- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Separation of Microspores from Anthers of Lilium longiflorum (Lily) and Subsequent RNA Extraction
Published: Vol 5, Iss 4, Feb 20, 2015 DOI: 10.21769/BioProtoc.1400 Views: 10553
Reviewed by: Pablo Bolanos-VillegasArsalan DaudiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Dot Blot Analysis of N6-methyladenosine RNA Modification Levels
Lisha Shen [...] Hao Yu
Jan 5, 2017 29525 Views

Low-cost and High-throughput RNA-seq Library Preparation for Illumina Sequencing from Plant Tissue
Marta Bjornson [...] Pingtao Ding
Oct 20, 2020 8315 Views

A Highly Efficient System for Separating Glandular and Non-glandular Trichome of Cucumber Fruit for Transcriptomic and Metabolomic Analysis
Lei Sun [...] Xingwang Liu
Jan 5, 2025 1478 Views
Abstract
This protocol has been designed in order to facilitate the isolation and extraction of total RNA from microspores collected from lily anther sacs. This protocol allows the extraction of high amounts of high quality RNA, as observed in agarose gels.
Keywords: Microspore separationMaterials and Reagents
- Young anthers (at the microspore stage of lily buds normally from 30 to 65 mm of bud length)
- Lithium chloride (LiCl) (Merck KGaA, catalog number: 105679 )
- Tris-base (United States Biological, catalog number: 75825 )
- Ethylenediaminetetraacetic acid (EDTA) (United States Biological, catalog number: 15699 )
- Sodium dodecyl sulfate (SDS) (AppliChem, catalog number: A2263 )
- Isoamylalcohol (J.T. Baker®, catalog number: JT9038-1 )
- Phenol (pH 4.0) (United States Biological, catalog number: 77510 )
- Chloroform (Merck KGaA, catalog number: 102445 )
- Deionized distilled water (ddH2O)
- Diethylpyrocarbonate (DEPC) (Sigma-Aldrich, catalog number: D5758 )
- Sodium acetate (NaOAc) (Sigma-Aldrich, catalog number: S2889 )
- Isopropanol (Merck KGaA, catalog number: 107022 )
- 75% ethanol (analytical grade)
- Liquid nitrogen
- 0.1% DEPC ddH2O (RNase-free water) (see Recipes)
- Extraction buffer (see Recipes)
- 3 M sodium acetate (see Recipes)
- 10 mM sodium acetate buffer (see Recipes)
- 4 M LiCl (see Recipes)
- 75% ethanol (see Recipes)
Equipment
- Scalpel
- Tweezers (stainless, length: 11.5 cm; tip: 0.1 mm)
- Ceramic mortar and pestle
- 1.5 ml Eppendorf
- Centrifuge (Eppendorf, model: 5424 )
- Fume hood
- Speed vacuum (Savant Systems LLC, model: SC110 )
Procedure
- Microspore collection
- Approximately 25 anthers of young lily buds (3.5 cm) are transversely sliced close to the edge of an anther with a scalpel.
- The locular fluid (microspore) is collected in a 1.5 ml Eppendorf tube containing 200 μl of 10 mM sodium acetate buffer (pH 5.2) from the cutting site using a tweezer that gently squeezes out the fluid by moving along the surface of the anther to the cutting side.Video 1. Separation of Microspore
- Centrifuge at 7,000 rpm (4,600 x g) for 5 min.
- Decant the supernatant and the pellet (microspore) in the tube is saved for RNA extraction.
- Approximately 25 anthers of young lily buds (3.5 cm) are transversely sliced close to the edge of an anther with a scalpel.
- RNA isolation
- Immerse the Eppendorf tube that contains the microspore pellet in liquid nitrogen.
- Transfer the frozen microspores (0.2 g) into mortar and pestle in liquid nitrogen and grind the sample into powder.
- Work in the fume hood. The tissue powder is added by 1 ml of a mix of hot (~80 °C) extraction buffer/phenol (1:1) and continually ground in a mortar in liquid nitrogen.
- After melting, the solution of sample mixture is transferred to a 1.5 ml Eppendorf tube.
- Add 0.2 ml of chloroform/isoamylalcohol (24:1, v/v) into an Eppendorf tube, close the lid, and hand-shake the tube vigorously for 30 sec.
- Centrifuge the sample at 12,000 rpm (13,500 x g) for 5 min at 4 °C.
- Transfer the solution of the upper phase to a new Eppendorf tube and add an equal volume of 4 M LiCl.
- Precipitate RNA at -80 °C for 2 h or -20 °C overnight (approximately 16 h) (Note 1).
- Centrifuge at 12,000 rpm for 10 min at 4 °C.
- Decant the supernatant.
- The RNA pellet is dissolved in 0.4 ml of RNase-free water by pipetting up and down for several times.
- Add 1/10 volume (40 μl) of 3 M sodium acetate (pH 5.2) and 1 volume (0.4 ml) of isopropanol.
- Precipitate RNA at -80 °C for 2 h or -20 °C overnight (approximately 16 h) (Note 1).
- Centrifuge at 12,000 rpm for 5 min at 4 °C.
- Decant the supernatant.
- Wash pellet with 0.6~1 ml of 75% ethanol by pipetting up and down for several times.
- Centrifuge at 12,000 rpm for 5 min at 4 °C.
- Decant the supernatant.
- Shortly dry the RNA pellet by speed vacuum.
- Dissolve RNA with an appropriate amount of RNase-free water (usually 20 μl or less) and incubate at 55-60 °C for 10 min.
- The RNA sample is stored at -80 °C.
- Immerse the Eppendorf tube that contains the microspore pellet in liquid nitrogen.
Representative data
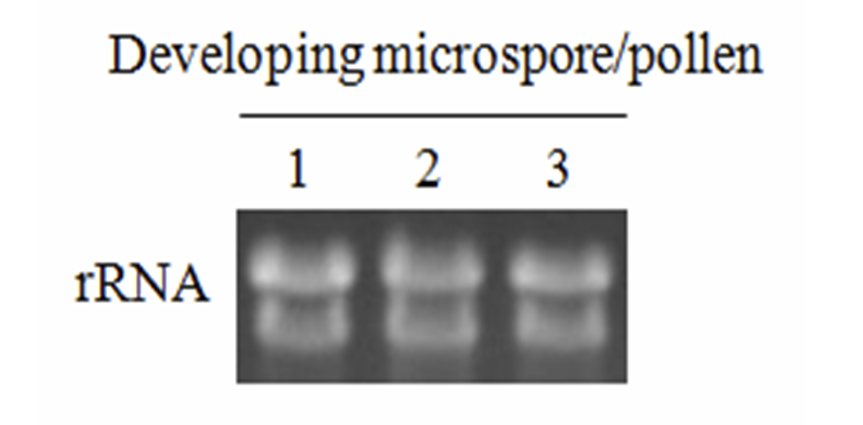
Figure 1. Total RNA (20 μg) was isolated from microspores isolated from lily flower buds with different lengths. 1. 34-36 mm buds; 2. 44-46 mm buds, both are at the microspore stage and 3. 60-65 mm buds at the transition between microspore and mature pollen. Total RNA was denatured, fractionated on a formaldehyde-agarose gel and stained with ethidium bromide.
Recipes
- 0.1% DEPC ddH2O (RNase-free water)
1 ml of DEPC is added into 999 ml of ddH2O, mixed at 37 °C for 1 h and autoclaved. - Extraction buffer 100 ml final conc.
4 M LiCl 5 ml 200 mM 1 M Tris-HCl (pH 8.0) 20 ml 200 mM 0.5M EDTA 4 ml 5 mM 10% SDS 20 ml 2% DEPC ddH2O 51 ml - 3 M sodium acetate (pH 5.2)
24.61 g of sodium acetate (MW = 82.03) is added into 100 ml of ddH2O, pH is adjusted to 5.2, and autoclaved. - 10 mM sodium acetate buffer (pH 5.2)
82.03 mg of sodium acetate (MW= 82.03) is added into 100 ml of ddH2O, pH is adjusted to 5.2, and autoclaved. - 4 M LiCl
16.96 g of LiCl (MW= 42.39) is added into 100 ml of ddH2O and autoclaved. - 75% ethanol
789.5 ml of 95% ethanol is added into 210.5 ml ddH2O.
Notes
- Precipitation of RNA at -20 °C overnight (approximately 16 h) may lead to a better yield of total RNA. However, if yield is not a concern, most RNAs can be precipitated at -20 °C for 2 h.
Acknowledgments
The protocol is an adaption from the protocol of Verwoerd et al. (1989). We greatly appreciate their original contribution.
References
- Liu, M. C., Yang, C. S., Yeh, F. L., Wei, C. H., Jane, W. N., Chung, M. C. and Wang, C. S. (2014). A novel lily anther-specific gene encodes adhesin-like proteins associated with exine formation during anther development. J Exp Bot 65(8): 2023-2037.
- Verwoerd, T. C., Dekker, B. M. and Hoekema, A. (1989). A small-scale procedure for the rapid isolation of plant RNAs. Nucleic Acids Res 17(6): 2362.
Article Information
Copyright
© 2015 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Liu, M., Chen, Y. and Wang, C. (2015). Separation of Microspores from Anthers of Lilium longiflorum (Lily) and Subsequent RNA Extraction. Bio-protocol 5(4): e1400. DOI: 10.21769/BioProtoc.1400.
Category
Plant Science > Plant biochemistry > RNA > RNA extraction
Plant Science > Plant physiology > Tissue analysis
Molecular Biology > RNA > RNA extraction
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










