Materials and Reagents
- Mouse embryos
- 4-6 μm paraffin sections of embryonic hearts
- 4% PFA
- Paraffin
- Xylene (Merck KGaA, catalog number: 8.08691.1000 )
- Ethanol (Merck KGaA, catalog number: 1.00983.1000 )
- PBS
- Wheat germ agglutinin (WGA) conjugates tetramethylrhodamine (Life Technologies, Molecular Probes®, catalog number: W849 ) (1:100)
- DAPI (Invitrogen, catalog number: D1306 )
- Distilled water
- Fluorescence mounting media (Fluoromont-G) (SouthernBiotech, catalog number: 0100-01 )
Equipment
- Microtome
- Fluorescence microscope
- Humid chamber
Software
- Image analysis software (Image J)
Procedure
- Embryos are fixed in 4% PFA and embedded in paraffin. (Mouse embryos are fixed ON at 4 °C and embedded in paraffin after dehydration with Ethanol and Xylene. E16.5 embryos were dehydrated 45 min ethanol 50%, 45 min ethanol 70%, 45 min ethanol 80%, 45 min ethanol 90%, 45 min ethanol 95%, 30 min ethanol 100%, 30 min ethanol 100%, 30 min xylene and 3 times 1-hour paraffin before orientation. Everything at room temperature but the paraffin which is done in a stove at 65 °C)
- Rehydrate the sections (65 °C 20 min, xylene 5 min, xylene 5 min, ethanol 100% 5 min, ethanol 100% 5 min, ethanol 96% 5 min, ethanol 90% 5 min, water 5 min).
- Wash with Distilled water 5 minutes three times at room temperature.
- Wash with 1x PBS 5 min twice at room temperature.
- Incubate WGA (1:100) 45 min at room temperature in humid chamber.
- Wash with 1x PBS 5 min three times.
- Incubate with DAPI (1:1,000) 10 min at room temperature in humid chamber and in darkness.
- Wash with 1x PBS 5 min twice.
- Mount with fluorescence mounting media (store at 4 °C in darkness before analysis).
- Make pictures under the microscope.
- Analyse pictures measuring the cell area using ImageJ. Measure at least 100 cells per section and three sections per embryo. Below image example of compact myocardium with the membranes stained with WGA where the relative area was measured. Myocites are identified ignoring the endocardial and endothelial cells, these cells form a monolayer covering the cardiomyocites. Cells within the myocardial wall are measured.
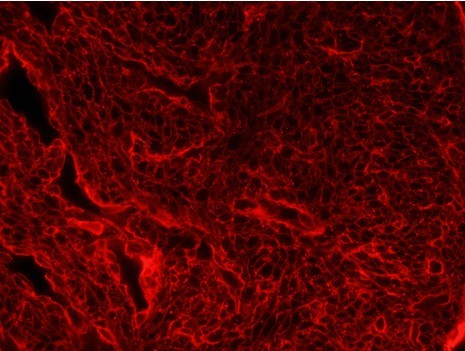
Figure 1. 7 µm transverse sections of a E15.5 WT heart stained with fluorophore-coupled wheat-germ agglutinin (WGA)
Acknowledgments
This study was funded by grants SAF2010-17555, RD06/0014/0038 (RECAVA) and, RD06/0010/1013 (TERCEL) from the Spanish Ministry of Economy and Competition (MINECO) and EU FP7-ITN 215761 (NotchIT) to J.L.d.l.P. G.L. had a PhD fellowship from the MINECO (FPI Program, BES-2008-002904).
References
- Luxan, G., Casanova, J. C., Martinez-Poveda, B., Prados, B., D'Amato, G., MacGrogan, D., Gonzalez-Rajal, A., Dobarro, D., Torroja, C., Martinez, F., Izquierdo-Garcia, J. L., Fernandez-Friera, L., Sabater-Molina, M., Kong, Y. Y., Pizarro, G., Ibanez, B., Medrano, C., Garcia-Pavia, P., Gimeno, J. R., Monserrat, L., Jimenez-Borreguero, L. J. and de la Pompa, J. L. (2013). Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med 19(2): 193-201.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
Category
Cell Biology > Cell imaging > Fluorescence














