- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Nictation Assays for Caenorhabditis and Other Nematodes
Published: Vol 5, Iss 7, Apr 5, 2015 DOI: 10.21769/BioProtoc.1433 Views: 10174
Reviewed by: Fanglian HeAnonymous reviewer(s)
Abstract
Nictation is a dauer-specific standing and waving behavior of the nematodes including Caenorhabditis species. Nictation enhances the capability of free-living nematodes to hitchhike to other animals as well as parasitic nematodes to infect their hosts. However, lack of an assay for this behavior has made it difficult to elucidate its underlying regulatory mechanisms and related genetic pathways. We have developed nictation assays that enable the quantification of the nictation behavior of individuals and groups of worms. Gauze assay is less quantitative but is an easier way to observe nictation behavior in plates with plenty of dauers. The micro-dirt chip made from PDMS mold is a more sophisticated method to quantify the nictation behavior. Nictation can be quantified on a micro-dirt chip either by measuring the average nictating time of individual dauers or by the fraction of nictating worms in a given dauer population.
Keywords: nictationMaterials and Reagents
- Dauer larva
- SU-8 photoresist 2008 (MicroChem Corp)
- Polydimethylsiolaxne (PDMS) (Sylgard® 184 Silicone Elastomer Kit, Dow-Corning)
- Agar powder (OCI Company Ltd, catalog number: 9002-18-0 )
- Cotton medical gauze
- M9 solution (see Recipes)
- Synthetic pheromone plates (see Recipes)
Equipment
- Microwave oven or autoclave
- Dissecting microscope (Leica Microsystems, model: S6E )
- Mouth pipette with glass capillary (Chase)
- 60Φ Petri dish (SPL Life Sciences, model: 10060 )
- Slide glass (Marienfeld)
- Spatula
- Glass flask
- Incubator (Vision Scientific Co. Ltd., model: VS-8480S )
Procedure
- Prepare dauers for nictation assay
- Dauers can be induced by various methods. Most easily, dauer can be prepared from starved plates. Dauer can also be induced by treating crude pheromone (Golden and Riddle, 1984). We usually use synthetic pheromone plates to induce dauers, and those dauers seem to give more reproducible data than starvation-induced dauers. Dauers of temperature-sensitive dauer-formation constitutive mutants can be easilyinduced by temperature shift. We usually let adult worms to lay eggs in15 °C and then shift temperature to 25 °C.
- Dauers can be induced by various methods. Most easily, dauer can be prepared from starved plates. Dauer can also be induced by treating crude pheromone (Golden and Riddle, 1984). We usually use synthetic pheromone plates to induce dauers, and those dauers seem to give more reproducible data than starvation-induced dauers. Dauers of temperature-sensitive dauer-formation constitutive mutants can be easilyinduced by temperature shift. We usually let adult worms to lay eggs in15 °C and then shift temperature to 25 °C.
- Gauze assay
- Count total number of dauers in the plate to assay.
- Cut cotton medical gauze to fit to the size of petri dish, and put it on. Dauers waving on filament would be observed within 5 min.
- Count the number of dauers waving or standing on gauze at a moment 3 times. Nictation ratio can be measured as [Average number of nictating dauers/Number of total dauers].
Video 1. Gauze assay
- Count total number of dauers in the plate to assay.
- Micro-dirt chip manufacture
- A micro-dirt master was fabricated by patterning an array of holes [25 μm (radius) x 25 μm (height) x 25 μm (distance)] onto a 4-inch silicon wafer by photolithography with SU-8. Negative replicas of the master were made by pouring 10 ml of PDMS prepolymer onto the master and curingthe polymer at 65 °C for 30 min (Xia and Whitesides, 1998). The cured polymeric layer was peeled off from the master and cut to fit in 60Φ petri dish. PDMS negative replicas function as a mold for micro-dirt chip. Make agar solution (3.5%~4%) with D.W. by boiling with microwave or autoclave. Cool it to about 70 °C, and pour agar solution onto the PDMS mold placed in a 60Φ petri dish with structure-side up.
- Remove air bubbles on the surface of PDMS mold with slide glass before agar solution is solidified. Optimize agar concentration considering quality of agar powder and stiffness of agar solution. If solution is too stiff, it hinders air bubble removal. If air bubbles are removed, wait enough until agar solution is fully solidified.
- Using spatula, take out the solidified part (PDMS mold + micro-dirt chip) frompetri dish, and detach PDMS mold from it. Remaining agar part is micro-dirt chip with positive array of posts.
- Dry micro-dirt chip in 37 °C incubator (without shaking) for 1.5 h before use.
- A micro-dirt master was fabricated by patterning an array of holes [25 μm (radius) x 25 μm (height) x 25 μm (distance)] onto a 4-inch silicon wafer by photolithography with SU-8. Negative replicas of the master were made by pouring 10 ml of PDMS prepolymer onto the master and curingthe polymer at 65 °C for 30 min (Xia and Whitesides, 1998). The cured polymeric layer was peeled off from the master and cut to fit in 60Φ petri dish. PDMS negative replicas function as a mold for micro-dirt chip. Make agar solution (3.5%~4%) with D.W. by boiling with microwave or autoclave. Cool it to about 70 °C, and pour agar solution onto the PDMS mold placed in a 60Φ petri dish with structure-side up.
- Micro-dirt chip assay
- Collect 30+ dauers with M9 loaded mouth pipette, and release them on the center of micro-dirt chip under dissecting microscope. It takes 10~30 min for dauers to start locomotion after transfer to micro-dirt chip in room temperature.
- Only dauers can nictate on micro-dirt chip. When dauer lift their neck, we regard the point as nictation initiation.When its body lands back to surface, we regard it as completion of nictation.
- For individual nictation assay, observe each moving dauer for 1 min and measure nictating time. Quiescent dauers (including standing dauers) are excluded from test. From these raw data, nictation ratio, initiation index, and average duration can be extracted accordingto Lee et al. (2011).
- For population nictation assay, scan micro-dirt chip (by eye) while counting number of nictating dauers and crawling dauers (Figure 1). Only moving (crawling and nictating) dauers should be counted. Initiation index and average duration cannot be extracted from population assay.
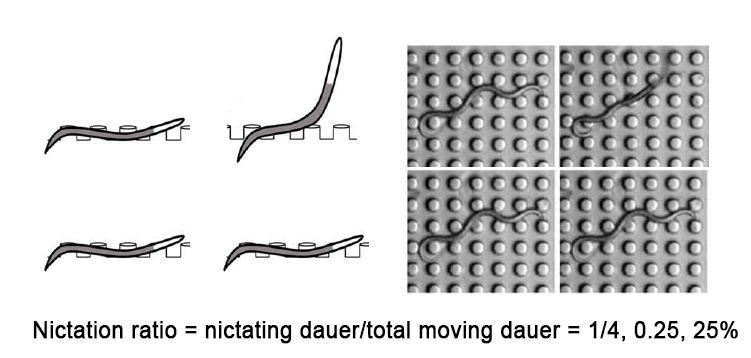
Figure 1. Micro-dirt chip population nictation assay. The surface of micro-dirt chip is scanned by eye to count the number of moving dauers and nictating dauers. Quiescent dauers are excluded. Nictation ratio can be measured as fraction of nictating dauers among moving dauers. We measure 3 times for same micro-dirt chip and average the result. For each strain, we usually make 5+ independent replicates to get reproducible nictation ratio.
Video 2. Micro-dirt chip manufacture and assay
- Collect 30+ dauers with M9 loaded mouth pipette, and release them on the center of micro-dirt chip under dissecting microscope. It takes 10~30 min for dauers to start locomotion after transfer to micro-dirt chip in room temperature.
Notes
- Nictation assay results exhibit considerable day to day variation, and the cause of stochastic results is unknown. To overcome the problem of variability and reproducibility, it is recommended to make multiple independent replicates rather than to test many worms at once. For instance, N2 might exhibit nictation ratio from 10% to 70% from one test (N>=10) by individual assay, but one will get stable average nictation ratio about 20%~40% from multiple tests.
- In nictation assay, we only measure moving dauers. For individual nictation assay, a waving worm can suddenly stand quiescently, then we exclude that worm from the result. However, we also count nictating worms which might stop after a moment in population assay. Presumably, this difference makes population assay to yield slightly higher nictation ratio than individual assay.
Recipes
- M9 buffer
3 g KH2PO4
6 g Na2HPO4
5 g NaCl
1 ml 1 M MgSO4
H2O to 1 L
Sterilized by autoclaving - Synthetic pheromone plate
1.5 g KH2PO4
0.25 g K2HPO4
1 g NaCl
10 g agar
(or alternatively, 5 g agar + 3.5 g agarose for digging strains like HW or npr-1 mutant)
4 mg cholesterol (add after autoclaving)
Acknowledgments
This work was supported by research grants through the National Research Foundation of Korea (NRF) (NRF-2014R1A4A1005259, NRF-2013K2A3A1000102 and NRF-2013R1A2A1A03070982). D. L. was supported by TJ Park Science Fellowship from the POSCO TJ Park Foundation. This protocol has been adapted and modified from our previous work, Lee et al. (2012).
References
- Golden, J. W. and Riddle, D. L. (1984). A Caenorhabditis elegans dauer-inducing pheromone and an antagonistic component of the food supply. J Chem Ecol 10, 1265–1280.
- Lee, H., Choi, M. K., Lee, D., Kim, H. S., Hwang, H., Kim, H., Park, S., Paik, Y. K. and Lee, J. (2012). Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat Neurosci 15(1): 107-112.
- Xia, Y. and Whitesides, G. M. (1998). Soft lithography. Angew Chem Int Edn Engl 28(1): 153-184.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Lee, D., Lee, H., Choi, M., Park, S. and Lee, J. (2015). Nictation Assays for Caenorhabditis and Other Nematodes. Bio-protocol 5(7): e1433. DOI: 10.21769/BioProtoc.1433.
Category
Neuroscience > Behavioral neuroscience > Nictation
Neuroscience > Sensory and motor systems
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link