μm cell strainer into a fresh 50 ml tube.
- Add PBS to the suspension to bring the volume up to 50 ml. Split this into two tubes of 25 ml.
- Underlay the cells with 10 ml Ficoll-Paque per tube by dispensing the Ficoll very slowly with a 10 ml pipet at the bottom of the cell suspension. Spin at 950 x g for 20 min at 21 °C without brake.
- The interface should be thick. Suck it out with a 5 ml pipet and transfer to another 50 ml tube. Then, equally split interface between 250 ml tubes.
- You should have two tubes of interface. Bring the volume of each up to 50 ml with PBS. Spin at 240 x g at 21 °C for 10 min. Aspirate supernatant carefully.
- Combine the two pellets as you wash three more times with 50 ml PBS containing 2% FBS.
- Resuspend the pellet in 50 ml RPMI + 10% FBS. Count your cells.
- Optional: Culture cells in a T150 flask or two at a concentration of 10 X 106 c/ml for 1 – 2 h to let adherent cells adhere. Supplement media with 450 μg/ml Pip/Tazo. Count cells after this incubation.
- Sort CD34+ cells using Miltenyi Biotec's CD34 MicroBead Kit, human, according to the manufacturer's protocol. Count cells. Culture the CD34+ cells at a concentration between 1-10 x 106 c/ml in RPMI + 10% FBS at room temperature until you are ready to transduce them.
- Culture the CD34- fraction overnight at 37 °C at a concentration of 10 x 106 c/ml in RPMI + 10% FBS supplemented with 450 μg/ml Pip/Tazo.
Transducing CD34+ Cells
Materials and Reagents
- RetroNectin (Clontech, catalog number: T100A/B ) 20 μg/ml in PBS (aliquot and freeze 1 mg/ml stock at -20 °C)
- Dulbecco's phosphate buffered saline (PBS) (Life Technologies, catalog number: 14190 )
- FACS Buffer (4% FBS in PBS)
- Infection medium – 2% Human Serum Albumin (American Red Cross, catalog number: NDC 52769-451-05) in Yseel's Serum-Free T-Cell Medium (Gemini, catalog number: 400-102 ) (Optional: Supplement with 50 ng/ml human MGDF, SCF, and Flt3-ligand)
- Cytokine medium – RPMI with 10% FBS, supplemented with 100 ng/ml human IL-3, IL-6, SCF
- Lentiviral vector, titered
- Cell Scraper
Equipment
- Biosafety cabinet
- 37 °C incubator
- Centrifuge
- 6-well Plate, Non-Treated (BD Biosciences, Falcon®, catalog number: 351146 )
Procedure
- Calculate the number of transduction wells required of a 6 well-tissue culture plate (1 well can handle no more than 8 X 106 cells). Coat the needed number of wells of a non-treated 6-well plate with RetroNectin: Dispense 1.25 ml of RetroNectin solution (20 μg/ml in PBS) into each well and allow the covered plate to stand for 2 h at room temperature in a clean biosafety cabinet.
- Remove RetroNectin solution from wells.
- Add 1.25 ml of FACS buffer to each well for blocking. Allow the plate to stand at room temperature for 30 min.
- Remove FACS Buffer and wash wells once with PBS.
- Keep PBS in the coated wells until the plate is ready for use. You can store the plate at 4 °C. Remove PBS from the wells immediately before plating cells.
- For transduction efficiency controls, aliquot ~1 x 105 untransduced CD34+ cells and culture in 200 μl Cytokine Medium in 96-well plate for 5-7 days at 37 °C.
- Plate remaining CD34+ cells in Infection Medium in RetroNectin-coated wells (~2 x 106 cells/ml) and incubate at 37 °C for one hour (see Alternate method in Step 9 below).
- Add your vector to the wells at a multiplicity of infection (MOI) between 2–10. Gently mix and incubate overnight at 37 °C.
- Alternate method (for lower-efficiency vectors): Add infection medium and vector to wells first, and spin at 3,000 x g for 90-120 min at 12 °C, then add cells in medium on top of this, gently swirl to mix, and incubate overnight at 37 °C.
- The following morning, harvest the cells: Gently scrape the bottoms of the wells, collect cells, and count.
- Aliquot ~1 x 105 cells from each condition and culture in 200 μl cytokine medium in 96-well plate for 5-7 days at 37 °C. After 5-7 days, check transduction efficiency by flow cytometry, looking at your reporter, for instance, using the previously cultured, untransduced aliquot (see step 6) as your control.
- For each mouse you will transplant, you will need 0.5 x 106 transduced CD34+ cells per implant, and 0.5 x 106 transduced CD34+ cells for injection.
- For the implanted cells, combine 0.5 x 106 transduced CD34+ cells per mouse with 4.5 x 106 CD34- cells per mouse, aliquot into sterile 1.5 ml screw-cap tubes. Spin the cells to pellet them, aspirate supernatant, spin them again and aspirate any remaining supernatant, and keep the dry pellets on ice.
- For the injected cells, spin 0.5 x 106 transduced CD34+ cells per mouse to pellet them, aspirate supernatant, and resuspend them in plain RPMI, 150 μl per mouse. Keep these on ice.
Tissue Transplants to Construct NSG-CTL Mice
Materials and Reagents
- Fetal thymus pieces
- CD34+ cells for implantation and injection
- NSG Mice, at least 6–8 weeks old
- Matrigel, High Concentration (BD Biosciences, catalog number: 354248 )
- Ketamine (Ketaject (Phoenix Pharmaceuticals, catalog number: NDC 57319-542-02), 100 mg/ml) / Xylazine (AnaSed Injection (Lloyd Laboratories, catalog number: NADA 139-236 ), 100 mg/ml), 2.5% each in PBS
- Isoflurane
- Betadine
- Sterile saline
- Carprofen (Rimadyl (Pfizer, catalog number: NADA 141-199 )) diluted 1:100 in sterile saline (keep dilution at 4 °C for up to 24 h)
- PBS
Equipment
- Flow Cytometer
- 15 ml conical tubes
- Sterile gauze pads
- 60 mm dish
- 15 ml conical tube
- Positive Displacement Pipette (1-20 μl) and Tips
- Oster clipper with No. 40 blade
- 60 mm Petri dish
- Isopropanol wipes
- 16 gauge cancer implant needle with a round-filed tip (cannula and trocar)
- Surgical tools: Needle-tipped forceps, curved forceps, scissors, hemostat, autoclip applier and wound clips
- Vicryl sutures, 4-0 (Owens & Minor, catalog number: 23000J304H )
- Insulin syringes
- 1 ml syringe
Procedure
- Up to 24 h prior to the surgery, perform total body irradiation on the mice to be used with a dose of 2.7 Gy.
- Pour thymus pieces and medium from the flask into a 60 mm dish. Pour some PBS into another dish.
- Fill a 1 ml syringe (no needle) with PBS.
- Chill positive displacement pipette tips by putting them in open sterile tubes in ice. Keep on ice with the dried cell pellets and Matrigel. It is important to keep the Matrigel and any tubes or tips that will touch it cold at all times until the implant needle is loaded.
- Anesthetize mice: Weigh mice and inject them interperitoneally with 15 μl of Ketamine/Xylazine per gram of body weight.
- When the mice have become sluggish from the Ketamine/Xylazine, place an animal's nose in a 15 ml conical tube that is stuffed with gauze soaked in isoflurane until the animal loses consciousness, its breathing slows to about half the normal rate, and it no longer responds to a paw pinch. Keep this tube on hand during the surgery to administer more isoflurane if the breathing starts to become more rapid.
- Punch the animal's ears for numbering or use your own preferred identification method.
- Using the Oster clipper with No. 40 blade, shave the left side of each mouse from hip to shoulder between the center of the back and the belly.
- Subcutaneously inject 0.3 ml of the diluted Carprofen up by the animal's shoulder, then place the mouse on a sterile dressing on its right side, facing left.
- Flush the cannula of the 16 gauge implant needle with PBS.
- Using a pair of needle-tipped forceps, place a piece of thymus from the dish into the opening of the cannula with the trocar just inside the opening, then pull back on the trocar to aspirate the tissue into the cannula.
- Use a positive displacement pipette and a chilled tip to put 5 μl of cold Matrigel into one tube with a dried cell pellet and gently stir just enough to get the cells into suspension. Do not pipette up and down. Pipette the Matrigel/cell suspension into the opening of the cannula as you slowly pull back on the trocar to load the needle. This is much easier with a helper to pipette while you manipulate the implant needle.
- Swab the bare skin of the mouse with Betadine, then wipe down the area with an isopropanol wipe two times.
- Determine the darkest spot under the skin, which indicates the location of the spleen. The kidney is approximately 5 mm dorsal to the spleen.
- Lift up the skin with curved forceps and make an incision about 15 mm long in the skin over the kidney parallel to the spleen. Make a similar cut in the peritoneum layer below.
- In male mice, the kidney should be visible, and can be extruded simply by pressing on the abdomen. You can support the kidney with a hemostat.
- In female mice, the ovary blocks the kidney. Using a hemostat, pick up the ovary and drag out the kidney.
- Use the needle-tipped forceps to pluck a tiny hole (1-2 mm) at the posterior end of the kidney capsule.
- Slide the implant needle into this hole and along the kidney until the opening of the cannula is completely covered by the kidney capsule.
- Gently extrude the cells and tissue under the kidney capsule, and pull the needle back out. The thymus pieces can be sticky, so you can use your curved forceps to make sure the thymus piece does not come out with the needle.
- Lift up the peritoneum with the forceps and gently use the hemostat to push the kidney back into place.
- Tie one stitch in the peritoneum with a double knot.
- Use two autoclips to close the skin.
- Mix the transduced CD34+ cells saved for injection and suck 150 μl (0.5 x 106 cells) up into an insulin syringe. Inject these cells into the mouse through retroorbital vein injection.
- Place one drop of PBS onto each eye and lay the mouse on its side back in a cage.
- When all the mice have been implanted, confirm that the animals are regaining consciousness before leaving them.
- Post-operational care: The day after the surgery, subcutaneously inject 0.3 ml of diluted Carprofen and 1.2 ml of sterile saline separately into each mouse. On days 2 and 3 following surgery, subcutaneously inject 1.5 ml of sterile saline into each mouse. Monitor the mice and the incisions for 10 days following surgery. Remove the autoclips and weigh the mice after 10 days.
- After 8-10 weeks, check engraftment by bleeding the mice and performing FACS analysis on the peripheral blood, staining for markers such as CD45, CD3, CD4, CD8, and any genes your vector should express. A typical flow cytometry profile, staining for human CD3 (T cell marker) and a transgenic, HIV specific TCR, is provided in Figure 2.
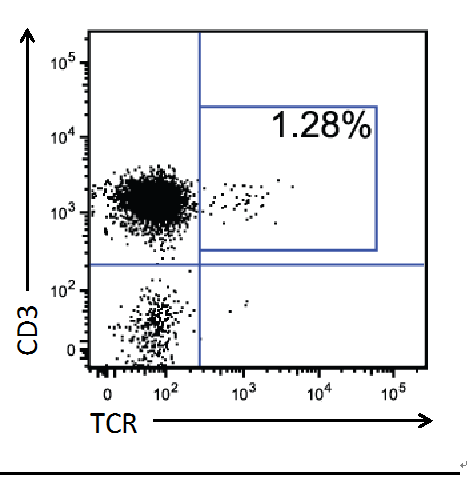
Figure 2. Sample of TCR + Cells in the Peripheral Blood
- For a visual reference on a similar type of transplantation procedure, please see Vatakis et al., 2012 in the Journal of Visualized Experiments
(http://www.jove.com/video/4181/using-blt-humanized-mouse-as-stem-cell-based-gene-therapy-tumor).
Variation
- Transduced CD34+ cells can be viably frozen instead of injected post-surgery. In this case, do not irradiate the mice prior to surgery. Instead, wait 4-6 weeks, thaw the cells, irradiate the mice (2.70 Gy), and inject the cells retro-orbitally, 0.5 x 106 cells/mouse. Post-operational care for these mice will be 0.3 ml Carprofen on the first day after surgery only. No saline injections will be required.
Acknowledgments
This work was funded by grants from the NIH (number R01AI078806 (S.G.K), the California HIV/AIDS Research Program (CHRP) (number 163893) (S.G.K.), the UC Multicampus Research Program and Initiatives from the California Center for Antiviral Drug Discovery (number MRPI-143226), and from funding by the UCLA Center for AIDS Research (CFAR) (number P30 AI28697).
References
- Kitchen, S. G., B. R. Levin, G. Bristol, V. Rezek, S. Kim, C. Aguilera-Sandoval, A. Balamurugan, O. O. Yang and J. A. Zack (2012). In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog 8(4): e1002649.
- Melkus, M. W., J. D. Estes, A. Padgett-Thomas, J. Gatlin, P. W. Denton, F. A. Othieno, A. K. Wege, A. T. Haase and J. V. Garcia (2006). Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med 12(11): 1316-1322.
- Vatakis, D. N., Bristol, G. C., Kim, S. G., Levin, B., Liu, W., Radu, C. G., Kitchen, S. G. and Zack, J. A. (2012). Using the BLT humanized mouse as a stem cell based gene therapy tumor model. J Vis Exp (70): e4181.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Levin, B., Bristol, G., Galic, Z. and Kitchen, S. G. (2013). Construction of NSG-CTL Mice. Bio-protocol 3(4): e329. DOI: 10.21769/BioProtoc.329.
- Kitchen, S. G., B. R. Levin, G. Bristol, V. Rezek, S. Kim, C. Aguilera-Sandoval, A. Balamurugan, O. O. Yang and J. A. Zack (2012). In vivo suppression of HIV by antigen specific T cells derived from engineered hematopoietic stem cells. PLoS Pathog 8(4): e1002649.
Category
Immunology > Immune cell function > Lymphocyte
Immunology > Animal model > Mouse
Cell Biology > Tissue analysis > Tissue isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link