- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Rapid Preparation of Unsheathed Bacterial Flagella
Published: Vol 5, Iss 6, Mar 20, 2015 DOI: 10.21769/BioProtoc.1425 Views: 11166
Reviewed by: Fanglian HeAmit DeyEsteban Paredes-Osses

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Mycobacterium smegmatis Ribosome Purification, Co-sedimentation, and Subunit Association Assay
Aneek Banerjee [...] Jayati Sengupta
May 20, 2025 1581 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1671 Views

Preparation and Negative Staining for Visualization of Cyanoglobule Lipid Droplets Using Transmission Electron Microscopy
Febri A. Susanto [...] Peter K. Lundquist
Dec 5, 2025 1311 Views
Abstract
The flagellum is required for bacterial swimming and swarming motility. In the biphasic Salmonella enterica serovar Typhimurium (S. Typhimurium), the flagellar filament is build up by two distinct monomeric subunits, flagellin FliC and FljB. S. Typhimurium has the ability to switch between two flagellins, FliC and FljB, in a phase-variable manner. The switch to FliC is called phase H1 and considered important for bacterial growth and survival in the spleen in a murine infection model of typhoid fever. Flagellin is secreted as monomeric subunits, but the majority of flagellin is polymerized upon secretion as the flagellar filament. Salmonella flagellin has traditionally been isolated through a process involving multiple steps of centrifugation and acid treatment. Here, we delineate a simplified protocol for preparing Salmonella´s flagellin for analytical purpose to determine the amount of flagellin without the aid of antibodies. The growth conditions used were stationary phase, logarithmic phase and a low oxygen and high salt condition mimicking the gastrointestinal tract. Flagellin expression of other source organisms, such as other serovars of Salmonella enterica and Escherichia coli, including flagellar phase- or genetic variants can be analysed. Flagellin expression analysis complements flagella-associated phenotype analysis such as swimming and swarming behaviour.
Keywords: BacteriaMaterials and Reagents
- Bacterial strains (S. Typhimurium)
- Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653 )
- 100% trichloroacetic acid (Sigma-Aldrich, catalog number: T6399 )
- β-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250 )
- Trizma® base (powder) (Sigma-Aldrich, catalog number: T1503 )
- 1 M HCl solution (Sigma-Aldrich, catalog number: H1758 )
- Glycerol 1 L (Sigma-Aldrich, catalog number: 49781 )
- Sodium dodecyl sulfate - 500 g (Sigma, catalog number: L4390 )
- Tryptone (BD-catalog number: 211705 )
- Yeast extract (BD, catalog number: 212750 )
- Agar (BD, catalog number: 281230 )
- Bromophenol blue (Shelton scientific, catalog number: IB74040 )
- Glycerol stock for one vial (see Recipes)
- LB medium (see Recipes)
- 1 M Tris (see Recipes)
- 2x SDS sample buffer (see Recipes)
Equipment
- Falcon tubes (50 ml and 15 ml) (TPP, catalog numbers: 91050 and 91115 )
- Filter Flasks (pore size 0.22 μm diameter) (TPP, catalog number: 99500 )
- Eppendorf tubes (1.5 ml) (Sarstedt, catalog number: 72.690.001 )
- Incubator (adjusted to 37 °C) (Memmert, model: ULE500 )
- Rotary shaker (HTINFORS, model: Minitron )
- Spectrophotometer (Hitachi, model: U1100 )
- Thermoblock (PEQLAB, model: 1202 )
- Vortex mixer (VWR International, model: 1719 )
- Luer lock tips (BD PLASTIKPAK, catalog number: 302187 )
- Needle (0.51 mm outer diameter) (BD Microlance sterile, model25 g x 5/8” x 100)
- Sterile petri dishes (90 mm) (Sartorius, catalog number: 14-555-735 )
- Centrifuge (HERAEUS, model: PICO17 )
- Microcentrifuge (NeoLab, model: microcentrifuge II )
- pH meter (Hanna instruments, model: pH211 )
Procedure
- Preparation of the pre-culture (to be used for all subsequent culture conditions)
Day 0
Streak bacterial strains from -80 °C glycerol stock [equal to 87% (v/v) glycerol in LB] on LB agar plates for single colonies. Use LB agar plates containing appropriates antibiotics, if required, and incubate overnight at 37 °C (approximately 16 to 24 h).
Day 1
- Take one bacterial colony and inoculate in 10 ml liquid LB medium (in a 50 ml Falcon tube).
- Incubate the culture overnight at 37 °C with shaking at 150 rpm. Put the lid loose on the tube. Add appropriate antibiotics, if required.
- Take one bacterial colony and inoculate in 10 ml liquid LB medium (in a 50 ml Falcon tube).
- Preparation of inoculum
Day 2
- Measure the optical density of the 1:10 diluted pre-culture in a spectrophotometer and calculate the optical density of the pre-culture. Adjust the OD600 of the pre-culture to OD600=1 for a 1 ml volume which corresponds to approximately 5 x 108 CFU.
- Add 100 μl of the adjusted pre-culture to 10 ml LB medium (1:100 dilution).
- Measure the optical density of the 1:10 diluted pre-culture in a spectrophotometer and calculate the optical density of the pre-culture. Adjust the OD600 of the pre-culture to OD600=1 for a 1 ml volume which corresponds to approximately 5 x 108 CFU.
- Culture conditions
Standard growth condition to logarithmic and stationary phase in LB medium
Add 100 µl of wild type, normalized test and control inoculum to 10 ml LB medium in a 50 ml Falcon tube (a 1:100 dilution of the inoculum). Add appropriate antibiotics and inducer if required.
- Logarithmic phase growth condition
- Incubate the samples for 3 h at 37 °C with shaking at 150 rpm. Put the lid loose on the tube. The OD600 will be approximately 0.6-0.8.
- Normalize each sample to OD600 = 0.6. Shear off flagella as described under D.
- Incubate the samples for 3 h at 37 °C with shaking at 150 rpm. Put the lid loose on the tube. The OD600 will be approximately 0.6-0.8.
- Stationary phase growth condition
- Incubate the samples for 16 to 24 h at 37 °C with shaking at 150 rpm. Put the lid loose on the tube.
- Normalize each sample to OD600 = 0.6. Shear off flagella as described under D.
- Incubate the samples for 16 to 24 h at 37 °C with shaking at 150 rpm. Put the lid loose on the tube.
S. Typhimurium cell culture invasion condition
- Low oxygen, high salt growth condition.
- Add 100 µl wild type, normalized test and control inoculum to 10 ml LB medium with high salt concentration in a 50 ml Falcon tube (a 1:100 dilution of the inoculum). Add appropriate antibiotics and inducer, if required.
- Incubate the samples at 37 °C with shaking at 150 rpm until OD600 = 0.6-0.7 (takes approximately 4.5 h). Put the lid loose on the tube.
- Normalize the OD600 of the samples to the wild type OD600 using LB medium with high salt concentration. Shear off flagella as described under D.
- Add 100 µl wild type, normalized test and control inoculum to 10 ml LB medium with high salt concentration in a 50 ml Falcon tube (a 1:100 dilution of the inoculum). Add appropriate antibiotics and inducer, if required.
- Logarithmic phase growth condition
- Shearing off cell-associated flagellin (flagella)
- Perform the following steps on ice.
- Place 5 ml of each normalized sample in an individual sterile petri dish plate.
- Lift the inoculum up from the sterile petri dish plate with a sterile Luer lock tip (syringe). Place the sterile needle on the Luer lock tip and eject the cell suspension back in the petri dish plate. Work on a clean bench as this procedure creates aerosol.
- Repeat the previous step 10 times with the same material to shear off cell-associated flagella.
- Take 1 ml of cell suspension and transfer to an Eppendorf tube. Centrifuge at 17,700 x g for 10 min at 4 °C.
- Transfer 800 µl of the supernatant to a new Eppendorf tube. The supernatant contains sheared-off flagella and minor amounts of secreted flagellin. Proceed with E, for flagellar preparation.
- Remove the rest of the supernatant. The bacterial pellet is used for analysis of cell-associated protein as described under E, for cell associated protein preparation.
- Perform the following steps on ice.
- Sample preparation
For flagellar preparation
- Centrifuge at 17,700 x g for 10 min at 4 °C.
- Transfer 750 µl to a new Eppendorf tube.
- Add 250 μl of cold trichloroacetic acid solution and mix gently.
- Place the sample for at least 1 h on ice or in a -20 °C freezer.
- Centrifuge at 17,700 x g for 40 min at 4 °C, remove the supernatant and remove the residual liquid (eventually through repeated centrifugation).
For cell associated protein preparation
- Centrifuge 17,700 x g for 1 min at 4 °C.
- Discard the rest of the supernatant and save the bacterial pellet.
Preparation of cell associated protein and flagellin samples for loading on protein gel
- Prepare the sample buffer with 90 (v/v)% 1x SDS sample pre-buffer and 10 (v/v)% β-mercaptoethanol.
- Resuspend the flagellar protein preparation in 45 μl sample buffer and 5 μl Tris-HCl (pH = 9).
- Resuspend the bacterial pellet in 100 μl sample buffer.
- Vortex the samples.
- Heat up the samples 5 min at 95 °C.
- Vortex the samples.
- Spin down with a microcentrifuge for few seconds.
The samples can be used immediately or stored at -20 °C for further utilization.
- Centrifuge at 17,700 x g for 10 min at 4 °C.
Representative data
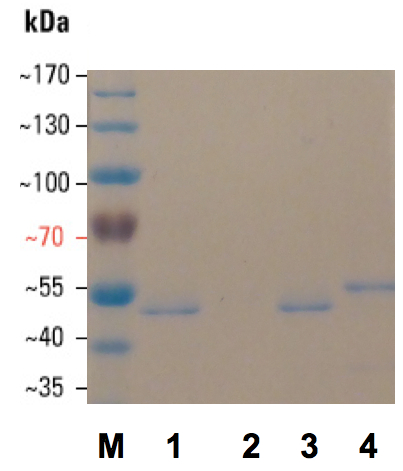
Figure 1. Coomassie brilliant blue stained 12% SDS PAGE of flagellar preparations from Salmonella enterica serovar Typhimurium UMR1 wild type and isogenic flagellin mutants. M. PageRuler prestained protein ladder 1. Wild type: ATCC14028-1s Nalr; 2. UMR1 fliC5050::MudJ fljB5001::MudCmr; 3. UMR1 fljB5001::MudCmr; 4. UMR1 fliC5050::MudJ. Molecular weight of flagellin: FliC: 52 kDa, FljB: 53 kDa
Notes
- Bacterial strains and controls.
This protocol has been developed using the wild type strain S. enterica serovar Typhimurium UMR1 (ATCC14028-1s Nalr). In this case, control strains are isogenic fljB fliC, fljB and fliC mutants. These strains are available upon request from the corresponding author.
- Medium volumes and culture conditions.
These factors can be subjected to change. Growth medium, medium volume and time of incubation can be adapted to the research question.
- Samples are normalized to OD600 = 1 in step C1b, 2b and 3c in order to enable growth culture independent estimation of flagellin expression.
- The cell associated protein samples.
The cell associated protein samples will be used to assess whether equal amounts of cells have been used for flagellar preparation.
- Precipitation of flagellar preparation samples.
The samples can be stored at -20 °C after adding trichloroacetic acid solution to the flagellin sample.
- A supplementary centrifugation of 5 min at 17,700 x g between step C and D can be used to separate polymerized and monomeric secreted flagellin.
Recipes
- Glycerol stock for one vial
250 μl 87% glycerol
750 μl bacterial overnight culture
- LB medium (1 L)
10 g tryptone
5 g yeast extract
10 g NaCl
Variant: with high salt concentration 17.5 g NaCl (0.3 M NaCl)
Fill up to 1 L with demineralized water and adjust the medium to pH = 7
Sterilize by autoclaving or filtration
- 1 M Tris (adjusted to pH = 9)
Add 12.14 g of Trizma® to deionized water for a total volume of 100 ml
Adjust the pH to 9 with 1 M HCl solution
- 2x SDS sample buffer
25 ml 0.5 M Trizma-base (pH = 6.8)
20 ml glycerol 87%
4 g SDS
0.2 g Bromophenol-blue
45 ml H2O
Before utilization, add 10% β-mercaptoethanol to 90% of 1x sample buffer
Acknowledgments
The development of this protocol was supported by research grants from Karolinska Institutet Doctoral funding (project no. C112130052) and the Swedish Research Council Natural Sciences and Engineering (project no. 621-2010-5755). The authors have no conflict of interest to declare. The protocol described above is adapted from one reported previously (Le Guyon et al., 2014; Ahmad et al., 2013). Kelly T. Hughes kindly provided fliC5050::MudJ and fljB5001::MudCmr mutant alleles in S. Typhimurium LT2.
References
- Ahmad, I., Wigren, E., Le Guyon, S., Vekkeli, S., Blanka, A., El Mouali, Y., Anwar, N., Chuah, M. L., Lünsdorf, H., Frank, R., Rhen, M., Liang, Z. X., Lindqvist, Y. and Römling, U. (2013). The EAL-like protein STM1697 regulates virulence phenotypes, motility and biofilm formation in Salmonella typhimurium. Mol Microbiol 90(6): 1216-1232.
- Ikeda, J. S., Schmitt, C. K., Darnell, S. C., Watson, P. R., Bispham, J., Wallis, T. S., Weinstein, D. L., Metcalf, E. S., Adams, P., O'Connor, C. D. and O'Brien, A. D. (2001). Flagellar phase variation of Salmonella enterica serovar Typhimurium contributes to virulence in the murine typhoid infection model but does not influence Salmonella-induced enteropathogenesis. Infect Immun 69(5): 3021-3030.
- Ibrahim, G. F., Fleet, G. H., Lyons, M. J. and Walker, R. A. (1985). Method for the isolation of highly purified Salmonella flagellins. J Clin Microbiol 22(6): 1040-1044.
- Guyon, S. L., Simm, R., Rehn, M. and Romling, U. (2014). Dissecting the cyclic di-guanylate monophosphate signalling network regulating motility in Salmonella enterica serovar Typhimurium. Environ Microbiol. (Epub ahead of print)
- Rochon, M. and Römling, U. (2006). Flagellin in combination with curli fimbriae elicits an immune response in the gastrointestinal epithelial cell line HT-29. Microbes Infect 8(8): 2027-2033.
- Römling, U. and Rohde, M. (1999). Flagella modulate the multicellular behavior of Salmonella typhimurium on the community level. FEMS Microbiol Lett 180(1): 91-102.
- Römling, U., Bian, Z., Hammar, M., Sierralta, W. D. and Normark, S. (1998). Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J Bacteriol 180(3): 722-731.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Guyon, S. L., Rhen, M. and Römling, U. (2015). Rapid Preparation of Unsheathed Bacterial Flagella . Bio-protocol 5(6): e1425. DOI: 10.21769/BioProtoc.1425.
Category
Microbiology > Microbial cell biology > Organelle isolation
Microbiology > Microbe-host interactions > Bacterium
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










