- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantification of Membrane Damage/Cell Death Using Evan’s Blue Staining Technique
Published: Vol 7, Iss 16, Aug 20, 2017 DOI: 10.21769/BioProtoc.2519 Views: 20594
Reviewed by: Michael EnosAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Leaf-Section Imaging for Visualizing Intracellular Chloroplast Movement and Analyzing Cell–Cell Interactions
Yuta Kato [...] Mitsutaka Taniguchi
Aug 5, 2025 2322 Views

Live-Cell Monitoring of Piecemeal Chloroplast Autophagy
Masanori Izumi [...] Shinya Hagihara
Nov 5, 2025 1690 Views

Chloroplast Movement Imaging Under Different Light Regimes With a Hyperspectral Camera
Paweł Hermanowicz [...] Justyna Łabuz
Dec 20, 2025 756 Views
Abstract
Membrane damage is a hallmark of both biotic and abiotic stress responses. The membrane determines the ability of a cell to sustain altered environmental conditions and hence can be used as a biomarker to assess stress-induced cell damage or death. We present an easy, quick, cost-effective, staining and spectrophotometric method to assess membrane stability of plant cells. In this method, Evan’s blue, an azo dye, is used to assay for cell viability. More specifically, Evan’s blue dye can penetrate through ruptured or destabilized membranes and stain cells. Thus, when plant cells are subjected to stress that compromises membrane integrity, the number of cells that are permeated by Evan’s blue dye will be increased compared to control cells that are not stressed. In contrast, live, healthy cells that are capable of maintaining membrane integrity do not take up Evan’s blue dye. Cells that have taken up Evan’s blue dye will have an accumulation of a blue protoplasmic stain and these stained cells can be qualitatively documented under bright field microscopy with or without the use of a camera. Furthermore, the dye can be extracted from cells that are stained by Evan’s blue dye and can be quantified spectrophotometrically. Using this analysis, the accumulation of dye in positively-stained cells correlates with the extent of cell membrane damage and thus the amount of cells that are stained with Evan’s blue dye under various conditions can be used as an indicator of cellular stress.
Keywords: Evan’s blueBackground
Plants are sessile organisms that are exposed to a diverse array of stress factors. The membrane is made up of lipids and glycoproteins and act as a physical, protective barrier. The fluidity of the cell membrane is altered when the cell is exposed to stress such as heat. Oxidative stress can damage cell membranes. The reactive oxygen species (ROS) associated with oxidative stress can act on membrane lipids to decrease membrane stability. An established protocol to assess membrane stability, known as Sullivan’s method, quantifies the extent of electrolyte leakage from the membrane (Sullivan and Ross, 1979). This method is time-consuming, tedious and involves several steps. Additionally, since this method usually requires exposure of the tissue to high temperature (Initial electrolyte leakage and final electrolyte leakage after boiling at high temperature), this method cannot be used to assess the instantaneous damage to membranes in plants exposed to stress. We adapted a reliable Evan’s blue staining technique that has been used by many researchers to assess cell death or membrane damage (Smith et al., 1982; Oprisko et al., 1990; Vemanna et al., 2017) for instantly monitoring stress. Evan’s blue is an acidic, non-permitting exclusion dye which stains dead or damaged cells. The dye does not enter live cells with stable membranes (Gaff and Okong’O-Ogala, 1971). One advantage of this method is that it does not subject the tissue to high temperature. Though microscopic visualization is effective, large sample sizes make this type of analysis too time consuming (Baker and Mock, 1994). We have altered our method to analyze spectrophotometrically. Evan’s blue stain can be extracted from intact cells and analyze by spectrophotometer. Our method is highly reproducible and it can be adapted to large scale phenotyping of genotypes. The other membrane penetrating dye phenosafranin can also be used but some difficulties have been reported that it will not stain the cells without nuclei (ghost cell) and also the uptake is affected by pH (Baker and Mock, 1994).
Materials and Reagents
- Eppendorf tubes
- Petri plates of 10 cm diameter
- 96-well plates or ELISA plate (Thermo Fisher Scientific, Thermo ScientifcTM, catalog number: 442404 ) or cuvette (Sigma-Aldrich, catalog number: Z276758 )
- Leaf or root tissue collected during stress period (approximately 10 discs or 250 mg)
- Distilled water
- Evan’s blue (Sigma-Aldrich, catalog number: E2129 )
- Sodium chloride (NaCl)
- Potassium nitrate (KNO3)
- Calcium nitrate tetrahydrate, Ca(NO3)2·4H2O
- Ammonium dihydrogen phosphate (NH4H2PO4)
- Magnesium sulfate heptahydrate (MgSO4·7H2O)
- Potassium chloride (KCl)
- Boric acid (H3BO3)
- Manganese sulfate (MnSO4·H2O)
- Zinc sulfate heptahydrate (ZnSO4·7H2O)
- Copper(II) sulfate pentahydrate (CuSO4·5H2O)
- Molybdic acid (H2MoO4)
- Na·Fe·DTPA
- Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016 )
- Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771 )
- Hydrochloric acid (HCl)
- Evan’s blue staining solution (see Recipe 1)
- Hoagland solution (see Recipe 2)
- 0.1 M CaCl2 of pH 5.6 (see Recipe 3)
- 1% SDS (see Recipe 4)
Equipment
- Pipettes
- (Optional) Pestle and mortar
- Tissue lyser (Tissue lyser II) (QIAGEN, catalog number: 69982 )
- Centrifuge (Thermo Fisher Scientific, model: SorvallTM ST16 , catalog number: 75004240)
- Light microscope (MAGNUS ANALYTICS, model: MLX )
- pH meter (Systronics, model: µController Based pH system 361, catalog number: 361 )
- Spectrophotometer/ELISA plate reader (Molecular Devices, model: SpectraMax Plus 384 )
- Orbital shaker (Shalom Instruments, model: SLM-GR-100 )
Procedure
- Prepare Evan’s blue solution (see Recipe 1)
- Stain tissue with Evan’s blue to assess extent of membrane stability
- Grow the plants under normal conditions.
- Impose the stress treatment to be determined for specific growth stages of plants and collect the tissue (leaf or root).
For example, we imposed the stress to leaves that are collected from 30-day-old plants of groundnut. We perform the leaf disc assay with small leaf discs of 1 cm diameter. We transfer ten leaf discs to a Petri plate and then impose methyl viologen (MV)-induced oxidative stress treatment. Methyl viologen at 5 µM is exposed to one set (stress) and another set is kept in pure water (control) and these leaf discs exposed to high light conditions (1,000 µmol m-2 sec-1) at room temperature (RT) for three hours. These leaf discs, were then stained with Evan’s blue stain (Figure 1A). - In another set of experiments, NaCl (150 mM in Hoagland solution [see Recipe 2]) stress is imposed to one set of rice seedlings (stress) and the other set was kept only in Hoagland’s solution (control) in a hydroponic experiment (Lopez and Satti, 1996). Three days after stress imposition collect the roots from control and stressed plants for Evan’s blue staining and membrane stability determination (Figure 1B). This method is highly reproducible with ~95% accuracy
- Transfer the collected leaf and root tissues to 2 ml Eppendorf tubes and add 2 ml of Evan’s blue solution to each tube.
Note: Adjust the volume to ensure that tissues are immersed in the solution.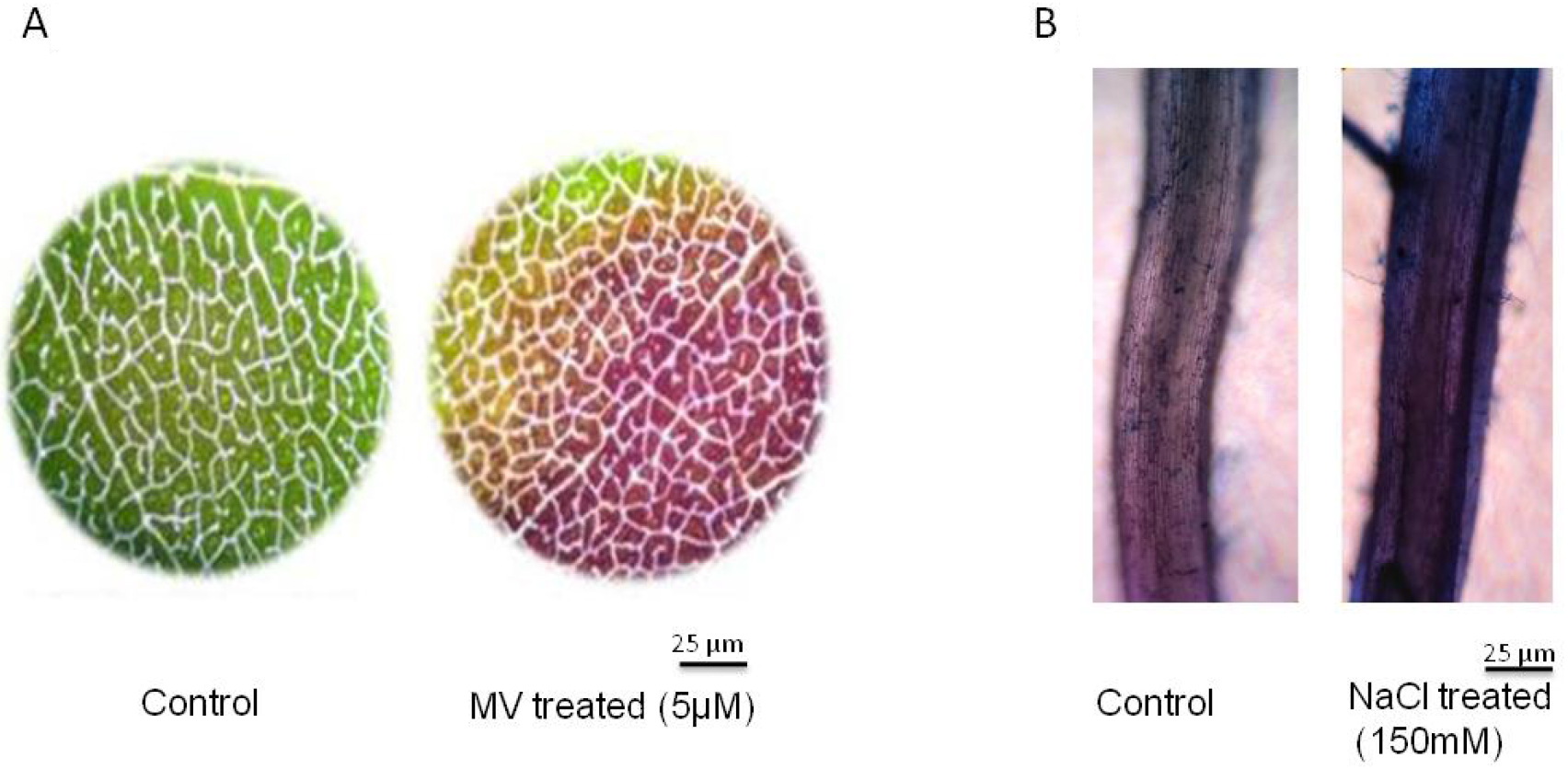
Figure 1. Determination of membrane stability using Evan’s blue staining. Microscopic detection of Evan’s blue stain in (A) methyl viologen (MV) 5 µM treated leaf discs and (B) 150 mM NaCl treated roots. Scale bar indicates 25 µm. The groundnut leaf discs from 30-day-old plants were incubated in MV for 3 h at high light intensity of 1,000 µmol m-2 sec-1 and subsequently the leaf discs were stained with Evan’s blue dye. For NaCl stress the rice seedlings of 30-days old were exposed to 150 mM NaCl for 3 days and the roots were stained with Evan’s blue dye to assess membrane integrity. - Shake the tubes in an orbital shaker at 50 oscillations/min for 20 min.
Note: Shaking is just to ensure that all of the tissue is in contact with the Evan’s blue dye solution. - Wash the roots and leaves thoroughly with distilled water thrice or until unbound dye washes out from surface.
Note: (Optional) Root or leaf tissue can also be washed with 0.1 M CaCl2 of pH 5.6 (see Recipe 3). - Observe the stained leaf or root under the normal, transmitted light microscope. Examine the leaves or roots and take photograph of images under a brightfield microscope. Furthermore, the Evan’s blue stained images can be quantified using ImageJ software to assess the viability of cells.
Note: If the sample size is large, it’s preferable to follow only spectrophotometric quantification and appropriate statistics can be applied to arrive at significance levels.
- Grow the plants under normal conditions.
- Quantification Evan’s blue stain taken up due to membrane destabilization
- To extract the Evan’s blue dye from the roots and leaves, use 1 ml of 1% SDS (see Recipe 4) cell lysis buffer which breaks up membrane structures.
- Grind 100 mg of tissue using a tissue lyser with a frequency of 15 strokes per second for 10 min, if the sample number is large. If only a few samples are needed, they can be ground in a pestle and mortar. After grinding, transfer the extract to 2 ml Eppendorf tubes.
- Centrifuge the extract at 7,168 x g for 5 min at RT to elute the dye into the solution and to remove the debris.
- Transfer an aliquot of supernatant to new tubes or directly transfer 250 µl of supernatant to a 96-well microtiter plate.
Note: Since Evan’s blue dye will not oxidize, it can be stored at RT for half an hour to measure large samples. - Measure the optical density at 600 nm spectrophotometrically using a cuvette or a plate reader by taking 1% SDS as blank.
- Concentration of Evan’s blue can be estimated by referring to a standard curve (Figure 2).
- Plot the graph of concentration of Evan’s blue on y-axis and plant number or stress treatment on x-axis (Figure 3A and 3B).
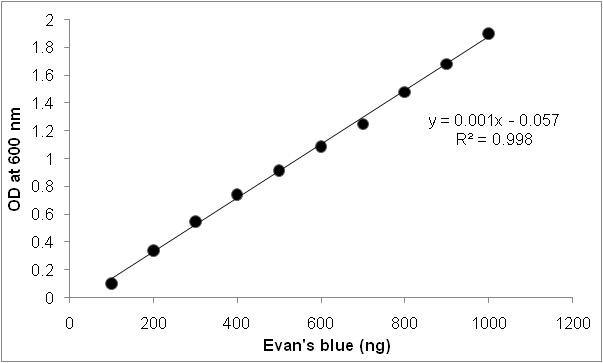
Figure 2. Standard graph for Evan’s blue staining. Standard graph is prepared using 1 µg ml-1 of Evan’s blue stock solution. Different concentrations of Evan’s blue were diluted and absorbance was recorded at 600 nm.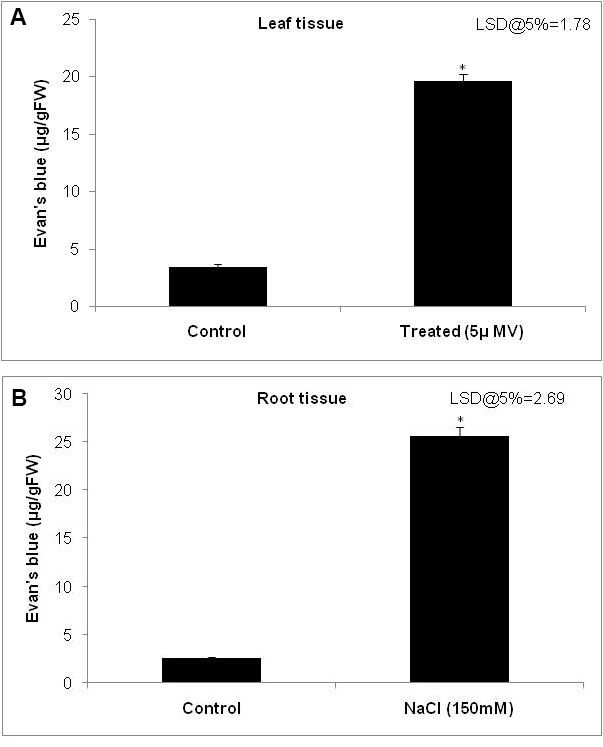
Figure 3. Quantification of membrane stability using the spectrophotometric method. Histogram showing the extent of Evan’s blue dye taken up by the (A) leaf tissue and (B) root tissue as a reflection of membrane damage. The extent of dye taken up by the cells was quantified by grinding the tissue in 1% sodium dodecyl sulfate (SDS) and measuring the absorbance at 600 nm and calculated using standard graph. Error bars indicate the minimum of three replicates data. * indicate the least significant difference (LSD) at P < 0.05 analyzed using GenStat statistical tool.
- To extract the Evan’s blue dye from the roots and leaves, use 1 ml of 1% SDS (see Recipe 4) cell lysis buffer which breaks up membrane structures.
- Preparation of standard curve using Evan’s blue:
Dissolve 1 µg of Evan’s blue in 1 ml of distilled water to get 1 µg ml-1 stock solution. From stock solution make five concentrations by making up the volume to 1 ml. The absorbance was measured at 600 nm in spectrophotometer (Figure 2).
Data analysis
A minimum of three biological replicates were used to quantify the uptake of Evan’s blue dye and the data were analyzed using the GenStat program (Marcos et al., 2013). Significance level was tested using analysis of variance (ANOVA) depending on the number of genotypes and treatment levels.
Recipes
- Evan’s blue staining solution
Dissolve 0.25 g of Evan’s blue dye in 100 ml of 0.1 M CaCl2 solution at pH 5.6 and mix well until it is dissolved
Note: Evan’s blue solution should be freshly prepared each time. - Hoagland solution
Composition of Hoagland solution used in this experiment is given in Table 1.
Table 1. Composition of Hoagland solution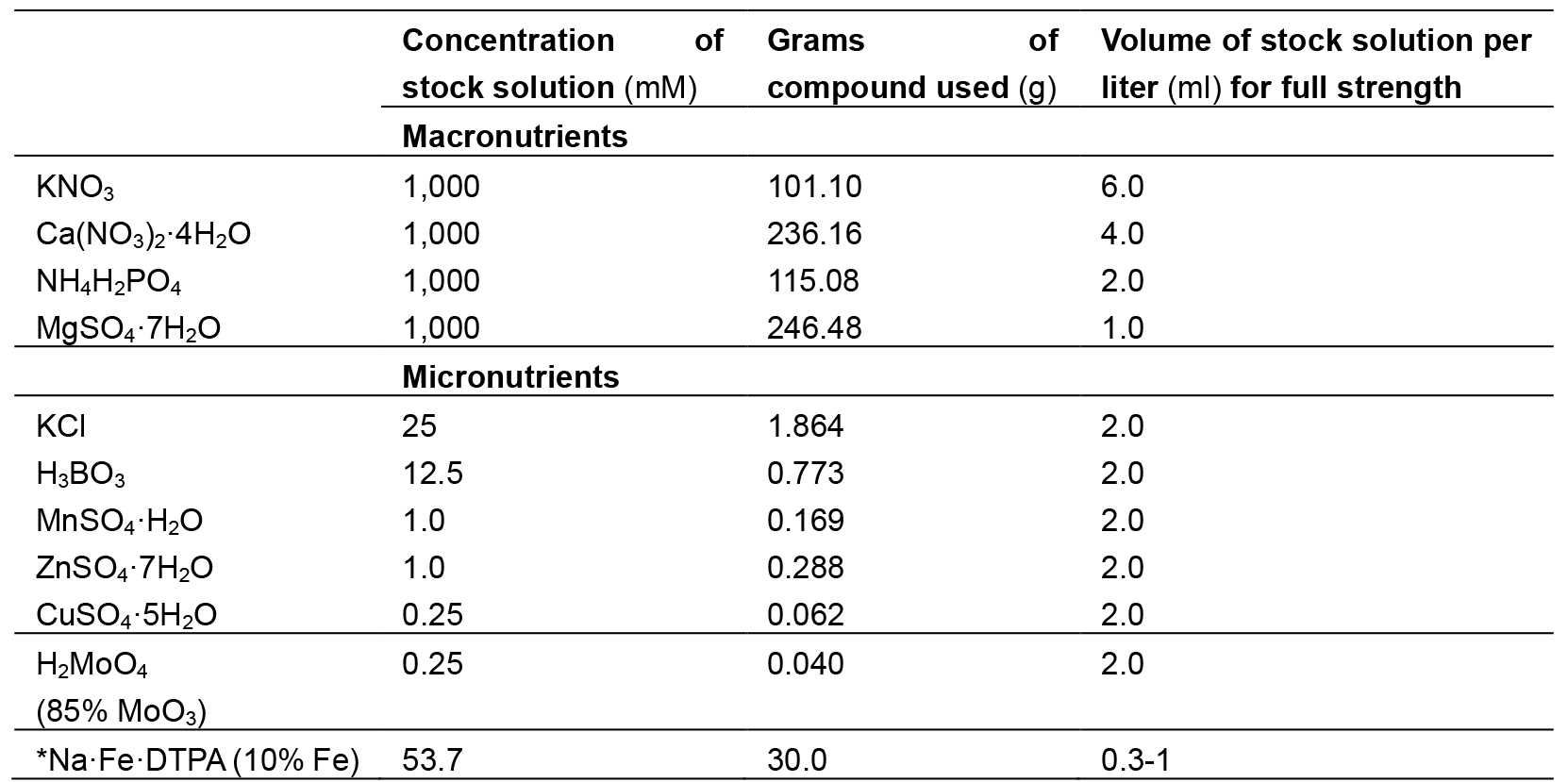
*Note: This should be avoided in salinity stress treatment imposition. - 0.1 M CaCl2 of pH 5.6
To prepare 0.1 M CaCl2, dissolve 1.10 g of CaCl2 (Molecular Weight = 110.989) in distilled water (1 L) and adjust pH to 5.6 using 0.2 N HCl. CaCl2 solution can be stored for a month in RT - 1% SDS
To prepare 1% SDS, add 1 g of sodium dodecyl sulfate to 80 ml distilled water and make up the volume to 100 ml. SDS should be prepared freshly before the use. Optionally it can be stored for a month in RT
Acknowledgments
Indian Council of Agricultural Research–Niche Area of Excellence program (F. No. 10-(6)/2005 EPD) and (F. No. 10 (15) 2012 EPD). MUK acknowledges the support for platinum jubilee fellowship from NASI, India.
References
- Baker, C. J. and Mock, N. M. (1994). An improved method for monitoring cell death in cell suspension and leaf disc assay using Evan’s blue. Plant Cell Tissue Organ Cult 39(1): 7-12.
- Gaff, D. F. and Okong'O-Ogola, O. (1971). The use of non-permeating pigments for testing the survival of cells. J Exp Bot 22: 756-758.
- Lopez, M. V. and Satti, S. M. E. (1996). Calcium and potassium-enhanced growth and yield of tomato under sodium chloride stress. Plant Science 114: 19-27.
- Marcos, M., Jean-Marcel, R. and Fred, A. V. (2013). The statistical analysis of multi environment data: modelling genotype-by-environment interaction and its genetic basis. Front Physiol 4: 44.
- Oprisko, M. J., Green, R. L., Beard, J. B. and Gates, C. E. (1990). Vital staining of root hairs in 12 warm-season perennial grasses. Crop Sci 30: 947-950.
- Vemanna, R. S., Babitha, K. C., Solanki, J. K., Amarnatha Reddy, V., Sarangi, S. K. and Udayakumar, M. (2017). Aldo-keto reductase-1 (AKR1) protect cellular enzymes from salt stress by detoxifying reactive cytotoxic compounds. Plant Physiol Biochem 113: 177-186.
- Smith, B. A., Reider, M. L. and Fletcher, J. S. (1982). Relationship between vital staining and subculture growth during the senescence of plant tissue cultures. Plant Physiol 70: 1228-1230.
- Sullivan, C. Y. and Ross, W. M. (1979). Selecting for drought and heat resistance in grain sorghum. In: Mussell, H. and Staples, R.C. (Eds.). Stress Physiology in Crop Plants. John Wiley and Sons pp: 263-281.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
NV, P., PA, V., Vemanna, R. S., MS, S. and Makarla, U. (2017). Quantification of Membrane Damage/Cell Death Using Evan’s Blue Staining Technique. Bio-protocol 7(16): e2519. DOI: 10.21769/BioProtoc.2519.
Category
Plant Science > Plant cell biology > Cell staining
Plant Science > Plant cell biology > Cell imaging
Cell Biology > Cell viability > Cell death
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









