- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
2D Diagonal Redox SDS-PAGE of Proteins
Published: Vol 3, Iss 11, Jun 5, 2013 DOI: 10.21769/BioProtoc.781 Views: 16579
Reviewed by: Tie Liu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Heterologous Expression and Purification of Catalytic Domain of CESA1 from Arabidopsis thaliana
Venu Gopal Vandavasi and Hugh O’ Neill
Oct 20, 2016 7518 Views

Expression, Purification and Crystallization of Recombinant Arabidopsis Monogalactosyldiacylglycerol Synthase (MGD1)
Joana Rocha [...] Christelle Breton
Dec 20, 2016 8811 Views

X-ray Crystallography: Seeding Technique with Cytochrome P450 Reductase
Bixia Zhang [...] ChulHee Kang
Nov 5, 2022 2289 Views
Abstract
2D diagonal redox SDS-PAGE of proteins is used to detect intramolecular or intermolecular disulfide bridges using Chlamydomonas in this example (Stroeher and Dietz, 2008; Schwarz et al., 2012). Both dimensions consist of a conventional SDS-PAGE, except that the sample buffer for the first dimension lacks a reducing agent. Intermolecular disulfide bridges increase the apparent molecular weight of a protein in the first dimension, whereas intramolecular bridges decrease the apparent weight of the protein.
Keywords: 2d Diagonal SDS PAGEMaterials and Reagents
- All material for a conventional Laemmli SDS-PAGE
- Non-reducing Laemmli sample buffer
- KCl (Applichem, catalog number: A1039 )
- Triton X-100 (Applichem, catalog number: A1388 )
- DTT (Applichem, catalog number: A1101 )
- SDS (SERVA Electrophoresis GmbH, catalog number: 20765 )
- Acrylamide 40% 37.5:1 (SERVA Electrophoresis GmbH, catalog number: 10681 )
- Ammoniumperoxodisulfate (Carl Roth, catalog number: 9592 )
- TEMED (Carl Roth, catalog number: 2367 )
- Beta-mercaptoethanol (Carl Roth, catalog number: 4227 )
- Iodoacetamide (Applichem, catalog number: A1666 )
- Tris (Applichem, catalog number: A1379 )
- Na2EDTA (Carl Roth, catalog number: 8043 )
- Tricine (Carl Roth, catalog number: 6977 )
- Glycine (SERVA Electrophoresis GmbH, catalog number: 23390 )
- Agarose (SERVA Electrophoresis GmbH, catalog number: 11380 )
- Bromophenol blue (Carl Roth, catalog number: T116 )
- Filter paper (Munktell, catalog number: 2.519.580600N )
- Page Ruler unstained protein ladder (Thermo Fisher Scientific, catalog number: 26614 )
- 2x Cell lysis buffer (see Recipes)
- 5x non-reducing Laemmli sample buffer (see Recipes)
- 5x reducing Laemmli sample buffer (see Recipes)
- Laemmli electrophoresis buffer (see Recipes)
Equipment
- All equipment for a conventional Laemmli SDS-PAGE
Procedure
- Cell lysis (perform all lysis steps at 4 °C)
- Break your cells with 2x cell lysis buffer (use 1 ml of lysis buffer per pellet of a 1 L culture) by repeated pipetting for up to 5 min.
- Solubilize proteins with a final concentration of 1% SDS (30 min, 4 °C, no agitation required).
- Prevent thiol-reshuffling of your sample by alkylation with a final concentration of 0.1 M iodoacetamide (30 min, 4 °C, in the dark as iodoacetamide is not stable in light, no agitation required).
- Break your cells with 2x cell lysis buffer (use 1 ml of lysis buffer per pellet of a 1 L culture) by repeated pipetting for up to 5 min.
- First dimension (keep in mind that each lane of the first dimension requires a separate gel for the second dimension when calculating the number of samples that you want to process)
- Add appropriate amount of non-reducing Laemmli sample buffer to each sample while staying within the size limits of your gel comb, use enough material to enable the analysis of your protein by your detection method.
- Denature sample according to the best conditions for your protein (e.g. many soluble proteins can be denatured at 95 °C for 6 min).
- Perform Laemmli SDS-PAGE with your sample (Figure 1).
- Add appropriate amount of non-reducing Laemmli sample buffer to each sample while staying within the size limits of your gel comb, use enough material to enable the analysis of your protein by your detection method.
- Second dimension
- Excise lanes from gel as gel stripes (Figure 1).
- Reduce disulfide bridges by agitating your gel stripes in an amount of Laemmli electrophoresis buffer that covers your gel stripe completely (+ 0.1 M DTT [final concentration], 15 min, room temperature).
- Prevent thiol-reshuffling through alkylation by agitating your gel stripes in Laemmli electrophoresis buffer (containing 0.1 M idoacetamide [final concentration], 15 min, room temperature, in the dark).
- Prepare a separate gel for the 2nd dimension SDS-PAGE of each gel stripe with a shorter stacking gel (approximately 50% shorter than your stacking gel in the first dimension and no wells) to accommodate the gel stripe of the first dimension into your gel apparatus.
- Place gel stripe from first dimension in horizontal orientation (higher molecular weight on the left side, lower molecular weight on the right side) above stacking gel of the 2nd dimension (Figure 1).
- Boil 0.5% agarose (in Laemmli electrophoresis buffer with bromophenol blue [0.75 g L-1]) and fill your gel of the second dimension with the agarose solution (Figure 1).
- Press gel stripe against stacking gel of the second dimension (do not change orientation of gel stripe, avoid or remove air bubbles between gel stripe and stacking gel) before agarose solidifies (Figure 1).
- Pipet protein size marker on a small stripe of filter paper and stick that piece of paper beside your gel stripe into the agarose solution before agarose solidifies.
- Run Laemmli SDS-PAGE as the second dimension (bromophenol blue from the solidified agarose serves as running dye, a 17 cm gel of 1.5 mm thickness requires ~16 h at 14 mA) (Figure 1).
- Analyze your protein of interest (e.g. western blotting, MS).
- Proteins with intermolecular disulfide bridges appear on the left side of the diagonal, as their apparent weight shrank from the first to the second dimension.
- Proteins without disulfide bridges appear directly on the diagonal of your 2D PAGE, as there is no difference in their electrophoresis pattern between the two dimensions.
- Proteins with intramolecular disulfide bridges appear on the right side of the diagonal, as their apparent weight increased from the first to the second dimension.
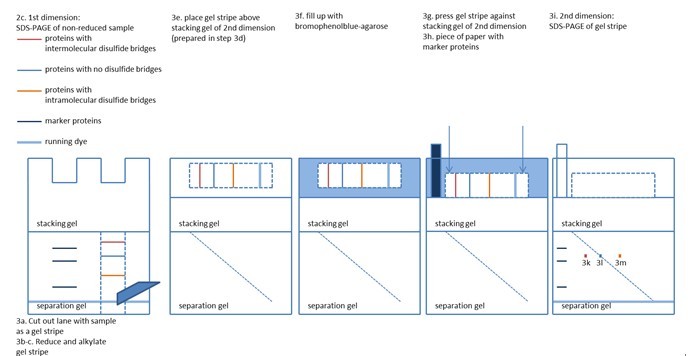
Figure 1. A scheme of the steps 2c - 3i with the results for the proteins described in 3k - 3m.
- Excise lanes from gel as gel stripes (Figure 1).
Recipes
- 2x cell lysis buffer
~200 ml dH2O
2.24 g KCl
97 mg EDTA
0.89 g Tricine
2.5 ml Triton X-100
Adjust pH to 7.8
Fill up with dH2O to 250 ml - 5x non-reducing Laemmli sample buffer
~40 ml dH2O
1.51 g Tris
3.75 g SDS
0.125 g Bromophenol blue
Adjust pH to 6.8
Fill up with dH2O to 50 ml - 5x reducing Laemmli sample buffer
Add 0.44 ml beta-mercaptoethanol to 50 ml of 5x non-reducing Laemmli sample buffer - Laemmli electrophoresis buffer
~800 ml dH2O
3.03 g Tris
14.41 g glycine
1.5 g SDS
Fill up with dH2O to 1,000 ml
References
- Schwarz, C., Bohne, A. V., Wang, F., Cejudo, F. J. and Nickelsen, J. (2012). An intermolecular disulfide-based light switch for chloroplast psbD gene expression in Chlamydomonas reinhardtii. Plant J 72(3): 378-389.
- Stroher, E. and Dietz, K. J. (2008). The dynamic thiol-disulphide redox proteome of the Arabidopsis thaliana chloroplast as revealed by differential electrophoretic mobility. Physiol Plant 133(3): 566-583.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Schwarz, C. and Nickelsen, J. (2013). 2D Diagonal Redox SDS-PAGE of Proteins. Bio-protocol 3(11): e781. DOI: 10.21769/BioProtoc.781.
Category
Plant Science > Plant biochemistry > Protein > Structure
Biochemistry > Protein > Electrophoresis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









