- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Affinity Pulldown of Biotinylated RNA for Detection of Protein-RNA Complexes
Published: Vol 6, Iss 24, Dec 20, 2016 DOI: 10.21769/BioProtoc.2062 Views: 26371
Reviewed by: Antoine de MorreeXiaoyi ZhengEmily Cope

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
In situ Hybridization (ISH) and Quantum Dots (QD) of miRNAs
Sajni Josson [...] Leland W.K. Chung
Feb 20, 2017 11570 Views
miRNA Characterization from the Extracellular Vesicles
Sajni Josson [...] Leland W.K. Chung
Feb 20, 2017 8945 Views
Detection of Individual RNA in Fixed Cells and Tissues by Chromogenic ISH
Meng Jiang [...] Rongqin Ke
Feb 5, 2020 5119 Views
Abstract
RNA-binding proteins (RBPs) have recently emerged as crucial players in the regulation of gene expression. The interactions of RBPs with target mRNAs control the levels of gene products by altering different regulatory steps, including pre-mRNA splicing and maturation, nuclear mRNA export, and mRNA stability and translation (Glisovic et al., 2008). There are several methodologies available today to identify RNAs bound to specific RBPs; some detect only recombinant molecules in vitro, others detect recombinant and endogenous molecules, while others detect only endogenous molecules. Examples include systematic evolution of ligands by exponential enrichment (SELEX), biotinylated RNA pulldown assay, RNA immunoprecipitation (RIP) assay, electrophoretic mobility shift assay (EMSA), RNA footprinting analysis, and various UV crosslinking and immunoprecipitation (CLIP) methods such as CLIP, PAR-CLIP, and iCLIP (Popova et al., 2015). Here, we describe a simple and informative method to study and identify the RNA region of interaction between an RBP and its target transcript (Panda et al., 2014 and 2016). Its reproducibility and ease of use make this protocol a fast and useful method to identify interactions between RBPs and specific RNAs.
Keywords: Tagged RNABackground
RNA-protein interactions critically influence gene expression patterns. The identification of these ribonucleoprotein (RNP) complexes is essential for understanding the regulatory mechanisms governed by RNA-binding proteins (RBPs). Recently, extensive efforts have led to the development of methods for systematic analysis of RNA-protein interactions. Highly informative methods to identify RNP complexes include a number of different types of RNP immunoprecipitation (IP) analyses. RIP methods involve RNP IP without crosslinking, while CLIP methods involve crosslinking of the RNP before IP. While RIP is fast, inexpensive, and capable of assessing many endogenous RBPs and RNAs, it does not typically permit the identification of the precise RNA region that interacts with the RBP. CLIP analysis (including its variant forms HITS-CLIP, PAR-CLIP, and iCLIP) does allow the discovery of the precise RNA sequences that interact with an RBP, as it includes an RNase step that digests all unprotected RNA and yields the RNA bound to the RBP. However, CLIP analysis is costly, time-consuming, and technically challenging (Panda et al., 2016). Therefore, alternatives to testing the binding of endogenous proteins to RNAs of interest are needed.
The biotinylated RNA-pulldown method described here theoretically works for all RBPs, as this assay is performed in a cell-free system. The method involves the in vitro synthesis of RNAs of interest in the presence of biotinylated CTP; the RNA tagged in this manner is then incubated with a cell-free system to allow RBPs to recognize RNA regions to which it has affinity, while regions without affinity do not interact with RBPs. After the binding is complete, the biotinylated RNA is pulled down using streptavidin-coated beads and the RBPs are typically detected by Western blot analysis. This method can be used to map the RNA sequence with which the RBP interacts if the user tests progressively smaller RNA fragments in a systematic fashion, as described here. Furthermore, this method allows for the identification of all of the proteins that interact with the RNA of interest if the biotin-RNA pulldown is followed by mass spectroscopy. In summary, this approach can successfully identify the interaction of an endogenous (or recombinant) RBP with in vitro-synthesized RNAs of interest.
Materials and Reagents
- ThermoGridTM rigid strip 0.2-ml PCR tubes (Denville Scientific, catalog number: C18064 [1000859])
- Posi-Click 1.7-ml microcentrifuge tube (Denville Scientific, catalog number: C2171 )
- 1.5-ml tubes
- 10-cm dishes
- NucAway spin columns (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM10070 )
- Disposable cuvettes, 1.5-ml (Stockwell Scientific, catalog number: 2410 )
- Nuclease-free water (Thermo Fisher Scientific, AmbionTM, catalog number: AM9930 )
- cDNA prepared from total RNA
- DreamTaq DNA polymerase (5 U/µl) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: EP0701 )
- dNTP mix (10 mM each) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: R0193 )
- Agarose LE (Denville Scientific, catalog number: CA3510-8 )
- QIAquick Gel Extraction Kit (50) (QIAGEN, catalog number: 28704 )
- MEGAshortscriptTM T7 Kit with manual (for RNA shorter than 0.5 Kb) (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM1354 )
- RiboLock RNase inhibitor (40 U/µl) (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: EO0381 )
- Biotin-14-CTP (Thermo Fisher Scientific, InvitrogenTM, catalog number: 19519-016 )
- MEGAscript® T7 Transcription Kit (*for RNA longer than 0.5 Kb) (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM1333 )
- Novex® TBE-urea gels, 6% (Thermo Fisher Scientific, InvitrogenTM, catalog number: EC6865BOX )
- 1x TBE buffer
- Dulbecco’s phosphate-buffered saline (DPBS) (Thermo Fisher Scientific, GibcoTM, catalog number: 14040-133 )
- cOmplete protease inhibitor cocktail (Sigma-Aldrich, catalog number: 11697498001 )
- 2x Laemmli sample buffer (Bio-Rad Laboratories, catalog number: 1610737 )
- Dynabeads® M-280 streptavidin (Thermo Fisher Scientific, InvitrogenTM, catalog number: 11205D )
- 2-mercaptoethanol (β-mercaptoethanol/BME)
- 10x Tris/glycine/SDS running buffer (Bio-Rad Laboratories, catalog number: 1610732 )
- 4-20% Mini-PROTEAN® TGX Stain-FreeTM protein gels (Bio-Rad Laboratories, catalog number: 4568094 )
- Ethidium bromide solution (Sigma-Aldrich, catalog number: E1510 )
- SpectraTM multicolor broad range protein ladder (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 26634 )
- Bio-Rad protein assay dye reagent concentrate (Bradford reagent) (Bio-Rad Laboratories, catalog number: 500-0006 )
- Tris-HCl (pH 8.0)
- KCl
- MgCl2
- Nonidet P-40
- EDTA
- NaCl
- Triton X-100
- Polysome extraction buffer (PEB) (see Recipes)
- 2x Tris, EDTA, NaCl, Triton (TENT) buffer (see Recipes)
- 1x TENT (see Recipes)
Equipment
- PCR strip tube rotor, mini centrifuge C1201 (Denville Scientific, catalog number: C1201-S [1000806])
- Veriti® 96-Well thermal cycler (Thermo Fisher Scientific, Applied BiosystemsTM, catalog number: 4375786 )
- Ultraviolet transilluminator
- NanoDrop One spectrophotometer (Thermo Fisher Scientific, Thermo Scientific, catalog number: ND-ONE-W )
- Eppendorf Thermomixer® R (Eppendorf, catalog number: 022670581 )
- Incubator
- Vortexer
- Cell scrapers
- Refrigerated centrifuge (Eppendorf, model: 5430R )
- SmartSpec Plus spectrophotometer (Bio-Rad Laboratories, catalog number: 1702525 ) or other spectrophotometer with 595 nm wavelength
- MagneSphere(R) stand (Promega, catalog number: Z5342 )
- Trans-Blot® TurboTM transfer starter system (Bio-Rad Laboratories, catalog number: 1704155 )
- Mini-PROTEAN® Tetra vertical electrophoresis cell (Bio-Rad Laboratories, catalog number: 1658004 )
Procedure
- Primer design and template generation for in vitro transcription
- The forward and reverse primers with a length of 20-25 nt and melting temperature of ~60 °C are designed using Primer 3 online tool (http://bioinfo.ut.ee/primer3-0.4.0/) (Untergasser et al., 2012) (Note 1).
- As shown in Figure 1, add the T7 RNA polymerase promoter sequence (T7) [5’AGTAATACGACTCACTATAGGG] (red lines) upstream of the actual forward primer sequence (Figure 1).
- Dissolve the primers in nuclease-free water to a final concentration of 100 µM, then prepare a primer mix of forward and reverse primers at a final concentration of 1 µM each in nuclease-free water (e.g., 10 µl of each primer from the 100 µM stock into 980 µl of nuclease-free water and mix well).
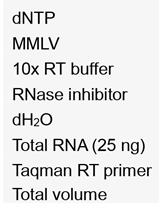
- For one PCR reaction of 40 µl in a 0.2-ml PCR tube, add 4 µl of 10x DreamTaq DNA polymerase buffer, 10 µl of 1 µM primer mix, 1 µl DreamTaq DNA polymerase, 1 µl of 10 mM dNTP mix, 0.2 µl (~2 ng) cDNA, and water to make the final volume 40 µl. (The cDNA was prepared from total RNA as described previously [Panda et al., 2016].)
- Mix the reaction by vortexing for 5 sec and centrifuge the PCR tube strip for 30 sec using minicentrifuge to settle the reactions at the bottom of the wells.
- Set up the Veriti® 96-Well Thermal Cycler program as follows: 5 min at 95 °C and 40 cycles of 5 sec at 95 °C and 60 sec at 60 °C.
- Resolve the PCR products on an ethidium bromide stained 2% agarose gel for 1 h at 100 volts and visualize with an ultraviolet transilluminator.
- Cut the desired bands from the gel. Purify the PCR products corresponding to each fragment using the QIAquick Gel Extraction Kit and dissolve in elution buffer provided with the kit.
- Measure the concentration of the PCR products/templates with a NanoDrop spectrophotometer.
- The PCR product (T7 DNA template) can be stored at -20 °C or -80 °C, or used immediately for in vitro transcription.
- The forward and reverse primers with a length of 20-25 nt and melting temperature of ~60 °C are designed using Primer 3 online tool (http://bioinfo.ut.ee/primer3-0.4.0/) (Untergasser et al., 2012) (Note 1).
- In vitro transcription of biotinylated RNA fragments
- The biotinylated RNA can be prepared using the MEGAshortscriptTM T7 Kit following the manufacturer’s protocol.

Figure 1. Schematic of biotinylated RNA pulldown assay - In vitro transcription reaction for each DNA template can be prepared in a 1.7-ml PCR tube containing 2 µl of 10x MEGAshortscriptTM T7 buffer, 2 µl of MEGAshortscriptTM T7 enzyme mix, 1 µl of RiboLock, 1 µl of 75 mM rATP, 1 µl of 75 mM rUTP, 1 µl of 75 mM rGTP, 0.8 µl of 75 mM rCTP, 1.5 µl of 10 mM Biotin-14-CTP, 100 ng of T7 DNA template and water to make the final volume 20 µl. (For longer RNA fragments [> 500 nt] the user should employ the MEGAscriptTM T7 Kit for in vitro RNA preparation) (Notes 5 and 6)
- Mix and centrifuge for ~5 sec at 500 x g to settle the reaction mixture at the bottom of the tube.
- Incubate the reaction at 37 °C for 4 h in a Thermomixer (Note 2).
- Add 1 µl of DNase provided in the kit and mix well followed by incubation for 15 min at 37 °C.
- Store the samples at -20 °C or -80 °C or proceed immediately to RNA purification.
- The biotinylated RNA can be prepared using the MEGAshortscriptTM T7 Kit following the manufacturer’s protocol.
- Purification and quality check of in vitro-transcribed biotinylated RNA fragments
- Dilute the entire 20 µl in vitro transcribed RNA by adding 50 µl of nuclease-free water and keep on ice.
- Use one column (NucAway Spin Columns Kit) for each RNA fragment.
- Tap the powders to the bottom of columns and add 650 µl of nuclease-free water followed by vortexing at the highest speed for 10 sec.
- Incubate the column for 15 min at room temperature followed by centrifugation for 2 min at 750 x g.
- Pipet the in vitro-transcribed RNA onto the top of the column and put the column in a fresh 1.5-ml tube.
- Centrifuge the column for 2 min at 750 x g to collect the flow-through containing biotinylated RNA.
- The RNA concentration can be determined using the NanoDrop spectrophotometer.
- Store the purified samples at -20 °C or -80 °C until the quality of RNA is checked.
- To check the quality of the RNA, transfer 1 µg of transcribed RNA to a fresh tube and denature for 5 min at 70 °C with RNA sample preparation buffer provided in the MEGAshortscript kit.
- Resolve on a 6% TBE-Urea gel with 1x TBE buffer and visualize the RNAs on an ultraviolet transilluminator after staining with ethidium bromide.
- All RNA fragments should show single bands of the expected size.
- Store the RNA samples at -20 °C or -80 °C or use in the pulldown reaction.
- Dilute the entire 20 µl in vitro transcribed RNA by adding 50 µl of nuclease-free water and keep on ice.
- Preparation of cell lysate
- Wash two 10-cm dishes of ~70% confluent cells of interest three times with PBS and collect them using a cell scraper. As the protein yield varies with the cell line, the user should adjust the number of cells needed to extract at least 500 µg protein for each pulldown assay.
- Pellet the cells in a 1.7-ml tube by centrifuging for 5 min at 500 x g.
- Disrupt the cell pellet by pipetting 10-20 times with 500 µl of polysome extraction buffer (PEB) containing protease and RNase inhibitors. Incubate for 10 min on ice.
- Collect the supernatant after centrifugation at 15,000 x g for 10 min at 4 °C using a refrigerated centrifuge.
- Determine protein concentrations by the Bradford protein assay following the manufacturer’s instructions using disposable cuvettes and a spectrophotometer at 595 nm wavelength.
- Store protein samples at -20 °C or -80 °C or use directly in pulldown reaction.
- Wash two 10-cm dishes of ~70% confluent cells of interest three times with PBS and collect them using a cell scraper. As the protein yield varies with the cell line, the user should adjust the number of cells needed to extract at least 500 µg protein for each pulldown assay.
- Pulldown of interacting RNA-binding protein
- Prepare the pulldown reaction in 1.5-ml tube containing 500 µg of cell lysate, 1 µg of purified biotinylated RNA, 1x protease inhibitor (add fresh), 5 µl of RiboLock, 250 µl of 2x TENT buffer (Tris, EDTA, NaCl, Triton; see Recipes) and PEB in 500 µl final volume.
- Mix the reaction by pipetting and incubate at room temperature for 30 min.
- In the meantime, wash 50 µl streptavidin-coupled Dynabeads for each reaction three times with 1x TENT buffer using the MagneSphere stand.
- Add 50 µl washed beads to the RNA-protein mixture prepared in step E1 (Note 7).
- Mix the beads by pipetting up and down.
- Incubate the reaction at room temperature for another 30 min with intermittent mixing by tapping the tube every ~5 min.
- Incubate the tube on the magnetic stand for 1 min to allow the magnetic beads to settle to one side of the tube.
- Discard the supernatant and mix the magnetic beads with 1 ml of ice-cold 1x TENT buffer.
- Repeat steps E7 and E8 3 more times.
- Add 40 µl of 1x Laemmli sample buffer supplemented with β-mercaptoethanol to the magnetic beads.
- Heat the samples for 5 min at 95 °C and proceed to Western blotting.
- Prepare the pulldown reaction in 1.5-ml tube containing 500 µg of cell lysate, 1 µg of purified biotinylated RNA, 1x protease inhibitor (add fresh), 5 µl of RiboLock, 250 µl of 2x TENT buffer (Tris, EDTA, NaCl, Triton; see Recipes) and PEB in 500 µl final volume.
- Detection of interacting RNA-binding proteins (RBPs)
- Use Western blotting techniques to detect the RBP interest in the pulldown. Briefly, load at least three lanes on a 4-20% SDS-PAGE gel: (1) 10 µg (or 2% of the protein used for pulldown) of cell lysate as input, (2) the entire sample from the pulldown assay, and (3) a pre-stained protein ladder. Resolve these samples by electrophoresis following the manufacturer’s protocol.
- Transfer the protein to a nitrocellulose membrane using a Trans-Blot transfer system following the manufacturer’s instructions.
- Use standard Western blotting techniques to detect the RBP of interest using primary antibodies recognizing that RBP (Mahmood and Yang, 2012).
- Incubate with the appropriate secondary antibody to recognize the primary antibody and detect the signals using Enhanced Chemiluminescence (ECL).
- Use Western blotting techniques to detect the RBP interest in the pulldown. Briefly, load at least three lanes on a 4-20% SDS-PAGE gel: (1) 10 µg (or 2% of the protein used for pulldown) of cell lysate as input, (2) the entire sample from the pulldown assay, and (3) a pre-stained protein ladder. Resolve these samples by electrophoresis following the manufacturer’s protocol.
Data analysis
As shown in Figure 1, Western blot analysis reveals the presence of the RBP in the input lysate (positive control) and in the pulldown material using biotinylated fragment C, suggesting that the sequence in fragment C includes a region with which this RBP interacts. This analysis includes negative control samples ‘Beads only’ and ‘Neg. control biot-RNA’, which do not pulldown the RBP, and shows that fragments A and B do not bind the RBP, supporting the conclusion that binding of the RBP to fragment C is specific. The experiment should be repeated at least three times to conclude the specificity of the RNA-RBP interaction.
Notes
- The size of the RNA fragment can be decided by the researcher, but an overlap of at least 20 bp between adjacent fragments is recommended.
- Longer (more than 4 h) incubation of in vitro transcription reaction may lead to degradation of the in vitro-transcribed RNA.
- As a control, use a ‘beads only’ sample without any biotinylated RNA to see if the magnetic beads alone will bind to RBP of interest in this pulldown condition.
- Use a negative control (NC) biotinylated RNA such as GAPDH/ACTB that does not bind the RBP studied in order to check the specificity of interaction between the RBP and the mRNA of interest.
- This protocol uses a ratio of 1 biotin-CTP:4 unlabeled CTP (i.e., 1 in 5 CTP is biotin-labeled). The ratio between biotin-CTP and unlabeled CTP is adjusted, and the user has to be mindful of the number of Cs present in the RNA product. For long transcripts, the biotin-CTP ratio can be lowered to 1 biotin-CTP:10 unlabeled CTP.
- Labeling the RNA at higher than 1:4 ratio of biotinylated to non-biotinylated CTP may inhibit RBP binding. The user must ensure that each RNA fragment has at least 1 or 2 biotinylated Cs in its sequence.
- Using higher amount of biotin-labeled RNA does not help and may be detrimental to the pulldown assay, as 50 µl of streptavidin magnetic beads can bind ~100 pmol of biotin-labeled RNA.
- Longer RNA may result in false positive pulldown result as the RBP of interest may interact with the RNA bait indirectly through another RBP.
- Lysis of cells in PEB isolates cytoplasmic fractions effectively. Thus, this protocol is best suited for studying the interactions of cytoplasmic proteins with RNAs of interest. RBPs interacting with the RNA bait in vitro may or may not interact in the cells due to differential subcellular localization of RNA or protein of interest.
Recipes
- Polysome extraction buffer (PEB) in nuclease-free water
20 mM Tris-HCl (pH 7.5)
100 mM KCl
5 mM MgCl2
0.5% Nonidet P-40 - 2x Tris, EDTA, NaCl, Triton (TENT) buffer in nuclease-free water
20 mM Tris-HCl (pH 8.0)
2 mM EDTA (pH 8.0)
500 mM NaCl
1% (v/v) Triton X-100 - 1x TENT
Equal volumes of 2x TENT and nuclease-free water mixed together
Acknowledgments
This work was supported by the National Institute on Aging Intramural Research Program, National Institutes of Health.
References
- Glisovic, T., Bachorik, J. L., Yong, J. and Dreyfuss, G. (2008). RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett 582(14): 1977-1986.
- Mahmood, T. and Yang, P. C. (2012). Western blot: technique, theory, and trouble shooting. N Am J Med Sci 4(9): 429-34.
- Panda, A. C., Abdelmohsen, K., Martindale, J. L., Di Germanio, C., Yang, X., Grammatikakis, I., Noh, J. H., Zhang, Y., Lehrmann, E., Dudekula, D. B., De, S., Becker, K. G., White, E. J., Wilson, G. M., de Cabo, R. and Gorospe, M. (2016). Novel RNA-binding activity of MYF5 enhances Ccnd1/Cyclin D1 mRNA translation during myogenesis. Nucleic Acids Res 44(5): 2393-2408.
- Panda, A. C., Abdelmohsen, K., Yoon, J. H., Martindale, J. L., Yang, X., Curtis, J., Mercken, E. M., Chenette, D. M., Zhang, Y., Schneider, R. J., Becker, K. G., de Cabo, R. and Gorospe, M. (2014). RNA-binding protein AUF1 promotes myogenesis by regulating MEF2C expression levels. Mol Cell Biol 34(16): 3106-3119.
- Popova, V. V., Kurshakova, M. M. and Kopytova, D. V. (2015). [Methods to study the RNA-protein interactions]. Mol Biol (Mosk) 49(3): 472-481.
- Untergasser, A., Cutcutache, I., Koressaar, T., Ye, J., Faircloth, B. C., Remm, M. and Rozen, S. G. (2012). Primer3—new capabilities and interfaces. Nucleic Acids Res 40(15): e115-e115.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Panda, A. C., Martindale, J. L. and Gorospe, M. (2016). Affinity Pulldown of Biotinylated RNA for Detection of Protein-RNA Complexes. Bio-protocol 6(24): e2062. DOI: 10.21769/BioProtoc.2062.
Category
Cancer Biology > General technique > Biochemical assays > RNA
Molecular Biology > RNA > RNA-protein interaction
Molecular Biology > Protein > Detection
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









