- Protocols
- Articles and Issues
- About
- Become a Reviewer
Past Issue in 2023
Volume: 13, Issue: 9
Biochemistry
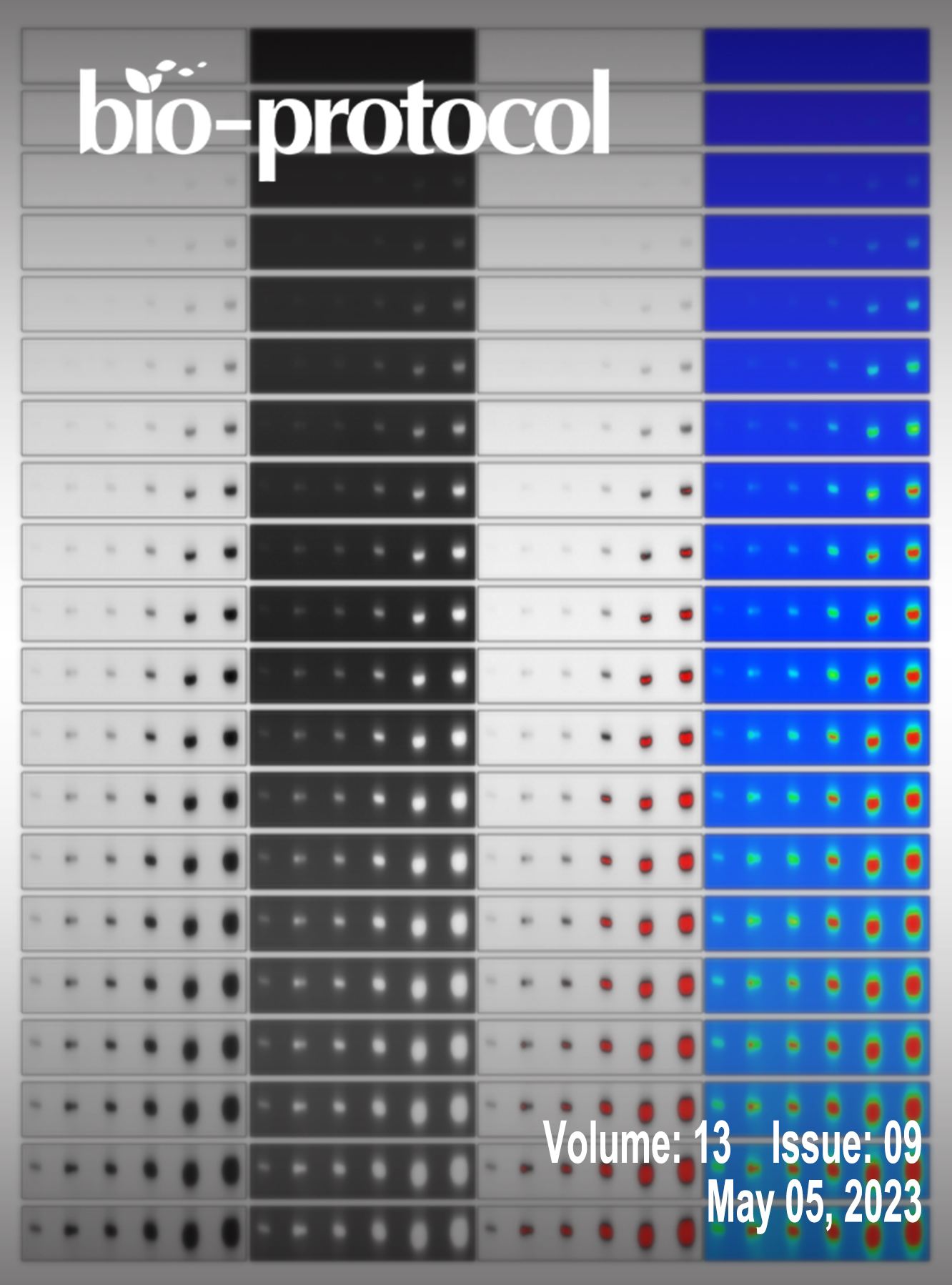
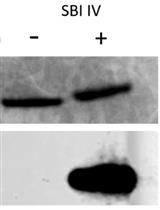
A Simple, Reproducible Procedure for Chemiluminescent Western Blot Quantification
Biological Engineering
Protocol for 3D Bioprinting Mesenchymal Stem Cell–derived Neural Tissues Using a Fibrin-based Bioink
Cell Biology
Induction of Skeletal Muscle Injury by Intramuscular Injection of Cardiotoxin in Mouse
Developmental Biology
E15.5 Mouse Embryo Micro-CT Using a Bruker Skyscan 1172 Micro-CT
Drug Discovery
Implementation of a Drug Screening Platform to Target Gch1 Expression in Injured Mouse Dorsal Root Ganglion Neurons
Medicine
A Novel Non-invasive Qualitative Assay Using Urinary Fluorescence Imaging to Assess Kidney Disease
Microbiology
Novel Antibody-independent Method to Measure Complement Deposition on Bacteria
Neuroscience
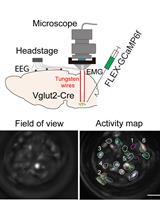
Simultaneous Microendoscopic Calcium Imaging and EEG Recording of Mouse Brain during Sleep
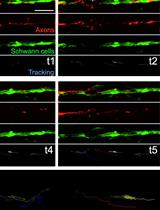
Long-term in toto Imaging of Cellular Behavior during Nerve Injury and Regeneration
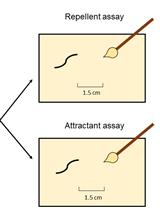
Assessment of Chemosensory Response to Volatile Compounds in Healthy, Aged, and Neurodegenerative Caenorhabditis elegans Models
Plant Science
Modified Pseudo-Schiff Propidium Iodide for Staining the Shoot Apical Meristem in Arabidopsis