- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
ARP2/3 Phosphorylation Assay in the Presence of Recombinant Bacterial Effectors
Published: Vol 7, Iss 7, Apr 5, 2017 DOI: 10.21769/BioProtoc.2208 Views: 9171
Reviewed by: Yurong XieTatsuki KunohAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Characterization of Protein Domain Function via in vitro DNA Shuffling
Kathy Hiu Laam Po [...] Sheng Chen
Jun 5, 2018 6636 Views

In vitro and in vivo Assessment of Protein Acetylation Status in Mycobacteria
Krishna K. Singh [...] Deepak K. Saini
Jul 5, 2019 5666 Views

In vitro Glutamylation Inhibition of Ubiquitin Modification and Phosphoribosyl-Ubiquitin Ligation Mediated by Legionella pneumophila Effectors
Alan G. Sulpizio [...] Yuxin Mao
Nov 5, 2020 3928 Views
Abstract
The Actin-Related Protein 2/3 (ARP2/3) complex is an actin nucleator that generates a branched actin network in mammalian cells. In addition to binding nucleation promoting factors, LeClaire et al. demonstrated that its phosphorylation state is essential key for its activity (LeClaire et al., 2008). In cells, the ARP2/3 complex is phosphorylated on threonine and tyrosine residues of the ARP2, ARP3, and ARPC1 subunits (Vadlamudi et al., 2004; LeClaire et al., 2008; Narayanan et al., 2011; LeClaire et al., 2015). In particular, phosphorylation of threonine 237 and 238 of the ARP2 subunit is necessary to allow a change in the ARP2/3 complex structure to its active conformation (Narayanan et al., 2011; LeClaire et al., 2015). While important for many functions in eukaryotic cells, ARP2/3 complex activity also benefits several cellular pathogens (Haglund and Welch, 2011; Welch and Way, 2013). Recently, we demonstrated that the bacterial pathogen, Legionella pneumophila, manipulates ARP2/3 complex phosphorylation state using a bacterial protein kinase injected in host cell cytoplasm (Michard et al., 2015). Here, we describe how to test the ability of a bacterial protein kinase or another protein kinase to phosphorylate the ARP2/3 complex in an in vitro context. First, the ARP2/3 complex and the bacterial protein kinase are produced and purified. Then, the purified proteins are incubated in the presence of ATP, and the ARP2/3 phosphorylation level is analyzed by Western blot.
Keywords: in vitro phosphorylation assayBackground
The ARP2/3 complex is phosphorylated on threonine and tyrosine residues (LeClaire et al., 2008). Four phosphorylation sites on the ARPC1 and ARP2 subunits of ARP2/3 complex are currently known: the threonine 21 of ARPC1, threonines 237/238 and tyrosine 202 of ARP2, each demonstrating an important role for ARP2/3 function (Vadlamudi et al., 2004; LeClaire et al., 2008; Narayanan et al., 2011). A recent study by our laboratory demonstrated that the Legionella kinase 2 (LegK2), an effector serine/threonine protein kinase of Legionella pneumophila, modifies the threonine phosphorylation state of ARPC1B and ARP3 subunits of ARP2/3 complex. ARP2/3 complex phosphorylation inactivates and blocks actin polymerization on the bacterial vacuole, preventing the degradation of bacteria by the endocytic pathway (Michard et al., 2015). This protocol describes an in vitro phosphorylation assay routinely used in our laboratory to test a potential substrate of protein kinases as subunits of the ARP2/3 complex. The protocol can be also adapted to assay other substrates as needed.
Materials and Reagents
- Overproduction and extraction of bacterial protein kinase
- 50 ml conical tubes (Greiner Bio One International, catalog number: 227261 )
- Sterile culture tubes (SARSTEDT, catalog number: 62.515.006 )
- Sterilization filters (0.22 µm) (Dutscher Scientific, catalog number: 51732 )
- Spectrophotometer cuvette (Dutscher Scientific, catalog number: 030101 )
- Escherichia coli BL21 (pREP4-groESL) (Amrein et al., 1995)
- pGEX-6P-3 (Smith and Johnson, 1988)/pGEX-protein kinase
- Prechilled dH2O
- Protease inhibitor cocktail (Sigma-Aldrich, catalog number: P8340 )
- LB medium (Lennox) (Carl Roth, catalog number: X964 )
- Ampicillin (Carl Roth, catalog number: K029 )
- Kanamycin (Sigma-Aldrich, catalog number: K4000 )
- Isopropyl β-D-1-thiogalactopyranoside (IPTG) (Carl Roth, catalog number: 2316 )
- Sodium chloride (NaCl) (Carl Roth, catalog number: 3957 )
- Potassium chloride (KCl)
- Sodium phosphate dibasic (Na2HPO4)
- Potassium phosphate monobasic (KH2PO4)
- LB medium + ampicillin + kanamycin
- LB medium (see Recipes)
- 100 µg/ml ampicillin (see Recipes)
- 25 µg/ml kanamycin (see Recipes)
- LB medium (see Recipes)
- 0.1 M IPTG (see Recipes)
- 1x phosphate-buffered saline (PBS) (see Recipes)
- 50 ml conical tubes (Greiner Bio One International, catalog number: 227261 )
- Purification of bacterial protein kinase
- Microtubes
1.5 ml (SARSTEDT, catalog number: 72.690.001 )
2 ml (SARSTEDT, catalog number: 72.694.006 ) - Polypropylene columns 1 ml (QIAGEN, catalog number: 34924 )
- Spectra/Por® dialysis membrane MWCO: 3.5-5 kD (Biotech CE Trial Kit) (Spectrum, catalog number: 131201T ), stored at 4 °C in humid atmosphere
- Protino Glutathione agarose-4B (Macherey-Nagel, catalog number: 745500.10 ), stored at 4 °C
- Glycerol (Carl Roth, catalog number: 3783 )
- Tris(hydroxymethyl)aminomethane (Tris) (Carl Roth, catalog number: 5429 )
- Glutathione (Sigma-Aldrich, catalog number: G4251 )
- Sodium chloride (NaCl) (Carl Roth, catalog number: 3957 )
- 1x PBS (see Recipes)
- GST elution buffer (see Recipes)
- Dialysis buffer (see Recipes)
- Microtubes
- Purification of ARP2/3 complex
- Polypropylene columns 1 ml (QIAGEN, catalog number: 34924 )
- PD-10 columns (GE Healthcare, catalog number: 17085101 )
- Actin-depleted cellular lysates prepared from Acanthamoeba castellanii (Zuchero, 2007)
- Liquid nitrogen (liquid N2)
- Tris (Fisher Scientific, catalog number: BP152-5 )
- Magnesium chloride (MgCl2) (Fisher Scientific, catalog number: BP214-500 )
- 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Fisher Scientific, catalog number: BP2939100 )
- Dithiothreitol (DTT) (Fisher Scientific, catalog number: BP172-5 )
- Glycerol (Fisher Scientific, catalog number: P2294 )
- Potassium chloride (KCl) (Fisher Scientific, catalog number: P217-500 )
- Adenosine triphosphate (ATP) (Fisher Scientific, catalog number: BP413-25 )
- N-WASP VCA-coupled-CH-Sepharose (Zuchero, 2007)
- Phenyl Sepharose (Sigma-Aldrich, catalog number: P2459 )
- Buffer A (see Recipes)
- ARP2/3 elution buffer (see Recipes)
- ARP2/3 storage buffer (see Recipes)
- Polypropylene columns 1 ml (QIAGEN, catalog number: 34924 )
- Dephosphorylation of ARP2/3 complex
- Microtubes (1.5 ml)
- 3,500 MWCO Mini dialysis units (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 69550 )
- 2 mM Tris, pH 8.0 (Fisher Scientific, catalog number: BP152-5 )
- Antarctic phosphatase (New England Biolabs, catalog number: M0289 )
- N-WASP VCA-coupled Sepharose (Zuchero, 2007)
- Tris (Fisher Scientific, catalog number: BP152-5 )
- dH2O
- Magnesium chloride hexahydrate (MgCl2) (Fisher Scientific, catalog number: BP214-500 )
- Zinc chloride (ZnCl2) (Sigma Aldrich, catalog number: Z3500 )*
- 5x HipH buffer (see Recipes)
- Buffer A (see Recipes)
- ARP2/3 elution buffer (see Recipes)
- Microtubes (1.5 ml)
- In vitro phosphorylation assays
- Microtubes (1.5 ml) (SARSTEDT, catalog number: 72.690.001 )
- Purified bacterial kinase and its catalytic variant
- Purified and purified/dephosphorylated ARP2/3 complex
- Tris (Carl Roth, catalog number: 5429 )
- Manganese chloride (MnCl2) (Fisher Scientific, catalog number: M87-100 )
- DTT (Carl Roth, catalog number: 6908 )
- ATP (Sigma-Aldrich, catalog number: A5394 )*
- dH2O
- Magnesium chloride hexahydrate (MgCl2) (Carl Roth, catalog number: 2189 )
- Zinc chloride (ZnCl2) (Sigma-Aldrich, catalog number: 211273 )
- Sodium dodecyl sulfate (SDS) (Carl Roth, catalog number: 2326 )
- Glycerol (Carl Roth, catalog number: 3783 )
- Bromophenol blue (Sigma-Aldrich, catalog number: B6131 )*
- β-mercaptoethanol (Sigma-Aldrich, catalog number: O34461-100 )
- Antarctic phosphatase (New England Biolabs, catalog number: M0289 )
- 10x phosphorylation buffer (see Recipes)
- ATP solution at 0.5 mg.ml-1 (see Recipes)
- 5x Laemmli loading buffer (see Recipes)
- 5x HipH buffer (see Recipes)
- Microtubes (1.5 ml) (SARSTEDT, catalog number: 72.690.001 )
- Detection of phosphorylation level with Western Blot
- Square Petri dish (Greiner Bio One International, catalog number: 688162 )
- Whatman 3 MM CHR paper (GE Healthcare, catalog number: 3030-917 )
- Nitrocellulose membrane Optitran BA-S85 reinforced NC (GE Healthcare, catalog number: 10439194 )
- 50 ml conical tubes
- Parafilm
- Plastic wrap
- Page RulerTM prestained protein ladder (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 26616 )
- Monoclonal mouse anti-phosphothreonine antibody, Clone PTR-8 (Sigma-Aldrich, catalog number: P6623 )
- Goat anti-mouse IgG peroxidase antibody (Sigma-Aldrich, catalog number: A0168 )
- Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 34080 )
- Rotiphorese Gel 30 (30% acrylamide, 0.8% bisacrylamide) (Carl Roth, catalog number: 3029 )
- Tris (Carl Roth, catalog number: 5429 )
- dH2O
- SDS (Carl Roth, catalog number: 2326 )
- Ammonium persulfate (APS) (Carl Roth, catalog number: 9592 )
- Tetramethylethylenediamine (TEMED) (Carl Roth, catalog number: 2367 )
- Glycine (Carl Roth, catalog number: 0079 )
- Glacial acetic acid
- Ethanol
- Coomassie Brilliant Blue R-250 (MP Biomedicals, catalog number: 1-800-854-0530 or MP Biomedicals, catalog number: 02190682 )
- Methanol
- 6-aminohexanoic acid
- Sodium chloride (NaCl) (Carl Roth, catalog number: 3957 )
- Bovine serum albumin (BSA) (Carl Roth, catalog number: T844 )
- Tween 20 (VWR, BDH®, catalog number: 663684B )
- Anti-phosphotyrosine antibody (EMD Millipore, catalog number: AB1607 )
- Migration buffer (see Recipes)
- 12% SDS-PAGE gels (see Recipes)
- Staining solution (see Recipes)
- Destaining solution (see Recipes)
- Assembly for the semi-dry transfer
a.Transfer solution 1 (see Recipes)
b.Transfer solution 2 (see Recipes)
c.Transfer solution 3 (see Recipes) - Tris-buffered saline (TBS) (see Recipes)
- TBS-5% BSA (see Recipes)
- TBS-0.1% Tween 20 (see Recipes)
- Anti-phosphothreonine antibodies solution (see Recipes)
- Anti-mouse-peroxydase antibodies solution (see Recipes)
- Square Petri dish (Greiner Bio One International, catalog number: 688162 )
*Note: These products have been discontinued.
Equipment
- Shaker/incubator at 37 °C and 20 °C for tubes and 500 ml Erlenmeyer flask
- Autoclave
- Ultrospec 10-cell density meter (GE Healthcare, Amersham Biosciences, model: Ultrospec® 10 )
- Centrifuges for conical and microtubes at 4 °C
- French pressure cell press (American instrument company)
- Tubes rotator for conical and microtubes
- Support of purification columns
- NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Thermo ScientificTM, model: NanoDropTM 2000 )
- Freezer -80 °C
- Lab water bath at 37 °C and 30 °C
- Dry block heater at 100 °C
- HoeferTM Dual Gel Caster system (GE Healthcare, Amersham Biosciences, model: Dual Gel Caster )
- Electrophoresis power supply
- Semi-dry blotter (C.B.S Scientific, catalog number: EBU-4000 ) with GD3000 D generator (Sebia)
- ChemiStart 5000 (Fisher Bioblock Scientific)
Procedure
- Production and extraction of bacterial protein kinase
To test the ability of a protein kinase to phosphorylate ARP2/3 complex, the protein must be overexpressed in the appropriate cell expression system and purified. For example, the gene encoding a protein kinase may be cloned in pGEX-6P-3 plasmid to produce a fusion protein with an N-terminal GST tag (the BamHI/SalI restriction sites can be often used in our cases). A catalytic variant of the protein kinase, with amino acid substitutions that abolish the kinase activity, should be generated from the same construct and used as a control. Mutagenesis may be accomplished using the QuikChange II site-directed mutagenesis kit (Stratagene), per the manufacturer’s instructions, to substitute the invariant lysine essential for donor-ATP binding with a methionine. The genetic constructs are then transformed in BL21 (pREP4-groESL) E.coli strain, which expresses the GroES and GroEL chaperon proteins to promote folding of the overproduced fusion protein of interest, here the GST-protein kinase.
Note: The pGEX-6P-3 plasmid is not always adaptable for the cloning and expression of your protein. In such cases, the pQE30 plasmid can also be used to generate a 6His affinity-tagged protein kinase. The purification protocol should then be adapted to include purification by immobilized metal affinity media (i.e., nickel beads).- Inoculate 5 ml of LB medium containing 100 µg/ml of ampicillin and 25 µg/ml of kanamycin with one colony of the overproducing strain described above.
- Incubate the culture overnight at 37 °C with agitation.
- Inoculate 100 ml of LB + ampicillin + kanamycin with 2 ml of overnight bacterial culture (dilution 1/50).
- Incubate the culture at 37 °C with agitation until the optical density reaches an OD600 nm of 0.7.
- Add 1 ml of 0.1 M IPTG (1 mM final concentration) in the culture to induce the overproduction of recombinant protein.
- Incubate for additional 4 h at 20 °C with agitation.
- Split the culture content equally in two conical tubes of 50 ml.
Note: For all remaining steps, the samples should be kept on in ice.
- Centrifuge the two tubes at 6,000 x g for 10 min at 4 °C.
- Decant the supernatants.
- Wash each bacteria pellet with 10 ml of prechilled dH2O.
- Pool the two pellets in one conical tube.
- Centrifuge at 6,000 x g for 10 min at 4 °C.
- Decant the supernatant.
- Resuspend the bacteria with 5 ml of prechilled 1x PBS and 50 µl of protease inhibitor cocktail.
- Lyse the bacteria by two passages through a French press.
- Centrifuge the extract for 30 min at 14,000 x g at 4 °C.
- Collect the supernatant that contains the soluble overproduced proteins.
- Inoculate 5 ml of LB medium containing 100 µg/ml of ampicillin and 25 µg/ml of kanamycin with one colony of the overproducing strain described above.
- Purification of bacterial protein kinase
- Equilibrate the glutathione agarose-4B resin:
- Pipette 250 µl resin in a microtube for each protein kinase to purify.
- Centrifuge 5 min at 1,000 x g.
- Discard the supernatants.
- Add 1 ml of 1x PBS.
- Gently invert the resins until well resuspended.
- Centrifuge 5 min at 1,000 x g.
- Repeat washing with 1x PBS 3 times.
- Load the supernatant containing overproduced proteins on the equilibrated glutathione resin.
- Incubate the proteins/resin mix for 4 h at 4 °C with gentle rotation.
- Load the proteins/resin mix on a column and allow the column to empty by gravity flow to separate the resin from the flow-through.
- Collect the flow-through and load into the column a second time to collect as much recombinant protein as possible.
- Wash the resin with 5 ml of 1x PBS. Allow PBS to move through the column by gravity flow. Repeat this washing step 3 times.
- Elute the GST tagged protein kinase with 1 ml of GST elution buffer twice and keep the sample in ice.
- Dialyze the eluate using a dialysis membrane:
- Cut the appropriate length of dialysis membrane.
- Incubate the membrane for 1 h in dH2O with gentle agitation to rinse.
- Place the eluate in a microtube, puncture a large hole in the cap and replace with the dialysis membrane.
- Invert the microtube (with dialysis membrane towards the buffer) in 1,000 volumes of dialysis buffer and dialyze overnight at 4 °C.
- Determine the protein concentration with a NanoDrop assay.
Note: Any method to determine the protein concentration may be used. - Dispense 100 µl aliquots of the protein into 1.5 ml prechilled microfuge tubes.
- Add 25 µl of glycerol (20% final).
- Store the proteins at -80 °C until needed.
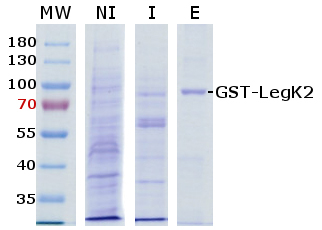
Figure 1. Example of purified bacterial protein kinase. Coomassie staining of SDS-PAGE gel with an overexpressed and purified bacterial protein kinase from the Legionella pneumophila bacterium, GST-LegK2. MW: molecular weight, NI: non-induced fraction of bacteria, I: induced fraction of bacteria, E: eluted fraction.
- Purification of ARP2/3 complex
- All reagents and the purification of ARP2/3 complex must be performed at 4 °C.
- Equilibrate a 1 ml polypropylene column containing VCA-coupled Sepharose with buffer A.
- Apply actin-depleted extracts of Acanthamoeba castellanii to the column at 4 °C.
Note: Actin-depleted extracts of Acanthamoeba castellanii prepared as previously described in Zuchero (2007). In vitro actin assembly assays and purification from Acanthamoeba. Methods Mol Biol 370: 213-226. - Wash with 20 column volumes of buffer A.
- Elute ARP2/3 with ARP2/3 elution buffer.
- Pass ARP2/3 enriched fractions over a 1 ml phenyl Sepharose column equilibrated in elution buffer followed by 1.5 column volumes of elution buffer.
- Collect the filtrate and identify fractions enriched in ARP2/3 by SDS-PAGE.
- Exchange ARP2/3 complex enriched fractions into ARP2/3 storage buffer using a PD10 column or dialysis.
- Aliquot ARP2/3 complex, flash freeze in liquid N2 and store at -80 °C.
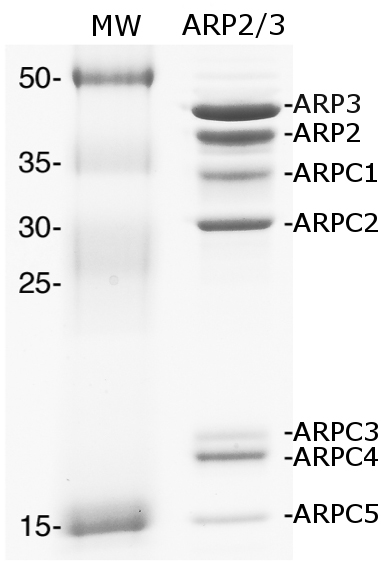
Figure 2. Purified ARP2/3 complex. Coomassie staining of SDS-PAGE gel with ARP2/3 complex purified from Acanthamoeba castellanii. MW: molecular weight.
- All reagents and the purification of ARP2/3 complex must be performed at 4 °C.
- Dephosphorylation of ARP2/3 complex
- Dilute ARP2/3 complex 1:1 with 2 mM Tris pH 8.0 and combine with HipH buffer containing 1 U Antarctic phosphatase.
- Incubate at 30 °C for 1.5 h.
- Mock-treated controls should be incubated with heat-inactivated phosphatase at 65 °C for 30 min.
- Affinity purify dephosphorylated ARP2/3 complex using N-WASP VCA-coupled to activated CH-Sepharose.
- Elute ARP2/3 complex from Sepharose with ARP2/3 elution buffer.
- Dialyze ARP2/3 against buffer A overnight at 4 °C.
- Dilute ARP2/3 complex 1:1 with 2 mM Tris pH 8.0 and combine with HipH buffer containing 1 U Antarctic phosphatase.
- In vitro phosphorylation assays
- Prepare the following reactions in duplicate in 1.5 ml microtubes:
Table 1. Reactions for the phosphorylation assays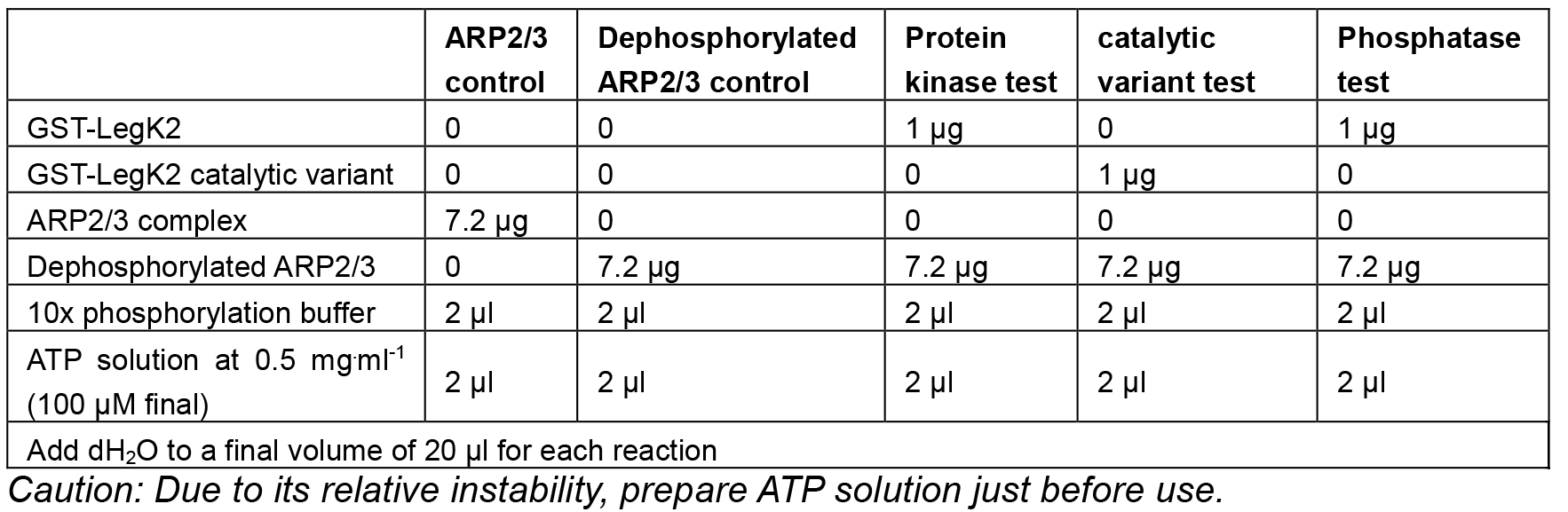
- Incubate the reactions for 30 min at 37 °C in water bath.
- In the phosphatase test tubes, add 2 µl of Antarctic phosphatase at 5,000 U/ml (10 U final) with 5 µl of its 5x HipH buffer and incubate for an additional 1 h at 30 °C in water bath.
- Stop the reactions by the addition of 5 µl of 5x Laemmli loading buffer.
- Prepare the following reactions in duplicate in 1.5 ml microtubes:
- Detection of phosphorylation level by Western blot with an anti-phosphothreonine antibody
- Prepare two SDS-PAGE gels at 12%.
- Heat the samples for 5 min at 100 °C in dry block heater.
- Load 20 µl of heated samples and 5 µl of protein ladder in gel and electrophoresis in SDS-PAGE migration buffer at 35 mA by gel for approximately 45 min.
Note: The amperage and time of migration are dependent of the gel size. - Stain one of the gels with Coomassie blue in a Petri dish.
- Stain the gel for 20 min in staining solution with gentle agitation.
- Wash the gel for 30 min to 1 h with gentle agitation in destaining solution.
- Analyze the different fractions.
- Perform a Western blot with the second gel.
- Transfer the proteins from the second gel to a nitrocellulose membrane in semi-dry conditions with 0.8 mA/cm2 for 1 h.
- Place the membrane in a dish adapted for agitation or a conical tube. The side of membrane with proteins must not be in contact with the container and the solutions must completely cover the membrane.
- Block the membrane for 1 h at room temperature with gentle rotation in TBS-5% BSA.
- Wash the membrane 3 x 5 min in TBS-0.1% Tween 20.
- Incubate the membrane for 1 h at room temperature with gentle rotation in anti-phosphothreonine antibodies solution.
Note: Following this protocol, we detect threonine phosphorylation level. Tyrosine phosphorylation level of ARP2/3 complex can also be detected using an anti-phosphotyrosine antibody (EMD Millipore, catalog number: AB1607 ) or another anti-phosphotyrosine antibody, adjust the procedure to follow the manufacturer’s recommendations if necessary. - Wash the membrane 3 x 5 min in TBS-0.1% Tween 20.
- Incubate the membrane for 1 h at room temperature with gentle rotation in anti-mouse-peroxidase antibody solution.
- Wash the membrane 3 x 5 min in TBS-0.1% Tween 20.
- Wash the membrane with TBS.
- Detect the anti-phosphothreonine labeling with SuperSignal West Pico Chemiluminescent Substrate Kit.
- Place 1 ml of chemiluminescent substrate (500 µl of stable peroxide solution + 500 µl of Luminol/Enhancer solution) on a sheet of Parafilm.
- Place the membrane on the chemiluminescent substrate directly so that the proteins are in direct contact with the peroxidase substrate.
Note: Caution, remove any bubbles that are present between the membrane and the substrate. - Incubate 5 min in the dark.
- Drain the solution from the membrane and place it on a support.
- Cover the membrane with plastic wrap to prevent drying.
- Image membrane at different exposures with a ChemiStart or appropriate detection system.
- Place 1 ml of chemiluminescent substrate (500 µl of stable peroxide solution + 500 µl of Luminol/Enhancer solution) on a sheet of Parafilm.
- Other methods for detecting the chemiluminescent signal may be used according to recommendations by the equipment manufacturer.
- You may also detect the autophosphorylation state of your protein kinase. However, we suggest that the analysis be performed at a different polyacrylamide concentration if the molecular weights of the kinase and its substrates are substantially different.
- The original publication describing ARP2/3 phosphorylation can also be help to set the conditions and for the analysis: LeClaire, L. L. 3rd, Baumgartner, M., Iwasa, J. H., Mullins, R. D., Barber, D. L. (2008). Phosphorylation of the Arp2/3 complex is necessary to nucleate actin filaments. J Cell Biol 182(4):647-54.
- Prepare two SDS-PAGE gels at 12%.
Notes
- To optimize the expression and purification of bacterial protein, all the fractions corresponding to each steps of protocol may be analyzed by Coomassie blue staining after SDS-PAGE.
- The bacterial pellet of step A13 can be frozen at -80 °C.
- For our ARP2/3 phosphorylation assays, we used the anti-phosphothreonine antibody from Sigma. However, we found that labeling by the antibody is inconsistent and requires optimization of the experimental conditions. To test the phosphorylation activity, we found that the polyclonal phosphothreonine antibody from Cell Signaling (#9381) showed more consistent labeling patterns and was more specific for labeling.
- Several steps of the Western blot analysis can be performed overnight; the transfer (at 0.2 mA/cm2); blocking (at 4 °C); or incubation with the primary antibody (at 4 °C).
- Wear gloves to protect the samples from proteases and/or contaminating proteins.
Recipes
- LB medium + ampicillin (100 µg/ml) + kanamycin (25 µg/ml)
0.2 ml of 100 mg/ml ampicillin stock solution
25 µl of 50 mg/ml kanamycin stock solution
200 ml of LB medium - LB medium
20 g of LB medium (Lennox)
1 L of dH2O
Aliquot by 200 ml
Sterilized by autoclaving - 100 mg/ml ampicillin stock solution
5 g of ampicillin
50 ml of dH2O
Sterilize by filtration
Aliquot and freeze the solution until needed - 50 mg/ml kanamycin stock solution
2.5 g of kanamycin
50 ml of dH2O
Sterilize by filtration
Aliquot and freeze the solution until needed - 0.1 M IPTG
238 mg of IPTG
10 ml of dH2O
Sterilize by filtration
Aliquot and freeze until needed - 10x PBS (1 L)
80 g NaCl
2 g KCl
14.4 g Na2HPO4
2.4 g KH2PO4
Adjust pH to 7.4
Add dH2O to 1 L - 1x PBS (1 L)
100 ml of 10x PBS
900 ml of dH2O - GST elution buffer
50 mM Tris HCl pH 8.0
10 mM glutathione - Dialysis buffer
50 mM Tris HCl pH 7.5
150 mM NaCl
10 % glycerol - ARP2/3 elution buffer
10 mM Tris, pH 8.0
400 mM MgCl2 - ARP2/3 storage buffer
1 mM HEPES
1 mM DTT
10% glycerol
Adjust pH to 7.0 - 10x phosphorylation buffer
250 mM Tris HCl pH 7.5
50 mM MnCl2
50 mM DTT - ATP solution at 0.5 mg/ml
1 mg of ATP
2 ml of dH2O
Prepare immediately before use - 5x HipH buffer
50 mM Tris pH 8.0
1 mM Mg2Cl2
0.1 mM ZnCl2 - Buffer A 10x solution
500 mM KCl
20 mM MgCl2
10 mM ATP
100 mM Tris, pH 7.5 - 5x Laemmli loading buffer
5 ml 10% SDS
6.25 ml 0.5 M Tris HCl pH 6.8
5 ml glycerol
7.5 ml dH2O
1 pinch of bromophenol blue
2.5 µl 1 M β-mercaptoethanol - 12% SDS-PAGE gels (for 2 mini-gels)
- Stacking gels
1 ml 30% ProtoGel (30% acrylamide, 0.8% bisacrylamide)
1.25 ml 0.5 M Tris HCl pH 6.8
2.65 ml dH2O
50 µl 10% SDS
50 µl 10% APS
5 µl TEMED - Separating gels
4 ml 30% ProtoGel (30% acrylamide, 0.8% bisacrylamide)
2.5 ml 1.5 M Tris HCl pH 8.8
3 ml dH2O
100 µl 10% SDS
100 µl 10% APS
10 µl TEMED - Migration buffer
25 mM Tris
192 mM glycine
0.1% SDS - Staining solution
10% glacial acetic acid
40% ethanol
0.04% Coomassie Brilliant Blue R-250 - Destaining solution
10% glacial acetic acid
5% ethanol - Transfer solution 1
300 mM Tris
20% methanol - Transfer solution 2
25 mM Tris
20% methanol - Transfer solution 3
25 mM Tris
40 mM 6-aminohexanoic acid
20% methanol - Assembly for the semi-dry transfer
Cathode (-)
Anode (+) - TBS
100 mM Tris
150 mM NaCl
Adjust pH to 8 - TBS-5% BSA
0.5 g of BSA
10 ml TBS - TBS-0.1% Tween 20
1 ml of Tween 20
1 L TBS - Anti-phosphothreonine antibodies solution
PBS-5% BSA-0.1% Tween 20
Monoclonal mouse anti-phosphothreonine antibody (1/500) - Anti-mouse-peroxydase antibodies solution
TBS-5% BSA-0.1% Tween 20
Goat anti-mouse IgG peroxydase antibody (1/5,000)
Acknowledgments
This work was performed within the framework of the LABEX ECOFECT (ANR-11-LABX-0042) of the Université de Lyon, within the program Investissements d’avenir (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR). This work was funded by the Centre National de la Recherche Scientifique (UMR 5308), the Institut National de la Recherche Médicale (U1111) and the Université Lyon 1.
This protocol was derived from previously described protocols: (Hervet et al., 2011; Zuchero, 2007 and LeClaire et al., 2008)
References
- Amrein, K. E., Takacs, B., Stieger, M., Molnos, J., Flint, N. A. and Burn, P. (1995). Purification and characterization of recombinant human p50csk protein-tyrosine kinase from an Escherichia coli expression system overproducing the bacterial chaperones GroES and GroEL. Proc Natl Acad Sci U S A 92(4): 1048-1052.
- Haglund, C. M. and Welch, M. D. (2011). Pathogens and polymers: microbe-host interactions illuminate the cytoskeleton. J Cell Biol 195(1): 7-17.
- Hervet, E., Charpentier, X., Vianney, A., Lazzaroni, J. C., Gilbert, C., Atlan, D. and Doublet, P. (2011). Protein kinase LegK2 is a type IV secretion system effector involved in endoplasmic reticulum recruitment and intracellular replication of Legionella pneumophila. Infect Immun 79(5): 1936-50.
- LeClaire, L. L., 3rd, Baumgartner, M., Iwasa, J. H., Mullins, R. D. and Barber, D. L. (2008). Phosphorylation of the Arp2/3 complex is necessary to nucleate actin filaments. J Cell Biol 182(4): 647-654.
- LeClaire, L. L., Rana, M., Baumgartner, M. and Barber, D. L. (2015). The Nck-interacting kinase NIK increases Arp2/3 complex activity by phosphorylating the Arp2 subunit. J Cell Biol 208(2): 161-170.
- Michard, C., Sperandio, D., Bailo, N., Pizarro-Cerda, J., LeClaire, L., Chadeau-Argaud, E., Pombo-Gregoire, I., Hervet, E., Vianney, A., Gilbert, C., Faure, M., Cossart, P. and Doublet, P. (2015). The legionella kinase LegK2 targets the ARP2/3 complex to inhibit actin nucleation on phagosomes and allow bacterial evasion of the late endocytic pathway. MBio 6(3): e00354-00315.
- Narayanan, A., LeClaire, L. L., 3rd, Barber, D. L. and Jacobson, M. P. (2011). Phosphorylation of the Arp2 subunit relieves auto-inhibitory interactions for Arp2/3 complex activation. PLoS Comput Biol 7(11): e1002226.
- Smith, D. B. and Johnson, K. S. (1988). Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene 67(1): 31-40.
- Vadlamudi, R. K., Li, F., Barnes, C. J., Bagheri-Yarmand, R. and Kumar, R. (2004). p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep 5(2): 154-160.
- Welch, M. D. and Way, M. (2013). Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe 14(3): 242-255.
- Zuchero, J. B. (2007). In vitro actin assembly assays and purification from Acanthamoeba. Methods Mol Biol 370: 213-226.
Article Information
Copyright
© 2017 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Michard, C., LeClaire, L. L. and Doublet, P. (2017). ARP2/3 Phosphorylation Assay in the Presence of Recombinant Bacterial Effectors. Bio-protocol 7(7): e2208. DOI: 10.21769/BioProtoc.2208.
Category
Microbiology > Microbial biochemistry > Protein > Modification
Cell Biology > Cell movement > Cell motility
Biochemistry > Protein > Modification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










