- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Transfection of Embryoid Bodies with miRNA Precursors to Induce Cardiac Differentiation
Published: Vol 6, Iss 3, Feb 5, 2016 DOI: 10.21769/BioProtoc.1726 Views: 9448
Reviewed by: Jingli CaoAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Isolation of Neural Stem Cells from the Embryonic Mouse Hippocampus for in vitro Growth or Engraftment into a Host Tissue
Oksana Rybachuk [...] Vitaliy Kyryk
Feb 20, 2019 10066 Views
Generation of Mouse Primitive Endoderm Stem Cells
Yasuhide Ohinata [...] Haruhiko Koseki
Nov 20, 2023 2440 Views
An Efficient Method for Immortalizing Mouse Embryonic Fibroblasts by CRISPR-mediated Deletion of the Tp53 Gene
Srisathya Srinivasan and Hsin-Yi Henry Ho
Jan 20, 2025 2721 Views
Abstract
In recent years, the utilization of stem cell therapy to regenerate cardiac tissue has been proposed as a possible strategy to treat cardiac damage (Gnecchi et al., 2012, Aguirre et al., 2013; Sanganalmath and Bolli, 2013). Although encouraging results have been obtained in experimental models, the efficiency of cardiac regeneration is very poor and one of the major barriers to progress in the area of cell therapy for damaged heart is represented by the limited capacity of cells to differentiate into mature cardiomyocytes (CMC) (Laflamme and Murry, 2011). Cell manipulation and transfection represent versatile tools in this context (Melo et al., 2005; Dzau et al., 2005). Murine P19 embryonal carcinoma cells are a well-established cell line capable of differentiating in vitro into spontaneously beating CMC. This cell system with its limited cell culture requirements, protocol reproducibility and ease in uptake and subsequent expression of ectopic genetic materials render it ideal for the study of the cardiac differentiation process. P19 cells have been successfully used to gain important insights into the early molecular processes of CMC differentiation (van der Heyden and Defize, 2003; van der Heyden et al., 2003). P19 cells can also be maintained in an undifferentiated state in a monolayer culture when grown in adherence; this condition allows the enrichment of large cell numbers useful for cardiac differentiation protocols (McBurney, 1993). On the other hand, when cultured in bacterial dishes, P19 cells will grow in suspension and generate embryoid bodies (EB). When exposed to dimethyl sulfoxide (DMSO), EB differentiate into spontaneously beating cells, which can be defined as CMC. This definition is based on their gene and protein expression and their electrophysiological properties (Wobus et al., 1994; van der Heyden et al., 2003). In our laboratory, we used this in vitro model to verify whether the over-expression of a defined combination of miRNA can synergistically induce effective cardiac differentiation (Pisano et al., 2015). We used miRNA1, miRNA133 and miRNA499 alone or in combination. Here, we describe how we transiently transfect P19 cells to over-express a single or a combination of miRNA precursors (pre-miRNA).
Keywords: MicroRNAMaterials and Reagents
- Bacterial dishes (100 x 15 mm) (Corning, catalog number: 351006 )
- Standard culture Petri dishes (100 x 15 mm) (Corning, catalog number: 70165-101 )
- 6 multiwell-plates (Corning, catalog number: 353224 )
- 50 ml tubes (Falcon®, catalog number: 352098 )
- P19 cells (Izsler-Istituto Zooprofilattico Sperimentale, Lombardy and Emilia Romagna “Bruno Ubertini”, Brescia, Italy) (Izsler, catalog number: BS-TCL 206 , Passage 0)
- Minimum Essential Medium alpha (α-MEM) (Sigma-Aldrich, catalog number: M8042 )
- Penicillin-streptomycin 10,000 U/ml (Thermo Fisher Scientific, GibcoTM, catalog number: 15140-122 )
- L-glutamine 2 nM (Thermo Fisher Scientific, GibcoTM, catalog number: 25030-081 )
- Dimethyl sulfoxide (Sigma-Aldrich, catalog number: D4540-100 ml )
- Fetal Bovine Serum (FBS) (Sigma-Aldrich, catalog number: F6178-50 ml )
- Trypsin-EDTA (0.5%), no phenol red (Thermo Fisher Scientific, GibcoTM, catalog number: 15400-054 )
- Optimem® (Thermo Fisher Scientific, GibcoTM, catalog number: 31985-070 )
- siPORT® NeoFX transfection agent (Thermo Fisher Scientific, Invitrogen™, catalog number: AM4511 )
- Pre-miRNA 1 (ID: 000385) (Thermo Fisher Scientific, Applied Biosystems®, catalog number: AM17100 ), Pre-miRNA 133 (ID: 000458) (Thermo Fisher Scientific, Applied Biosystems®, catalog number: AM17100), Pre-miRNA 499 (ID: 001045) (Thermo Fisher Scientific, Applied Biosystems®, catalog number: AM17100) molecules and scramble miRNA (negative control) (Thermo Fisher Scientific, Ambion™, catalog number: AM17110 )
- Culture medium (see Recipes)
- Standard differentiation medium (see Recipes)
- Mix A (see Recipes)
- Mix B (see Recipes)
Equipment
- Pipetus® Pipettes
- Laminar flow-hood (EuroClone S.p.A., model: S@feflow 1.8 )
- Humidified cell culture incubator set at 37 °C, 5% CO2 (Panasonic Corporation, Sanyo, model: MCO-18AC )
- Inverted bright light microscope equipped with a phase-contrast filter (ZEISS, model: Observer Z1 )
Procedure
- Expand the P19 cells form passage 0 to passage 5, in a standard Petri dish and feed the cells with culture medium. When the cells reach 80-90% confluence, harvest the P19 cells using 5% trypsin/EDTA for 5 minutes at 37 °C, 5% CO2 (Figure 1A).
- On day 1, transfer 4 x 105 cells to a 100 mm bacterial dish in 8 ml of standard differentiation medium and incubate the cells for 24 h at 37 °C, 5% CO2.
- On day 2, add 5 ml of fresh standard differentiation medium (total volume medium: 13 ml, fresh differentiation medium must be prepared just before the use).
- On day 3, the morphologic changes of P19 cells start to be visible: some cell clusters sediment at the bottom of the culture dish, become EB and begin to differentiate into CMC. Some isolated cells may remain in suspension: these cells are not committed toward the cardiac lineage and will not adhere at the bottom of the culture dish since bacterial dish are used. These undifferentiated P19 will be discarded when exchanging the cell culture medium.
- Replace 5 ml of fresh standard differentiation medium: with a 10 ml pipette gently aspirate 5 ml of medium, avoiding to aspirate also the EB; then, with a new 10 ml pipette, add 5 ml of fresh differentiation medium.
- On day 4, the EB are ready for transfection (Figure 1B). Prepare mix A and mix B in two separate 15 ml conical tubes.
- Gently add mix B to mix A, invert the tube 3 times and incubate at RT for 10 min.
- Using a 25 ml pipette, collect and transfer the EB contained in one bacterial dish to a 50 ml tube and let the EB to sediment.
- Aliquot 1 ml of transfection mix into each well of a 6-multiwell-plate; add only standard differentiation medium in those wells that will be used as control.
- EB previously collected in the 50 ml tube will now be settled. Carefully aspirate the medium and re-suspend the EB in 14.4 ml of fresh standard differentiation medium.
- Transfer 2.4 ml of re-suspended EB to each well and gently rock the multiwell-plate using a 1 ml pipette tip. Now shake back and forth the plate to obtain homogeneous distribution of the cells. Every time you pick an aliquot of the mixture, pipette up and down to re-suspend the EB. Incubate over-night at 37 °C, 5% CO2.
- On day 5, EB will have adhered to the bottom of the dish (Figure 1C). You can now exchange the medium with fresh differentiation medium to allow the expansion of EB. This step represents “day 1” of the cardiac induction protocol. A Real-Time PCR can be performed in order to quantify the miRNA transfection efficiency.
- On day 5 of the induction protocol, the first contracting EB should appear and will be visible at the microscope (Video 1).
Video 1. Beating embryoid bodies - Early cardiac differentiation can be documented by PCR and/or immunocytochemistry starting from day 7 of the induction protocol. More advanced cardiac differentiation will appear starting from day 14 of the induction protocol (Figure 1D) (Pisano et al., 2015 http://onlinelibrary.wiley.com/doi/10.1002/stem.1928/pdf, Figures 2, 3 and 6).
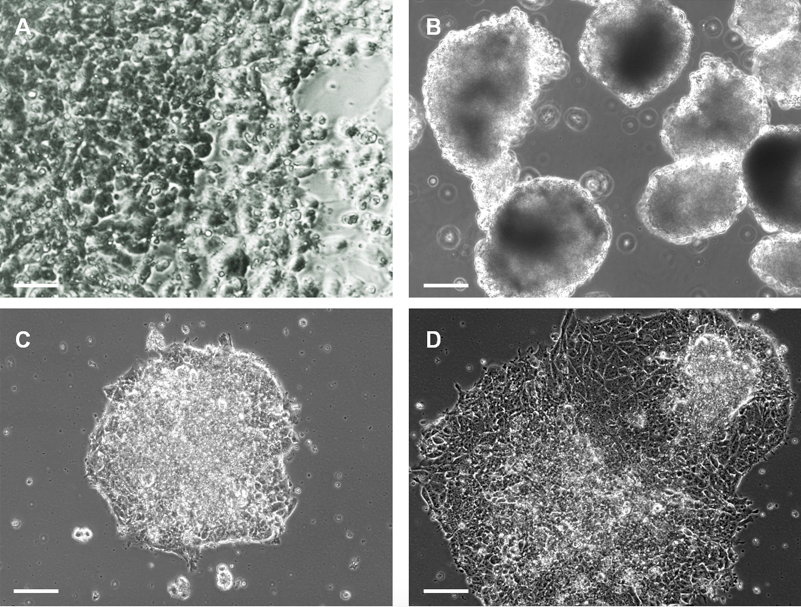
Figure 1. P19 cells and EB. A. Images of undifferentiated P19 maintained in culture medium; B. EB formation during the cardiac differentiation protocol, day 4; C. When EB are transferred from a bacterial dish to a Petri dish they adhere to the plate; D. After 7 days of cardiac differentiation the attached EB will have expanded and are beating.
Notes
- Manipulation of miRNA molecules requires the use of RNAse-free plastic and reagents. Moreover, if the flow-hood used for miRNA transfection is utilized also for other experiments, we suggest to pre-treat the surfaces with 10% NaClO.
- During the transfection protocol you must work carefully and aseptically under the flow-hood since Optimem® medium does not contain antibiotics.
Recipes
- Culture medium
Store the medium at 4 °C; when the phenol red contained in the α-MEM turns to pink (or to yellow) discard the medium and use a fresh culture medium.
α-MEM 500 ml
FBS 55 ml
L-glutamine 5.5 ml
Penicillin-streptomycin 5.5 ml - Standard differentiation medium (prepare fresh before use)
Culture medium
DMSO 0.5% - Mix A (prepare fresh before use)
Dilute the pre-miRNA solution using Optimem®
We use pre-miRNA1 and pre-miRNA499 at a concentration of 10 nM, and pre-miRNA133 and scramble miRNA at 5 nM.
Incubate the mix at room temperature (RT) for 10 min
For one 6-multiwell dish we use 3 ml of Mix A - Mix B (prepare fresh before use)
Dilute siPORT® 1:50 using Optimem®
Incubate at RT for 10 min
For one 6-multiwell dish we use 3 ml of Mix B
Acknowledgments
The development of this protocol was supported by the Fondazione IRCCS Policlinico San Matteo Pavia, Italy; the Fondazione Cariplo (2007-5984) and the Ministero Italiano della Sanità (GR-2008-114278). We want to thank Laurene Kelly for help with editing the manuscript.
References
- Aguirre, A., Sancho-Martinez, I. and Izpisua Belmonte, J. C. (2013). Reprogramming toward heart regeneration: stem cells and beyond. Cell Stem Cell 12(3): 275-284.
- Dzau, V. J., Gnecchi, M. and Pachori, A. S. (2005). Enhancing stem cell therapy through genetic modification. J Am Coll Cardiol 46(7): 1351-1353.
- Gnecchi, M., Danieli, P. and Cervio, E. (2012). Mesenchymal stem cell therapy for heart disease. Vascul Pharmacol 57(1): 48-55.
- Laflamme, M. A. and Murry, C. E. (2011). Heart regeneration. Nature 473(7347): 326-335.
- McBurney, M. W. (1993). P19 embryonal carcinoma cells. Int J Dev Biol 37(1): 135-140.
- Melo, L. G., Pachori, A. S., Gnecchi, M. and Dzau, V. J. (2005). Genetic therapies for cardiovascular diseases. Trends Mol Med 11(5): 240-250.
- Pisano, F., Altomare, C., Cervio, E., Barile, L., Rocchetti, M., Ciuffreda, M. C., Malpasso, G., Copes, F., Mura, M., Danieli, P., Viarengo, G., Zaza, A. and Gnecchi, M. (2015). Combination of miRNA499 and miRNA133 exerts a synergic effect on cardiac differentiation. Stem Cells 33(4): 1187-1199.
- Sanganalmath, S. K. and Bolli, R. (2013). Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113(6): 810-834.
- van der Heyden, M. A. and Defize, L. H. (2003). Twenty one years of P19 cells: what an embryonal carcinoma cell line taught us about cardiomyocyte differentiation. Cardiovasc Res 58(2): 292-302.
- van der Heyden, M. A., van Kempen, M. J., Tsuji, Y., Rook, M. B., Jongsma, H. J. and Opthof, T. (2003). P19 embryonal carcinoma cells: a suitable model system for cardiac electrophysiological differentiation at the molecular and functional level. Cardiovasc Res 58(2): 410-422.
- Wobus, A. M., Kleppisch, T., Maltsev, V. and Hescheler, J. (1994). Cardiomyocyte-like cells differentiated in vitro from embryonic carcinoma cells P19 are characterized by functional expression of adrenoceptors and Ca2+ channels. In Vitro Cell Dev Biol Anim 30A(7): 425-434.
Article Information
Copyright
© 2016 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Pisano, F. and Gnecchi, M. (2016). Transfection of Embryoid Bodies with miRNA Precursors to Induce Cardiac Differentiation. Bio-protocol 6(3): e1726. DOI: 10.21769/BioProtoc.1726.
Category
Stem Cell > Embryonic stem cell > Maintenance and differentiation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










