- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Protocol to Measure the Cytoplasmic Calcium in Arabidopsis Guard Cells
Published: Vol 5, Iss 9, May 5, 2015 DOI: 10.21769/BioProtoc.1462 Views: 11435
Reviewed by: Maria SinetovaAlexander JonesAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols
Cytohistochemical Determination of Calcium Deposition in Plant Cells
Wan-Jun Zhang and Tao Wang
Jan 20, 2016 8471 Views
Cation (Ca2+ and Mn2+) Partitioning Assays with Intact Arabidopsis Chloroplasts
Anna Harms [...] Anja Schneider
Jan 5, 2017 9008 Views
Quantitative Determination of Ca2+-binding to Ca2+-sensor Proteins by Isothermal Titration Calorimetry
Seher Abbas and Karl-Wilhelm Koch
Apr 5, 2020 6293 Views
Abstract
Cytoplasmic calcium ([Ca2+]cyt) acts as a stimulus-induced second messenger in multiple signal transduction cascades (Allen et al., 1999). In plant cells, a dramatic and readily assayed response to stimulus is the change of stomatal aperture. Changes in [Ca2+]cyt of stomatal guard cells were involved in stomatal movement in response to various stimuli and cellular processes. In general, there are two available ways to measure [Ca2+]cyt in guard cells, i.e., loading of calcium-sensitive fluorescence dyes such as fluo-3 AM and fura-2 or expressing genetically encoded calcium indicators such as yellow cameleon (Krebs et al., 2012). In this protocol, we aim at describing the experimental procedure to record [Ca2+]cyt fluctuation in guard cells with loading of fluo-3 AM upon ABA or PA treatment combining with fluorescence imaging performed with confocal laser scanning microscope.
Materials and Reagents
- Plant materials: Arabidopsis thaliana ecotype Columbia-0 (wild type) was obtained from ABRC at Ohio State University (Columbus)
- Glycine,N-[4-[6-[(acetyloxy)methoxy]-2,7-dichloro-3-oxo-3H-xanthen-9-yl]-2-[2-[2-[bis[2-[(acetyloxy)methoxy]-2-oxyethyl]amino]-5-methylphenoxy]ethoxy]phenyl]-N-[2-[(acetyloxy)methoxy]-2-oxyethyl]-, (acetyloxy) methyl ester (Fluo-3, AM) (Life Technologies, InvitrogenTM, catalog number: F-14218 )
- Potassium chloride (KCl) (Sangon Biotech, catalog number: PB0440 )
- Calcium chloride dihydrate (CaCl2·2H2O) (Sangon Biotech, catalog number: C0556 )
- 2-(N-morpholino) ethanesulfonic acid (MES) (free acid, monohydrate) (Sigma-Aldrich, catalog number: M3671 )
- Potassium hydroxide (KOH) (Sangon Biotech, catalog number: PT1159 )
- Egtazic acid, Glycol ether diamine tetraacetic acid (EGTA) (Sangon Biotech, catalog number: ED007 )
- Chloroform (Sinopharm Chemical Reagent, catalog number: 10006818 )
- Sodium hypochlorite solution (Sangon Biotech, catalog number: S1944 )
- Agar (Sangon Biotech, catalog number: AJ637 )
- Murashige & Skoog (MS) Basal Medium w/Vitamins (PhytoTechnology Laboratories®, catalog number: M519 )
- Abscisic acid (ABA) (Sigma-Aldrich, catalog number: A1049 )
- Phosphatidic acid (PA) (Avanti Polar Lipid, catalog number: 840858C )
- Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650 )
- Ethanol anhydrous (Sangon Biotech, catalog number: ET0737 )
- Tris (hydroxymethyl) aminomethane (Tris) (Sigma-Aldrich, catalog number: 252859 )
- Epidermal buffer (see Recipes)
- Fluo-3 AM stock solution (see Recipes)
- Loading buffer (see Recipes)
- ABA stock solution (see Recipes)
- ABA working solution (see Recipes)
- PA working solution (see Recipes)
Equipment
- Graduated centrifuge tubes (1.5 ml)
- Petri dish (6 cm diameter)
- Cover glass (20 mm * 20 mm, 0.13-0.17 μm thickness)
- Slides (76 mm * 26 mm, 1-2 mm thickness)
- Forceps
- Pipettor and matched tips
- Growth chamber (Percival, model: I-41LL )
- Zeiss LSM780 confocal laser scanning microscope
- Microprocessor pH meter (HANNA® Instruments, model: HI-2211 )
- Ultrasonic cell crusher (Scientz, model: JY92Ⅱ )
Software
- Zeiss confocal software ZEN 2012 (blue edition)
- Microsoft Excel
Procedure
- Sample preparation
- Seeds of Arabidopsis thaliana were surface-sterilized by soaking in 75% ethanol for 3 min and 2% sodium hypochlorite for 10 min. The seeds were washed for 3 to 5 times with sterile distilled water, and then were placed on MS medium (1% agar). Arabidopsis seedlings were grown in a growth chamber at 160 μmol/m2/s light intensity and 14/10 h (24/21 °C) day/night regimes. The cotyledons of 7-day-old seedlings were cut from the plants and used for experiments.
- The cotyledons were incubated with epidermal buffer at 24 °C for 2 h in growth chamber (160 μmol/m2/s light intensity) to induce stomatal opening prior to various treatments.
- The samples were then incubated in 10 μM fluo-3 AM loading buffer (diluted from 5 mM stock solution in 10 mM MES-KOH pH 6.1) for 2 h at 4 °C in darkness. Higher concentration of fluo-3 AM at room temperature for incubation may result in false positive signals from other ions, for example Mn2+, Zn2+ and Pb2+.
- The samples were rinsed with loading buffer for 3 times to remove fluo-3 AM and were kept in growth chamber (24 °C) for 1 h before ABA or PA addition.
- For ABA or PA treatment, the cotyledons were incubated with 10 μM ABA or 50 μM PA working solution, and observed by the confocal laser scanning microscope.
- Seeds of Arabidopsis thaliana were surface-sterilized by soaking in 75% ethanol for 3 min and 2% sodium hypochlorite for 10 min. The seeds were washed for 3 to 5 times with sterile distilled water, and then were placed on MS medium (1% agar). Arabidopsis seedlings were grown in a growth chamber at 160 μmol/m2/s light intensity and 14/10 h (24/21 °C) day/night regimes. The cotyledons of 7-day-old seedlings were cut from the plants and used for experiments.
- [Ca2+]cyt imaging
- [Ca2+]cyt imaging was performed with a 488 nm excitation and a 510-545 nm emission filter to record [Ca2+]cyt images. Increases in [Ca2+]cyt occurred after ABA or PA addition. Fluorescence intensity of Fluo-3 dye increases when bound to [Ca2+] in the cytosol. The Kd of Fluo-3 for calcium is 325 nM. Fluo-3 can also detect calcium near the chloroplasts membrane and plasma membrane, and display higher fluorescence in cytosol because of higher concentration of calcium. Changes in [Ca2+]cyt were visualized in pseudocolour as indicated by the color bars. The color bar showed the pixel intensity as the arrow indicated (Figure 1).
- The imaging parameters were as follows: Plan-Apochromat 10×/0.45 M27 objective, image dimension 1,024 x 1,024, pinhole 3 Airy unit, scanning speed 1936.28 ms. The images were acquired at the time indicated. Fluorescence pixel intensity values in guard cells were measured using the Zeiss confocal software ZEN 2012 (blue edition). The pixel intensity value of background is about 4.5 for subtraction.
Please note that the laser power, gain, offset, zoom, scan speed, etc. should not be changed across experimental conditions if using intensity to distinguish phenotype. Set values at the brightest sample and continue imaging with same conditions to get an appropriate dataset. If the fluorescence is high enough, it can be performed with a fluorescence scope. The confocal scanning will be more sensitive and reproducible.
- [Ca2+]cyt imaging was performed with a 488 nm excitation and a 510-545 nm emission filter to record [Ca2+]cyt images. Increases in [Ca2+]cyt occurred after ABA or PA addition. Fluorescence intensity of Fluo-3 dye increases when bound to [Ca2+] in the cytosol. The Kd of Fluo-3 for calcium is 325 nM. Fluo-3 can also detect calcium near the chloroplasts membrane and plasma membrane, and display higher fluorescence in cytosol because of higher concentration of calcium. Changes in [Ca2+]cyt were visualized in pseudocolour as indicated by the color bars. The color bar showed the pixel intensity as the arrow indicated (Figure 1).
Representative data
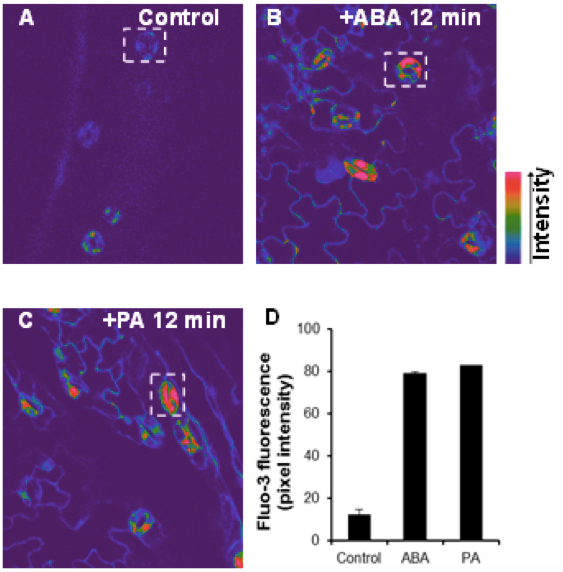
Figure 1. Elevation of cytoplasmic calcium induced by ABA and PA in Arabidopsis guard cells. The confocal images of [Ca2+]cyt in guard cells were monitored by fluo-3 AM dye before (A) or after treatment with10 μM ABA (B) or 50 μM PA (C) for 12 min. The color bars showed the intensity as the arrow indicated. D. Changes in the relative levels of [Ca2+]cyt in ABA- or PA-treated guard cells. Regions of interests used to measure the intensities were indicated by white rectangle with background subtraction. Values are the mean ± SD (n = 50-60 from not less than 10 cotyledons) from three independent experiments.
Recipes
- Epidermal buffer
10 mM KCl
0.2 mM CaCl2
0.1 mM EGTA
10 mM MES-KOH
Adjust the pH to 6.15 with KOH (0.1 M)
- Fluo-3 AM stock solution
5 mM Fluo-3 AM in DMSO
- Fluo-3 AM Loading buffer
10 mM MES
Adjust the pH to 6.1 with KOH (0.1 M)
10 μM Fluo-3 AM
- ABA stock solution
Dissolve 26.432 mg ABA powder in 1 ml ethanol to a concentration of 100 mM
- ABA working solution (for treatment)
Add ABA stock solution (10,000x) to the epidermal buffer to a concentration of 10 μM ABA
- PA working solution (for treatment)
PA in chloroform were dried under N2 and suspended in 1 ml of epidermal solution by sonication for preparing the stock solution (50 mM, 1,000x). The PA stock solution was diluted into 50 μM with epidermal solution before treatment.
Acknowledgments
The methods were adapted from (Jiang et al., 2014). Techniques were also adapted from all of the references cited. This work was supported by grants from National Basic Research Program of China (31100194 and 31470364) and the Fundamental Research Funds for the Central Universities (KYZ201423) to Q Zhang.
References
- Allen, G. J., Kwak, J. M., Chu, S. P., Llopis, J., Tsien, R. Y., Harper, J. F. and Schroeder, J. I. (1999). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J 19(6): 735-747.
- Jiang, Y., Wu, K., Lin, F., Qu, Y., Liu, X. and Zhang, Q. (2014). Phosphatidic acid integrates calcium signaling and microtubule dynamics into regulating ABA-induced stomatal closure in Arabidopsis. Planta 239(3): 565-575.
- Krebs, M., Held, K., Binder, A., Hashimoto, K., Den Herder, G., Parniske, M., Kudla, J. and Schumacher, K. (2012). FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca(2)(+) dynamics. Plant J 69(1): 181-192.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Li, L., Lin, F., Qu, Y. and Zhang, Q. (2015). A Protocol to Measure the Cytoplasmic Calcium in Arabidopsis Guard Cells. Bio-protocol 5(9): e1462. DOI: 10.21769/BioProtoc.1462.
Category
Plant Science > Plant physiology > Ion analysis
Biochemistry > Other compound > Ion > Calcium
Cell Biology > Cell imaging > Confocal microscopy
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









