- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Teratoma Formation Assay for Assessing Pluripotency and Tumorigenicity of Pluripotent Stem Cells
Published: Vol 7, Iss 16, Aug 20, 2017 DOI: 10.21769/BioProtoc.2518 Views: 16385
Reviewed by: Andrea PuharKevin Patrick O’RourkeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
In vitro and in vivo Limiting Dilution Assay for Colorectal Cancer
Lauren Agro and Catherine A O’Brien
Nov 20, 2015 25197 Views
Isolation and Separation of Epithelial CD34+ Cancer Stem Cells from Tgfbr2-deficient Squamous Cell Carcinoma
Heather A. McCauley and Géraldine Guasch
Sep 5, 2017 9916 Views
Abstract
Pluripotent stem cells such as induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) form teratomas when transplanted into immunodeficient mice. As teratomas contain all three germ layers (endoderm, mesoderm, ectoderm), teratoma formation assay is widely used as an index of pluripotency (Evans and Kaufman, 1981; Hentze et al., 2009; Gropp et al., 2012). On the other hand, teratoma-forming tumorigenicity also represents a major risk factor impeding potential clinical applications of pluripotent stem cells (Miura et al., 2009; Okano et al., 2013). Recently, we reported that iPSCs derived from naked mole-rat lack teratoma-forming tumorigenicity when engrafted into the testes of non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice due to an ES cell-expressed Ras (ERAS) and Alternative reading frame (ARF)-dependent tumor-suppression mechanism specific to this species (Miyawaki et al., 2016). Here, we describe a method for transplanting pluripotent stem cells into the testes of NOD/SCID mice to generate teratomas for assessing the pluripotency and tumorigenicity.
Keywords: Pluripotent stem cellsBackground
iPSCs and ESCs are exploited for applications in cell transplantation therapy for regenerative medicine. However, these cells form tumors called teratoma containing differentiated tissues when transplanted into immune-deficient mice. Therefore, the risk of their teratoma-forming tumorigenicity limits their clinical application. Several studies have reported the methods to overcome the risk of teratoma-forming tumorigeniticy (Itakura et al., 2017; Vazquez-Martin et al., 2012). Recently, we reported that iPSCs derived from naked mole-rats lack teratoma-forming tumorigenicity when engrafted into the testes of NOD/SCID mice due to species-specific activation of tumor-suppressor ARF and a disruption mutation of the oncogene ERAS (Miyawaki et al., 2016). In this protocol, we describe a method for transplanting pluripotent stem cells into the testes of NOD/SCID mice to generate teratomas. This approach can minimize the immune rejection due to the presence of the testicular–blood barrier (Cheng and Mruk, 2012). In addition, this approach is advantageous because transplanted cells are easily identified around the injection site even when they do not form tumors. Thus, the technique described herein is useful for assessing the pluripotency and tumorigenicity of pluripotent stem cells.
Materials and Reagents
- Falcon 15 ml conical tube (Corning, Falcon®, catalog number: 352196 )
- BIO-BIK 1.5 ml centrifuge tube (Ina-optika, catalog number: CF-0150 )
- 26-gauge needle (Terumo Medical, catalog number: NN-2613S )
- Laboratory wipe (Kimwipes) (KCWW, Kimberly-Clark, catalog number: 62011 )
- 0.22-μm filter unit (TPP Techno Plastic Products, catalog number: 99155 )
- 10 cm tissue culture dish (Corning, Falcon®, catalog number: 353046 )
- Pluripotent stem cells stably expressing a fluorescent marker, such as green fluorescent protein (GFP), for detection of injected cells
Note: We used naked mole-rat (NMR) iPSCs (clones 24 and 27), mouse iPSCs (clones 20D17 and 38C2: Okita et al., 2007), and human iPSCs (clone 201B7: Takahashi et al., 2007), as described in our previous study (Miyawaki et al., 2016). - Sterile phosphate-buffered saline (PBS) (NACALAI TESQUE, catalog number: 27575-31 )
- 0.1% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 15090046 )
- Fetal bovine serum (Biowest, catalog number: S1820-500 )
- Dulbecco’s modified Eagle’s medium (Sigma-Aldrich, catalog number: D5796 )
- Penicillin/streptomycin (Wako Pure Chemical Industries, catalog number: 168-23191 )
- Non-essential amino acids (NACALAI TESQUE, catalog number: 06344-56 )
- β-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- L-Glutamine (NACALAI TESQUE, catalog number: 04260-64 )
- DMEM/F12 (Sigma-Aldrich, catalog number: D6421 )
- KnockOutTM Serum Replacement (Thermo Fisher Scientific, GibcoTM, catalog number: 10828028 )
- Fibroblast growth factor 2 (PeproTech, catalog number: 100-18B )
- Isoflurane (Wako Pure Chemical Industries, catalog number: 099-06571 )
- 70% ethanol
- Ampicillin (Wako Pure Chemical Industries, catalog number: 012-23303 )
- Paraformaldehyde (Junsei Chemical, catalog number: 58295-1201 )
- Sodium hydroxide (NACALAI TESQUE, catalog number: 31511-05 )
- Paraffin
- Hematoxylin and eosin (HE)
- iPS medium for mouse iPSCs (see Recipe 1)
- iPS medium for NMR and human iPSCs (see Recipe 2)
- 4% paraformaldehyde (PFA) (see Recipe 3)
Equipment
- Two humidified 5% CO2 cell culture incubators: 32 °C for NMR iPSCs, 37 °C for mouse and human iPSCs (Thermo Fisher Scientific, Thermo ScientificTM, model: HeracellTM 150i )
- Centrifuge (TOMY DIGITAL BIOLOGY, model: AX-501 )
- Coulter counter (Beckman Coulter, catalog number: 6605698 )
- Isoflurane vaporizer, chamber, and nose cone (Shinano, catalog number: SN-487-0T )
- Heating pad (Nissin, model: NHP-M30N )
- Operating scissors and tweezers (see Figure 1)
- 25 µl Hamilton syringe (Hamilton, model: 702 N, catalog number: 80400 )
- Microbalance (Shimadzu, model: ATX84 )
- Icebox
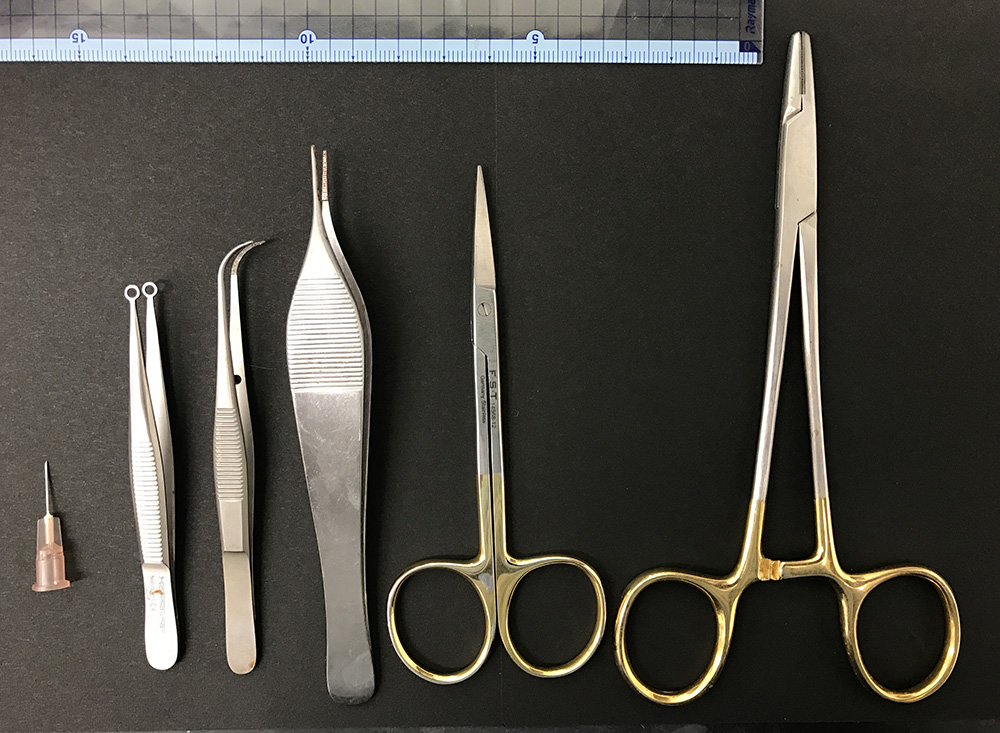
Figure 1. Surgical instruments used in this protocol
Software
- GraphPad Prism software (GraphPad software, model: version 6.0)
Procedure
- Preparing cells for transplantation
- Maintain iPSCs according to the previous reports (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Miyawaki et al., 2016). It is necessary to prepare at least 2 x 106 cells.
- Replace with fresh culture medium 1 h before dissociation.
- Aspirate culture medium and wash twice with PBS.
- Detach the cells using trypsin-EDTA (1 ml/10 cm dish).
- Stop the trypsin reaction with 15% fetal bovine serum-containing medium for mouse iPSCs (see Recipe 1) or trypsin inhibitor for NMR and human iPSCs (see Recipe 2).
- Collect cells in a 15 ml conical tube, and re-suspend cells in 4 ml of PBS.
- Count cells using a Coulter counter.
- Centrifuge cells at 200 x g for 5 min at room temperature.
- Aspirate supernatant and re-suspend iPSCs in an appropriate volume of PBS to yield a final concentration to 5 x 107 cells/ml (equal to 1 x 106 cells/20 μl).
- Transfer cells into a 1.5 ml tube.
- Place cells on ice until used for injection.
- Maintain iPSCs according to the previous reports (Takahashi and Yamanaka, 2006; Takahashi et al., 2007; Miyawaki et al., 2016). It is necessary to prepare at least 2 x 106 cells.
- Injection into mice
- (Optional) Administer ampicillin-containing water (1 g/L) orally to mice from 3 days before until 1 week after surgery.
- Place NOD/SCID mice on a heating pad and anesthetize with isoflurane.
- Wash surgical area with 70% ethanol and remove hair from the dorsal region.
- Make a 1 cm incision above the top of the preputial gland and open the abdomen.
- Carefully pull out the epididymal fat pad along with the testes (Figure 2A).
- Fill a Hamilton syringe with 20 µl (1 x 106 cells) of cell suspension.
- Cut the tunica vaginalis of the testis using a 26-gauge needle (Figure 2B).
- Use the Hamilton syringe to inject 20 μl of cell suspension (Figure 2C).
- Slowly remove the needle to avoid backflow of cells.
- Return the testes to their original location.
- Suture the wound.
- (Optional) Administer ampicillin-containing water (1 g/L) orally to mice from 3 days before until 1 week after surgery.
- Defining pluripotency and tumorigenicity
- At 4, 10, 20, or 28 weeks after transplantation, anesthetize and sacrifice the mice in accordance with the Guide for the Care and Use of Laboratory Animals. Thereafter, dissect the tumors and testes (Figures 2D and 2E).
Note: Tumors could be observed 4 weeks and 10 weeks after the injection of mouse iPSCs and human iPSCs, respectively.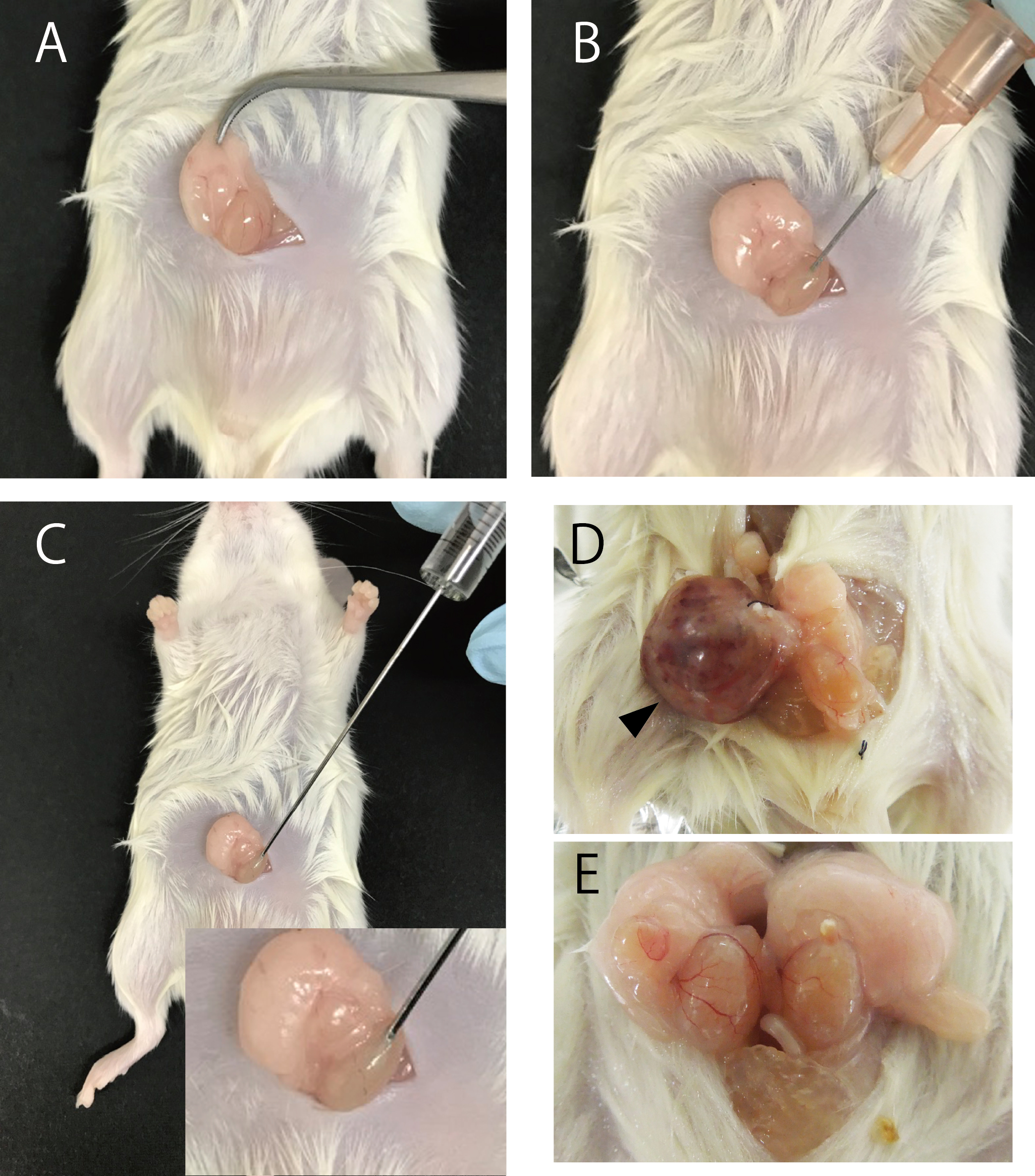
Figure 2. Injection iPSCs into testis of NOD/SCID mice. Transplant procedure (A-C). Representative photographs of teratoma (D) and testis without tumor formation (E). Arrowhead indicates teratoma formed by mouse iPSCs. - After removing moisture within the laboratory wipe, measure the weight of the tumor.
- Fix the tumors or testes overnight in PBS containing 4% PFA (see Recipe 3) for embedding in paraffin.
- Stain sections with hematoxylin and eosin (HE), or subject to immunohistochemical analysis, to detect expression of GFP and differentiation markers. The section of tumors and testes in our previous study are shown in Figure 3 (Miyawaki et al., 2016).
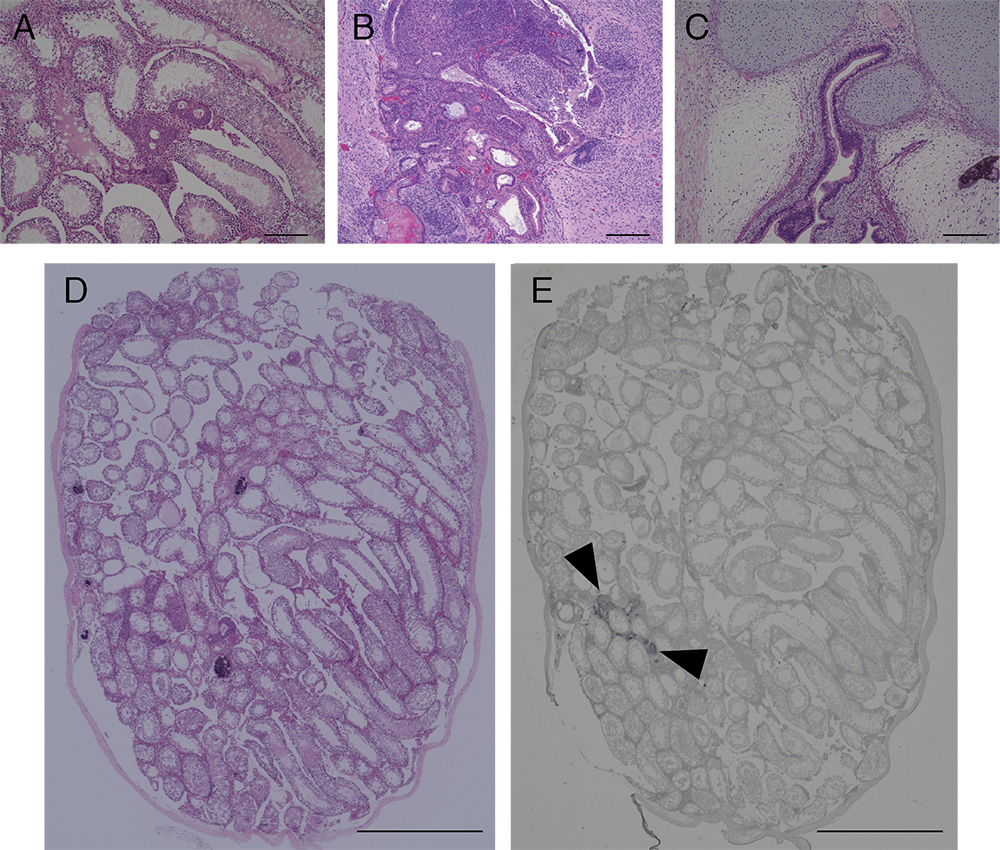
Figure 3. Image of HE and immunohistochemical stained section of tumors and testis after transplantation of iPSCs. HE staining section of tumors and testis of mice transplanted with NMR iPSCs 10 weeks after transplantation (A and D), mouse iPSCs 4 weeks after transplantation (B) or human iPSCs 10 weeks after transplantation (C). Immunohistochemical analysis of GFP (E). Arrowheads indicate the transplanted GFP-labeled NMR iPSCs. Scale bar, 200 µm (A-C) and 1 cm (D and E).
- At 4, 10, 20, or 28 weeks after transplantation, anesthetize and sacrifice the mice in accordance with the Guide for the Care and Use of Laboratory Animals. Thereafter, dissect the tumors and testes (Figures 2D and 2E).
Data analysis
The weights of tumors or testes were analyzed after logarithmic transformation (arbitrary units: 6 + log2). The Bartlett test was used to verify equal variances across populations. The statistical significance of the difference between experimental groups was determined by one-way analysis of variance or the Kruskal Wallis test followed by the Dunn’s method using GraphPad Prism 6 software. Statistical significance was considered when P < 0.05 and all data are displayed as mean ± SEM as in Miyawaki et al., 2016.
Weight of tumors and testes in our previous study are shown in Table 1 for reference (Miyawaki et al., 2016).
Table 1. Weight of each teratoma or testis, and mean weight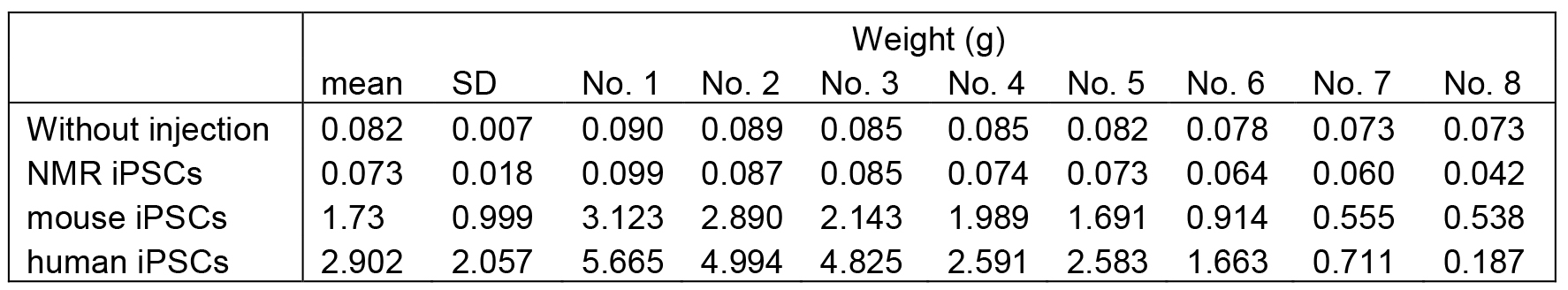
Recipes
- Mouse iPSCs medium
Dulbecco’s modified Eagle’s medium (Sigma-Aldrich)
15% fetal bovine serum (JRH or BioWest)
1% penicillin/streptomycin (Wako Pure Chemical Industries)
2 mM L-glutamine (NACALAI TESQUE)
0.1 mM non-essential amino acids (NACALAI TESQUE)
0.1 mM β-mercaptoethanol (Sigma-Aldrich)
Human recombinant leukemia inhibitory factor (NACALAI TESQUE)
Filtered by 0.22-μm filter unit, stored at 4 °C - NMR and human iPSCs medium
DMEM/F12 (Sigma-Aldrich)
20% KnockOut Serum Replacement (Thermo Fisher Scientific)
1% penicillin/streptomycin (Wako Pure Chemical Industries)
2 mM L-glutamine (NACALAI TESQUE)
0.1 mM non-essential amino acids (NACALAI TESQUE)
0.1 mM β-mercaptoethanol (Sigma-Aldrich)
4 ng/ml fibroblast growth factor 2 (PeproTech)
Filtered by 0.22-μm filter unit, stored at 4 °C - 4% PFA
4 g PFA
100 ml of PBS
Heat on hot plate stirrer
Add 20 μl of 1 M sodium hydroxide to dissolve residual PFA
Aliquot and freeze at -20 °C for storage
Note: The investigator must wear a facemask, gloves, lab coat and eye protection during PFA preparation.
Acknowledgments
This work was supported in part by PRESTO of the Japan Science and Technology Agency, Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Grant-in-Aid for Scientific Research on Innovative Areas ‘Oxygen Biology: a new criterion for integrated understanding of life’ from the MEXT to K.M., and K.M. and S.M. were Research Fellows of the Japanese Society for the Promotion of Science.
References
- Cheng, C. Y. and Mruk, D. D. (2012). The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64(1): 16-64.
- Evans, M. J. and Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292(5819): 154-156.
- Gropp, M., Shilo, V., Vainer, G., Gov, M., Gil, Y., Khaner, H., Matzrafi, L., Idelson, M., Kopolovic, J., Zak, N. B. and Reubinoff, B. E. (2012). Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS One 7(9): e45532.
- Hentze, H., Soong, P. L., Wang, S. T., Phillips, B. W., Putti, T. C. and Dunn, N. R. (2009). Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Res 2(3): 198-210.
- Itakura, G., Kawabata, S., Ando, M., Nishiyama, Y., Sugai, K., Ozaki, M., Iida, T., Ookubo, T., Kojima, K., Kashiwagi, R., Yasutake, K., Nakauchi, H., Miyoshi, H., Nagoshi, N., Kohyama, J., Iwanami, A., Matsumoto, M., Nakamura, M. and Okano, H. (2017). Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Reports 8(3): 673-684.
- Miura, K., Okada, Y., Aoi, T., Okada, A., Takahashi, K., Okita, K., Nakagawa, M., Koyanagi, M., Tanabe, K., Ohnuki, M., Ogawa, D., Ikeda, E., Okano, H. and Yamanaka, S. (2009). Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27(8): 743-745.
- Miyawaki, S., Kawamura, Y., Oiwa, Y., Shimizu, A., Hachiya, T., Bono, H., Koya, I., Okada, Y., Kimura, T., Tsuchiya, Y., Suzuki, S., Onishi, N., Kuzumaki, N., Matsuzaki, Y., Narita, M., Ikeda, E., Okanoya, K., Seino, K., Saya, H., Okano, H. and Miura, K. (2016). Tumour resistance in induced pluripotent stem cells derived from naked mole-rats. Nat Commun 7: 11471.
- Okano, H., Nakamura, M., Yoshida, K., Okada, Y., Tsuji, O., Nori, S., Ikeda, E., Yamanaka, S. and Miura, K. (2013). Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res 112(3): 523-533.
- Okita, K., Ichisaka, T. and Yamanaka, S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature 448(7151): 313-317.
- Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K. and Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5): 861-872.
- Takahashi, K. and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4): 663-676.
- Vazquez-Martin, A., Cufi, S., Lopez-Bonet, E., Corominas-Faja, B., Oliveras-Ferraros, C., Martin-Castillo, B. and Menendez, J. A. (2012). Metformin limits the tumourigenicity of iPS cells without affecting their pluripotency. Sci Rep 2: 964.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Miyawaki, S., Okada, Y., Okano, H. and Miura, K. (2017). Teratoma Formation Assay for Assessing Pluripotency and Tumorigenicity of Pluripotent Stem Cells. Bio-protocol 7(16): e2518. DOI: 10.21769/BioProtoc.2518.
Category
Cancer Biology > Cancer stem cell > Tumor formation
Stem Cell > Pluripotent stem cell > Proliferation
Cell Biology > Tissue analysis > Macroscopic observation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










