- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Formalin Murine Model of Pain
Published: Vol 7, Iss 23, Dec 5, 2017 DOI: 10.21769/BioProtoc.2628 Views: 13022
Reviewed by: Xi FengJingang HuangAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Protocol for Measuring Free (Low-stress) Exploration in Rats
Wojciech Pisula and Klaudia Modlinska
Jan 20, 2020 4452 Views

Operant Vapor Self-administration in Mice
Renata C. N. Marchette [...] Khaled Moussawi
May 20, 2021 4553 Views

Construction of Activity-based Anorexia Mouse Models
Maria Consolata Miletta and Tamas L. Horvath
Aug 5, 2023 1757 Views
Abstract
Pain research is mostly based on experimental assays that use animal models, which may allow deciphering the physiopathology of this condition and to propel drug discovery. The formalin nociception test is considered one of the most predictive approaches to study acute pain in rodents. This test permits monitoring pain-related responses (i.e., itch) caused by a subcutaneous injection of an inflammatory agent, namely 2.5% formalin solution, in the hind paw. After the injection, two distinct time periods or phases of licking/biting behaviour occur, which are separated by a quiescent period. Importantly, these phases differ in duration and underlying mechanisms. Hence, the initial acute phase (phase I), commonly recorded for 5 min just after formalin administration, reflects acute peripheral pain, probably due to direct activation of nociceptors through TRPA1 channels. On the other hand, the phase II, which starts after the quiescent period (5-15 min) and is commonly recorded for 15-30 min, is due to the ongoing inflammatory input and central nociceptive sensitization. Here, we describe in detail the protocol used to perform a reliable and reproducible formalin test in mice.
Keywords: PainBackground
The formalin test is an experimental assay that permits determining mice nocifensive behaviour. Thus, the mice response (i.e., licking and flinching) is assessed after a subcutaneous injection of formalin normally in the plantar hind paw (Tjolsen et al., 1992). The test was originally described in the late seventies (Dubuisson and Dennis, 1977), and, initially, it consisted of the injection of 50 µl of 5% formalin in the dorsal surface of one forepaw of a rat or a cat. Since then, the formalin test has been extensively used to assess nociception and inflammation-related responses, thus being adapted according to each study’s aim. Mice and rats are frequently used due to their innate grooming behaviour (i.e., forepaw licking). Accordingly, a 2.5% formalin (in physiological saline solution) injected in the mid-plantar surface of the rodent hind paw is commonly used to induce nociceptive responses (i.e., paw ‘flinching’ and licking) lasting for 45-90 min (Tjolsen et al., 1992).
The formalin test is considered a very powerful tool in preclinical research to develop novel analgesic drugs (Mogil, 2009). The main advantage of the test consists of the very objective quantification of the pain-associated behaviour in response to the noxious stimuli (i.e., formalin). Furthermore, the formalin test allows the study of two different kinds of pain typologies: i) acute peripheral pain mediated by the direct activation of nociceptors through TRPA1 channels, and ii) inflammatory and central nociceptive sensitization. Indeed, the existence of these two phases lead to the possibility of studying different analgesic drugs, including NSAIDs and other mild analgesics, and, therefore, may allow deciphering the drug mechanism of action along the pain neuraxis.
Overall, the formalin test may be considered an easy-going experimental approach to ascertain the analgesic activity of any named drug. Here, we provide a complete description of the formalin test in order to facilitate its implementation by other scientists.
Materials and Reagents
- Syringes (BD Micro-FineTM Demi, U-100 Insulin, 30 G x ½’’–0.33 x 8 mm) (BD, catalog number: 324826 ) (Figure 1d)
- Eppendorf tubes (Figure 1b)
- Animals: Adult male CD-1 mice (Charles River Laboratories, L’Arbresle, France; RRID: MGI: 3785721) with 8 weeks of age and weighing 20-25 g were used
Note: Animals were housed in standard cages with free access to food and water, and were maintained under controlled standard conditions (12 h dark/light cycles starting at 7:30 AM, 22 °C temperature, and 66% humidity). All manipulations were carried out between 9:00 and 16:00 h. Procedures in this study were performed in accordance with relevant guidance from the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23), the Guide for the Care and Use of Laboratory Animals (Clark et al., 1997) and European Union directives (2010/63/EU). The ethics committees of the relevant institutions (CEUA/UFSC and CEEA/UB) approved the protocol. Efforts were made to minimize suffering and reduce the number of animals used in the experiments. To reach statistical significance, at least 10 mice per group are highly recommended. - 70% ethanol
- Formalin (A 36.5-38% formaldehyde solution in H2O) (Sigma-Aldrich, catalog number: F8775-500ML )
Note: It was used to prepare the final 2.5% formalin in physiological saline solution. - Analgesic drug to be tested
Note: In the example provided here we used raseglurant, a negative allosteric modulator of the metabotropic glutamate type 5 (mGlu5) receptor (Font et al., 2017). - Vehicle. Sterile physiological saline (0.9% NaCl) solution (Grifols, catalog number: 605137 )
- Sterile cleaning solution
Note: A 70% ethanol in H2O was used. - 2.5% formalin solution (see Recipes)
Equipment
- Observational chamber
Circular (25 cm ø x 30 cm height) glass container (i.e., glass beaker) (Figure 1a). The glass container should be cleaned with 70% ethanol first and then with sterile water to remove any animal/ethanol odour. Air dry for 5 min before testing another animal. No access to food or water should be allowed
Note: A recording system (i.e., camera) could be optionally used. Thus, place the recording system behind, below and/or above the observational chamber. Ensure that the hind paw of the animal is clearly visible from all angles of observation, thus a set of mirrors surrounding the observational chamber could be used for this purpose. - Weighing machine (Figure 1c)
- Coloured surface (Figure 1e)
- Restrain apparatus for mice (Figure 1f)
Note: Alternatively, a 50-ml Falcon tube could also be used. - PIPETMAN ClassicTM Pipets (Gilson, model: P20, P200, and P1000, catalog numbers: F123600 , F123601 and F123602 , respectively)
- Timer (Figure 1g) and chronometer (Figure 1h)
- Personal protective equipment (Figure 1i) (Laboratory coat, gloves, masks, etc.)
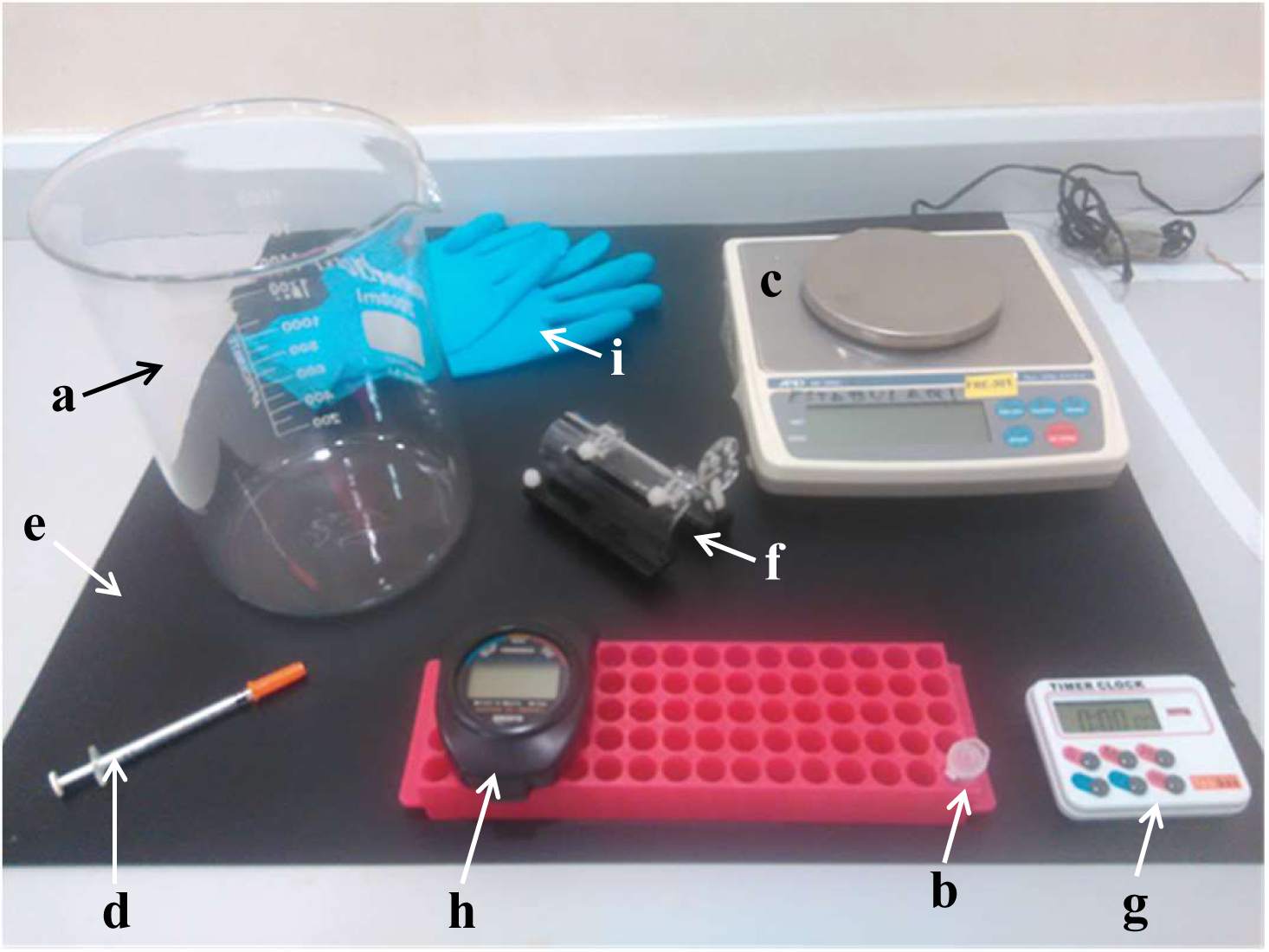
Figure 1. Material needed for the formalin test. Glass container (a), freshly prepared 2.5% formalin solution (b), balance (c), insulin syringe (d), black plastic surface (e), restrain apparatus for mice (f), timer (g), chronometer (h) and gloves (i).
Procedure
- Habituation
One day before the experiment, the animals to be tested are individually exposed to the observational chamber (Figure 1a) for 1 h. - Reagents preparation
The day of the experiment, prepare the 2.5% formalin solution (see Recipes) (Figure 1b) and the drug to be tested (not shown). Clean all the material needed. - Weighing animals
Remove the mouse from the house cage and measure its body weight using a weighing machine (Figure 1c). - Administration of the analgesic drug
Administer (i.e., intraperitoneally, i.p.) the analgesic drug to be tested (i.e., raseglurant; Figure 2) or the same volume of vehicle (physiological saline solution) using an insulin syringe (Figure 1d).
Note: The amount (400-500 μl) of drug to be administered should be adjusted according to the dose to be tested (i.e., 10 mg/kg of raseglurant) and the mouse body weight (20-25 g). For example, for a mouse of 20 g of weight we will administer (i.p.) 400 μl of a 500 mg/L raseglurant solution in saline. In addition, all animal experimentation should be carried out by a researcher blind to drug treatments. - Acclimation
The animal is placed in the observational chamber for 20 min before recording formalin administration (Figure 2).
Note: A coloured plastic surface (i.e., black) could be placed below the observational chamber for contrasting the animal’s hair (i.e., CD-1 mice have white hair) (Figure 1e).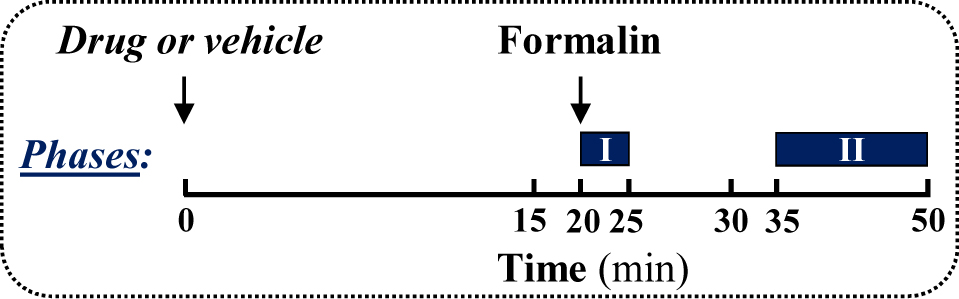 Figure 2. Treatment schedule depicting the administration regimen of drug, formalin and the behavioural testing (phase I and II)
Figure 2. Treatment schedule depicting the administration regimen of drug, formalin and the behavioural testing (phase I and II) - Formalin loading
Load 20 µl of 2.5% formalin solution directly in the 0.3 ml insulin syringe equipped with a 30 G needle.
Note: Optionally, the 20 µl of 2.5% formalin solution could be taken with a 2-20 µl micropipette, placed in a 100 µl Eppendorf tube and then load the insulin syringe with. - Formalin administration
Take the animal from the observational cage and place it carefully inside the restrain module (Figure 1f) with the selected (i.e., right) hind paw exposed outside (Figure 3A). Inject slowly the 20 µl of 2.5% formalin solution using the insulin syringe in the mid-plantar surface of the hind paw (Figure 3B).
Notes:- The needle should be placed in between toes and ankle and inserted beneath the surface of the skin, thus any tissue damage should be avoided.
- The injection should be done firmly and time-optimized. Animals could perceive the restrain as stressor stimulus and that can increase the variability of the results.
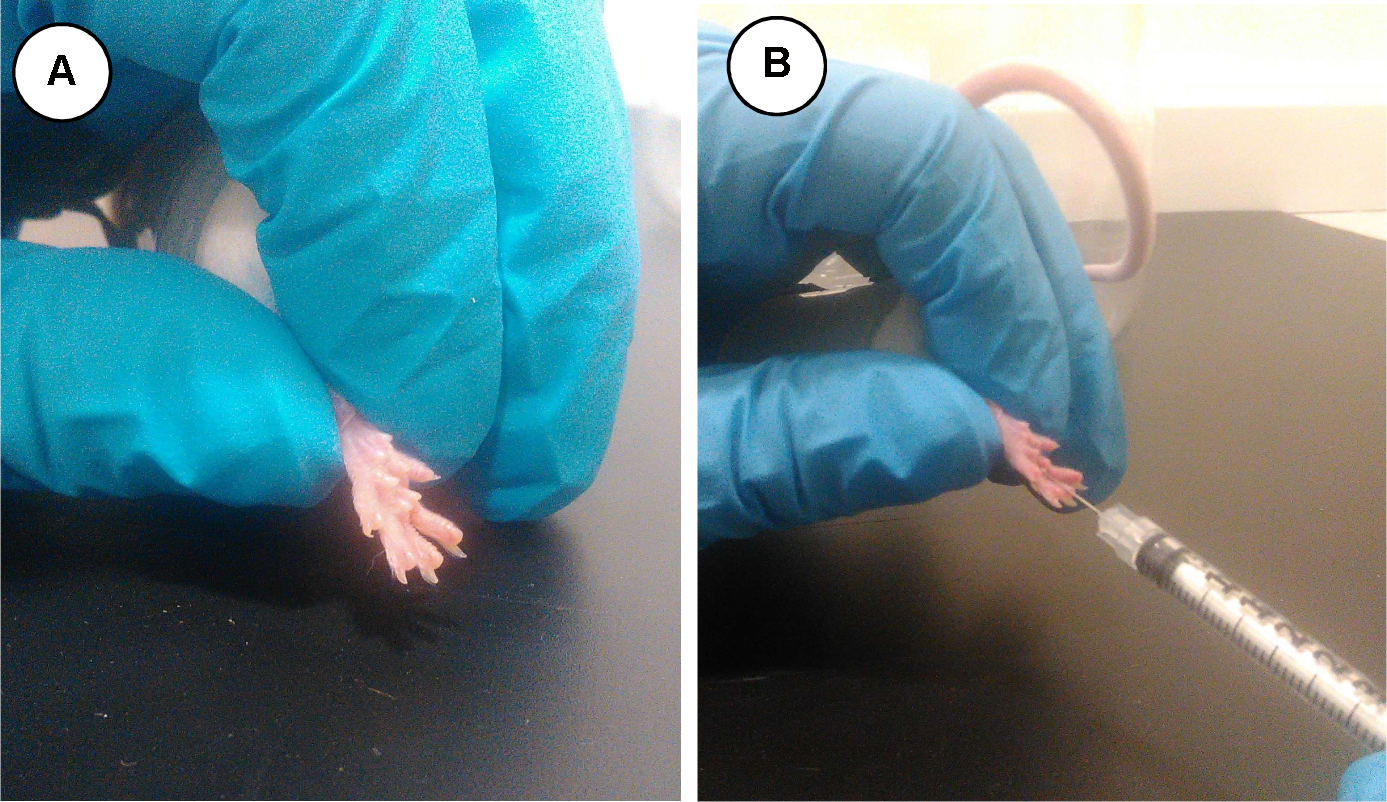
Figure 3. Hind paw of a restrained mouse (A) and administration of formalin solution (B)
- The needle should be placed in between toes and ankle and inserted beneath the surface of the skin, thus any tissue damage should be avoided.
- Phase I (0-5 min)
Return the injected animal to its observational chamber. Set the timer for 5 min and start it to mark the beginning of the phase I (Figure 2). Use a chronometer to visually measure the total time the animal spends licking or biting the injected paw during this period (5 min) (Video 1). Annotate the result.
Notes:- If a recording system is used, switch on and off the system accordingly.
- We strongly recommend the investigator to perform some training at this step to familiarize themselves with the licking/biting behavior.
- If a recording system is used, switch on and off the system accordingly.
- Quiescent period (5-15 min)
Stop the timer and leave the animal without disturbing it for 10 min (Figure 2). - Phase II (15-30 min)
Set the timer for 15 min and start it to mark the beginning of the phase II (Figure 2). Use a chronometer to visually measure the total time the animal spends licking or biting the injected paw during this period (15 min) (Video 1). Annotate the result. - Finishing
Return the animal to its home cage.
Note: If a recording system is used switch off the camera. Check whether the animal needs any special attention or health care (i.e., bleeding, extra tissue damage, etc.). - Cleaning
Clean all the material used.
Note: Repeat the experiment with another animal if required. If a camera is used, visualize the videos and measure the total time the animal spends licking or biting the injected paw during each period (phase I and II) (Video 1).Video 1. Video showing formalin-induced hind paw licking in mouse
Data analysis
The antinociception induced by drug treatment in the formalin test can be calculated by the following formula:![]()
where, LTV and LTD represent the licking/biting time in the vehicle- and drug-treated animals, respectively.
The antinociceptive effect is expressed as a percentage (mean ± SEM) of the maximum effect observed (Figure 4). The results are analysed either by Student’s t-test or by one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test using vehicle-treated animals as a control (Figure 4). Statistical significance was set as P < 0.05.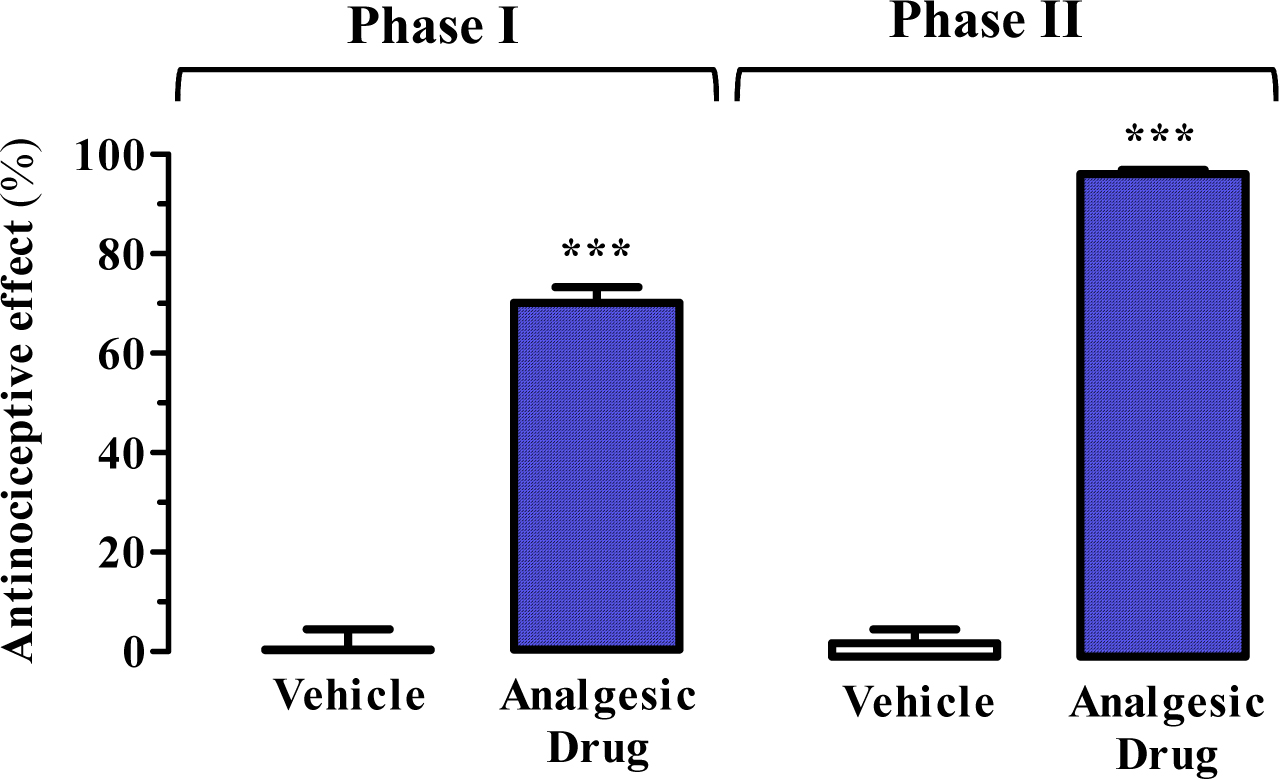
Figure 4. Representative results. Antinociceptive effect of raseglurant (10 mg/kg) as analgesic drug using the formalin test. ***P < 0.001 Student’s t-test when compared to the vehicle (physiological saline solution) treated animals (see Figure 2). Extracted from Font et al., 2017.
Recipes
- 2.5 %formalin solution (corresponds to a 0.93 % formaldehyde in physiological saline solution)
25 µl formaldehyde solution (36.5-38% in H2O)
975 µl sterile physiological saline solution
Acknowledgments
This work was supported by MINECO/ISCIII (SAF2014-55700-P and PIE14/00034), the Catalan government (2014 SGR 1054), ICREA (ICREA Academia-2010), Fundació la Marató de TV3 (Grant 20152031) and IWT (SBO-140028) to FC. MINECO (PCIN-2013-017-C03-01 and CTQ2014-57020-R), the Catalan government (2014SGR109 and 2014CTP0002) to AL. ERANET Neuron project ‘LIGHTPAIN’. This protocol was adapted from previous work: Font et al., 2017. The authors declare not conflict of interest.
References
- Clark, J. D., Gebhart, G. F., Gonder, J. C., Keeling, M. E. and Kohn, D. F. (1997). Special report: The 1996 guide for the care and use of laboratory animals. ILAR J 38(1): 41-48.
- Dubuisson, D. and Dennis, S. G. (1977). The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain 4(2): 161-174.
- Font, J., Lopez-Cano, M., Notartomaso, S., Scarselli, P., Di Pietro, P., Bresoli-Obach, R., Battaglia, G., Malhaire, F., Rovira, X., Catena, J., Giraldo, J., Pin, J. P., Fernandez-Duenas, V., Goudet, C., Nonell, S., Nicoletti, F., Llebaria, A. and Ciruela, F. (2017). Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. Elife 6: e23545.
- Mogil, J. S. (2009). Animal models of pain: progress and challenges. Nat Rev Neurosci 10(4): 283-294.
- Tjolsen, A., Berge, O. G., Hunskaar, S., Rosland, J. H. and Hole, K. (1992). The formalin test: an evaluation of the method. Pain 51(1): 5-17.
Article Information
Copyright
López-Cano et al. This article is distributed under the terms of the Creative Commons Attribution License (CC BY 4.0).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- López-Cano, M., Fernández-Dueñas, V., Llebaria, A. and Ciruela, F. (2017). Formalin Murine Model of Pain. Bio-protocol 7(23): e2628. DOI: 10.21769/BioProtoc.2628.
- Font, J., Lopez-Cano, M., Notartomaso, S., Scarselli, P., Di Pietro, P., Bresoli-Obach, R., Battaglia, G., Malhaire, F., Rovira, X., Catena, J., Giraldo, J., Pin, J. P., Fernandez-Duenas, V., Goudet, C., Nonell, S., Nicoletti, F., Llebaria, A. and Ciruela, F. (2017). Optical control of pain in vivo with a photoactive mGlu5 receptor negative allosteric modulator. Elife 6.
Category
Neuroscience > Behavioral neuroscience > Animal model > Mouse
Neuroscience > Sensory and motor systems > Animal model
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









