- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Calcium Mobilization Assay to Measure the Activity of Gq-coupled Receptors
Published: Vol 3, Iss 12, Jun 20, 2013 DOI: 10.21769/BioProtoc.790 Views: 27172
Reviewed by: Cheng Zhang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of Extracellular Ca2+ Influx and Intracellular H+ Efflux in Response to Glycerol and PEG6000 Treatments
Tao Li and Baodong Chen
Sep 20, 2013 10227 Views

Analyzing the Quenchable Iron Pool in Murine Macrophages by Flow Cytometry
Michael Riedelberger and Karl Kuchler
Mar 20, 2020 6233 Views

Double Labeling of PDGFR-β and α-SMA in Swine Models of Acute Kidney Injury to Detect Pericyte-to-Myofibroblast Transdifferentation as Early Marker of Fibrosis
Alessandra Stasi [...] Giuseppe Castellano
Oct 5, 2020 4345 Views
Abstract
Calcium mobilization assay is a cell-based second messenger assay to measure the calcium flux associated with Gq-protein coupled receptor activation or inhibition. The method utilizes a calcium sensitive fluorescent dye that is taken up into the cytoplasm of most cells. In some cell lines in which organic-anion transporters are particularly active (e.g. CHO, HeLa), addition of probenecid, an inhibitor of anion transport, is required for retention of this dye in the cells. The dye binds the calcium released from intracellular store and its fluorescence intensity increases. The change in the fluorescence intensity is directly correlated to the amount of intracellular calcium that is released into cytoplasm in response to ligand activation of the receptor of interest. This protocol can be applied to most mammalian cell lines expressing both endogenous and transiently/stably transfected receptors. The method is sensitive enough to be used for low-expressing systems or high throughput screening of target of interest.
Note: The method does not differentiate the Ca2+ mobilization induced by Gqα from the Ca2+ mobilization induced by Gβγ.
Materials and Reagents
- Cells of choice: HEK293 cells stably expressing Angiotensin II Type 1 Receptor (AT1R)
- Ligands of choice: Sar1-Angiotensin II (Bachem, catalog number: H1740 )
- Poly-L-lysine (Sigma-Aldrich, catalog number: P4707 )
- 1x phosphate-buffered saline (PBS)
- FLIPR Calcium 5 assay kit (Molecular Devices, catalog number: R8185 )
- 96-well, FlexStation pipet tips, black (Molecular Devices, catalog number: 9000-0911 )
- Probenecid (Life Technologies, Invitrogen™, catalog number: P36400 )
- Cell growth media (see Recipes)
Equipment
- Incubator (5% CO2, 37 °C)
- FlexStation® 3 Multi-Mode Microplate Reader (Molecular Devices)
- Assay plate: 96-well microplate, tissue culture treated, black/clear, with lid (Greiner bio one, catalog number: 655090 )
- Ligand plate: Clear 96-well microtest plate, tissue culture treated, U-bottom (BD Biosciences, Falcon®, catalog number: 353077 )
Software
- SoftMax® Pro Microplate Data Acquisition & Analysis Software (supplied with the FlexStation® 3 Multi-Mode Microplate Reader)
Procedure
- Preparing the assay plate:
- Pre-coat the assay plate with 0.01% poly-L-lysine (50 μl/well) for 30 min at 37 °C (or 2 h at room temperature).
- Remove and wash excess poly-L-lysine with PBS.
- Prepare uniform suspension of cells of choice expressing the receptor and seed 50,000 cells per well in 50 μl medium.
Notes:
a. The cell number needs to be optimized for a particular cell line so that a 90% to 100% confluent cell monolayer is formed on the day of the assay.
b. If the cell line is transiently transfected with the receptor of interest, it is useful to measure the expression level of the receptor on the plasma membrane. - Incubate the cells overnight at 37 °C, 5% CO2.
- Aspirate the medium and add 100 μl serum-free medium into each well. Incubate the cells for 2 h at 37 °C in the CO2 incubator.
- Prepare the FLIPR loading dye by mixing 10 ml of component A with component B (see manufacturer's instructions for more details) and mix well.
Note: If your cells require probenecid, add probenecid into the loading dye at a final working concentration of 2.5 mM. Probenecid by Invitrogen is water-soluble and dissolves quickly in assay buffer. Probenecid should be added freshly on the day of the experiment. - Remove the assay plate from the CO2 incubator. Do not aspirate serum free medium. Add an equal volume (100 μl) of FLIPR loading dye into each well and incubate for 30 min at 37 °C, 5% CO2 followed by a 30 min incubation at room temperature if the assay will be performed at room temperature. The plate can be incubated at 37 °C, 5% CO2 for an hour if the assay will be performed at 37 °C.
Note: The incubation time and temperature needs to be optimized for a particular cell line. - After incubation transfer the assay plate to the FlexStation® 3 Multi-Mode Microplate Reader.
Note: The temperature should be set to optimum assay condition.
- Pre-coat the assay plate with 0.01% poly-L-lysine (50 μl/well) for 30 min at 37 °C (or 2 h at room temperature).
- Preparing the ligand plate:
- Prepare ligands (activator/inhibitor) at 5x concentration in 1x PBS in a final volume of 200 μl in ligand plate.
Notes:- You can test either one or several different concentrations of ligand of interest.
- You need to have at least duplicates of each ligand concentration to do a statistical analysis of the data.
- Plan your experiment carefully so that the ligand plate should be loaded by the time the cells are ready to be assayed.
- You can test either one or several different concentrations of ligand of interest.
- Prepare ligands (activator/inhibitor) at 5x concentration in 1x PBS in a final volume of 200 μl in ligand plate.
- Setting up the instrument (FlexStation® 3 Multi-Mode Microplate Reader):
- Place the assay plate, the ligand plate and the pipette tips as directed by the manufacturer's instructions.
- Open the SoftMax® Pro Microplate Data Acquisition & Analysis Software.
- Click on settings and select the FLEX mode. Recommended experimental setup parameters are given below:
- Read mode: Fluorescence
- Wavelengths: Ex: 485; Em: 525; Cut off: Auto (515)
- Sensitivity: High (Choose medium or low sensitivity if you expect very strong signals, especially with cell lines stably overexpressing the receptor.)
- Timing: 120 s reading with an interval of 2 sec (or choose accordingly)
- Assay plate type: 96 well Greiner blk/clr btm
- Wells to read: entire plate (or choose accordingly)
- Compound transfer:
- Initial volume: 200 μl
- Transfers: 1 (or choose accordingly for multiple transfers)
- Pipette height: 220 μl (adjusted slightly more than initial volume)
- Pipette volume: 50 μl (to make the final volume of the ligand in the assay plate as 1x)
- Rate: 1
- Time point: 18 sec (or choose accordingly)
- Compound source: Costar 96 well Ubtm clr. 3 ml
- Autocalibrate: on
- Autoread: off
- Read mode: Fluorescence
- Read
- Place the assay plate, the ligand plate and the pipette tips as directed by the manufacturer's instructions.
Representative data
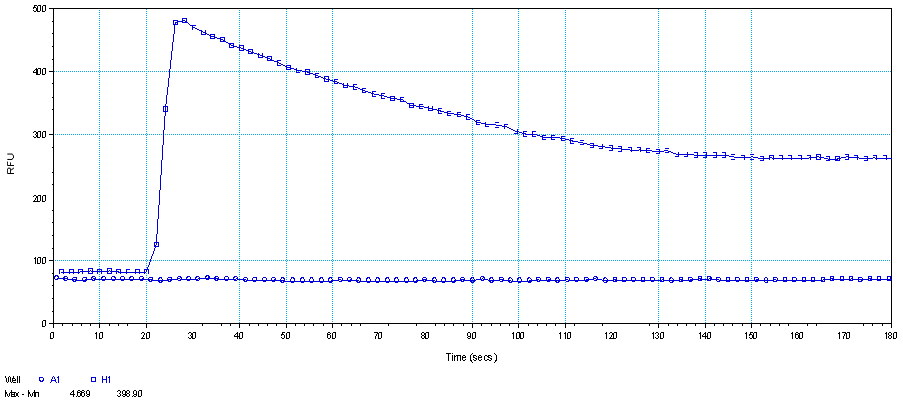
Figure 1. HEK293-AT1R cells stimulated with (well H1) and without (well A1) 1 μM Sar1-Angiotensin II at 18th second. The decrease in the signal with time is most likely resulted from receptor internalization.
Recipes
- Cell growth media (for HEK293 cells stably expressing Angiotensin II Type 1 Receptor (AT1R)) Dulbecco's modified eagle medium (DMEM) supplied with 10% fetal bovine serum (FBS).
Acknowledgments
This work was supported by the National Institutes of Health grants HL47570 and HL115964 (Sadashiva Karnik, Ph. D.) and National Research Service Award HL007914 (Hamiyet Unal, Ph. D.).
References
- Unal, H., Jagannathan, R., Bhatnagar, A., Tirupula, K., Desnoyer, R. and Karnik, S. S. (2013). Long range effect of mutations on specific conformational changes in the extracellular loop 2 of angiotensin II type 1 receptor. J Biol Chem 288(1): 540-551.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Unal, H. (2013). Calcium Mobilization Assay to Measure the Activity of Gq-coupled Receptors. Bio-protocol 3(12): e790. DOI: 10.21769/BioProtoc.790.
- Unal, H., Jagannathan, R., Bhatnagar, A., Tirupula, K., Desnoyer, R. and Karnik, S. S. (2013). Long range effect of mutations on specific conformational changes in the extracellular loop 2 of angiotensin II type 1 receptor. J Biol Chem 288(1): 540-551.
Category
Cell Biology > Cell-based analysis > Ion analysis > Calcium
Cell Biology > Cell staining > Other compound
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








