- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Alcian Blue – Alizarin Red Staining of Mouse Skeleton
Published: Vol 2, Iss 8, Apr 20, 2012 DOI: 10.21769/BioProtoc.162 Views: 32423
Abstract
Our lab has used the Alcian blue – Alizarin red staining method with certain modifications to characterize skeleton deformities in mice lacking Pek/Perk, encoding a translational control eIF2alpha kinase.
Materials and Reagents
- Neural buffered formalin (Sigma-Aldrich, catalog number: HT5014 )
- Alcian blue 8GX (Sigma-Aldrich, catalog number: A5268 )
- Trypsin (Sigma-Aldrich, catalog number: T1426 )
- Saturated sodium borate (Sigma-Aldrich, catalog number: S9640 )
- Alizarin red (Sigma-Aldrich, catalog number: A3882 )
- Thymol (EM Life Science, catalog number: TX0615-1 )
- Ethanol
- Glacial acetic acid
- Potassium hydroxide
- Glycerol
- KOH
- Trypsin solution (see Recipes)
Equipment
- Conical tube
Procedure
- Adults:
- Fix mouse skeleton in 10% neutral buffered formalin for at least 24 h.
- Rinse the sample in ddH2O O/N (1 h for embryos) with gentle shaking, and post-fix it in 70% ethanol.
Note: At this point, samples can be stored in 70% ethanol for a long period. - Remove skins and internal organs carefully from the sample.
Note: Remove all skins, even those on the small toes. - Stain the sample with 0.02% Alcian blue 8GX (prepared in ethanol/ glacial acetic acid, 7:3) for 1 to 2 days.
Note: Cartilage tissues will be stained blue. - Wash the sample with plain ethanol/ glacial acetic acid (7:3) for 1 h.
- Soak the sample in 100% ethanol O/N, and then in ddH2O for 1 to 2 days.
- Treat the sample with 1.0% trypsin (prepared in water solution containing 30% saturated sodium borate) O/N.
- Should limp and blue cartilage be readily observed at this point, proceed to stain the sample with Alizarin red (prepared in 0.5% KOH) O/N.
Note: Add enough (no specific amount) saturated Alizarin red until the solution appears dark purple. Mineralized bones will be stained red. - Treat the sample with a gradient series of 0.5% KOH/ glycerol (i.e., 2:1, 1:1, 1:2 and 100% glycerol, 2 days for each step), and store it in glycerol with a crystal of thymol.
- Fix mouse skeleton in 10% neutral buffered formalin for at least 24 h.
- Embryos:
- A similar protocol can be used to stain embryos. To do this, fix embryos in 90% ethanol for at least 1 week.
- Treat the sample with 0.01% Alcian blue 8GX for 3 days, and then perform rehydration through a gradient series of ethanol (70% ethanol, 2 to 3 h, twice; 40% ethanol, 2 to 3 h; 15% ethanol, 2 to 3 h; ddH2O, until the sample sinks to the bottom of a conical tube). Treat the sample further with fresh 1% KOH for 1 to 2 days until it becomes clear.
- Treat the sample with 0.001% Alizarin red for 2 to 3 days until the bone becomes purple.
- Rinse the sample ~ 3 times in 1% KOH, several hours each time.
- Treat the sample through a gradient series of glycerol-KOH (20% glycerol/ 1% KOH, 24 h; 50% glycerol/ 1% KOH, 24 h; 80% glycerol/ 1% KOH, 24 h; 100% glycerol, 24 h x 2).
- A similar protocol can be used to stain embryos. To do this, fix embryos in 90% ethanol for at least 1 week.
Representative data
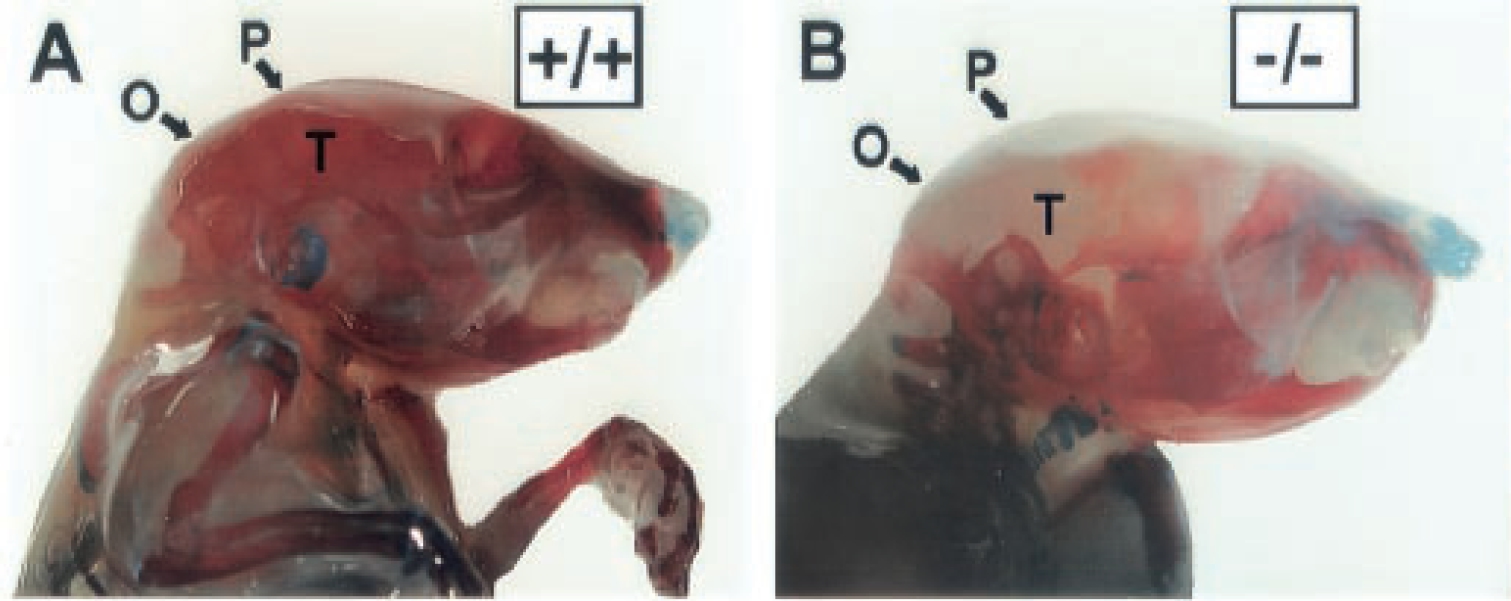
Figure 1. This figure is adapted from the original (Zhang et al., 2002). Shown here is Alizarin Red (mineralized bone) and Alcian Blue (cartilage) skeletal staining of 18-day-old wild-type A and Perk-/- mutant mice B. The mineralization of the flat bones of the skull (P, parietal; O, occipital; T, temporal) is greatly reduced in the Perk-/- mutant mouse.
Recipes
- Trypsin solution
1.0% trypsin
30% saturated sodium borate
H2O
Acknowledgments
This protocol was adapted from previously described work by Hanken and Wassersug (Hanken and Wassersug, 1981). PZ was supported by a research assistantship in the Cavener lab at the Pennsylvania State University. This work was supported by an NIH R01 grant awarded to DC.
References
- Hanken, J., Wassersug, R. J. (1981). The visible skeleton. Funct Photogr 16(4): 22-26, 44.
- Zhang, P., McGrath, B., Li, S., Frank, A., Zambito, F., Reinert, J., Gannon, M., Ma, K., McNaughton, K. and Cavener, D. R. (2002). The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol 22(11): 3864-3874.
Article Information
Copyright
© 2012 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhang, P. (2012). Alcian Blue – Alizarin Red Staining of Mouse Skeleton. Bio-protocol 2(8): e162. DOI: 10.21769/BioProtoc.162.
Category
Developmental Biology > Morphogenesis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









