- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cell Isolation of Spleen Mononuclear Cells
Published: Vol 3, Iss 9, May 5, 2013 DOI: 10.21769/BioProtoc.689 Views: 33295
Reviewed by: Lin Fang

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Identification and Sorting of Adipose Inflammatory and Metabolically Activated Macrophages in Diet-Induced Obesity
Dan Wu [...] Weidong Wang
Oct 20, 2025 2235 Views

Selective Enrichment and Identification of Cerebrospinal Fluid-Contacting Neurons In Vitro via PKD2L1 Promoter-Driven Lentiviral System
Wei Tan [...] Qing Li
Nov 20, 2025 1335 Views

Revisiting Primary Microglia Isolation Protocol: An Improved Method for Microglia Extraction
Jianwei Li [...] Guohui Lu
Dec 5, 2025 1462 Views
Abstract
This method allows you to isolate different subclass mononuclear cells, like B-cells, T cells, CD4+ and CD8+ T, from mouse spleen. By conjugating cells with specific antibodies and subsequently magnetic beads isolation, using the technique from Miltenyi, this allows a high purity.
Keywords: T cellMaterials and Reagents
- Antibody
- FITC-conjugated anti-CD3 antibody (BD Biosciences, catalog number: 553058 , clone 145-2C11)
- PE-conjugated anti-CD19 antibody (BD Biosciences, catalog number: 557399 , clone 1D3)
- FITC-conjugated anti-CD4 antibody (BD Biosciences, catalog number: 557667 , clone RM4-5)
- PE-conjugated anti-CD8 antibody (BD Biosciences, catalog number: 561095 , clone 53-6.7)
- FITC-conjugated anti-CD3 antibody (BD Biosciences, catalog number: 553058 , clone 145-2C11)
- Microbeads
- Anti-CD43 microbeads (Miltenyi Biotec, catalog number: 130-049-801 )
- Anti-CD90 microbeads (Miltenyi Biotec, catalog number: 130-091-376 )
- Anti-CD4 microbeads (Miltenyi Biotec, catalog number: 130-049-201 )
- Anti-CD8 microbeads (Miltenyi Biotec, catalog number: 130-091-112 )
- Anti-CD43 microbeads (Miltenyi Biotec, catalog number: 130-049-801 )
- Others
Equipment
- Scissors and forceps
- MiniMACS separation unit (Miltenyi Biotec, MiniMACS Separator, catalog number: 130-090-312 )
- Separation column (Miltenyi Biotec, separation column, Type MS, catalog number: 130-042-201 )
- Cell strainer (BD Biosciences, catalog number: 352360 )
- Flow cytometer (BD Biosciences, Coulter)
- Shaker
Procedure
B-cells, total T cells and/or single positive CD4+ and CD8+ T cells, were purified from spleen cells by magnetic separation with the Mini-MACS system [Miltenyi, 1990] (http://www.miltenyibiotec.com). The scheme illustrates how to manage the procedure.
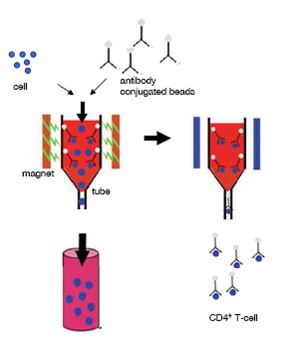
Figure 1. Schematic view of the experimental strategy using magnetic MACS beads to isolate CD4+ cells. Cells were incubated with CD4-,CD8-,CD90- and CD43-antibody conjugated magnetic beads. The cell suspensions were subjected to column selection and placed in the magnetic separator. Flow-through was discarded. Then the column was washed with separation puffer to increase purity and removed afterwards from the magnetic separator. With a plunger the magnetically labelled cells were flushed out of the column.
- Sacrifice mouse and isolate the complete spleen.
- Pass spleen through a 100-μm cell strainer to get single cells suspension by crushing with forceps and collecting the cell suspension in 5 ml PBS.
- Wash with 1x PBS & centrifuge the cells (100 x g for 5 min), then resuspend spleen cells in 80 μl ice-cold separation buffer per 107 cells. From a normal spleen you will get approximately 8 x 107 cells. The overall operation temperature is room temperature. The buffers should be ice cold.
- Add a 20 μl aliquot of antibody-conjugated microbeads per 107 cells incubate for 30 min at 4-8 °C at a shaker. No prewash is needed. The number of cells is depending from the animal.
The following microbeads were used: anti-CD43 microbeads for negative isolation of resting B cells, anti-CD90 microbeads for positive isolation of total T cells, anti-CD4 and anti-CD8 microbeads for positive isolation of the respective T-cell subset.
- Wash the column by putting 5 ml 1x PBS on the top. The liquid passes the column by gravity. Then pipette the labelled cell suspension on top of a separation column, which had been washed three times with separation buffer and placed in the MiniMACS separation unit. Pass the suspension through the column.
- In case of negative selection of CD43- B cells the effluent was collected as a B-cell fraction and washed respectively centrifuged three times with 5 ml PBS.
In case of positive selection of CD90+, CD4+ or CD8+ T-cells the effluent was discarded and the columns were washed twice with 500 μl separation buffer. Subsequently, remove columns from the separator and wash magnetically labelled cells out with 1 ml separation buffer using a plunger.
- Wash the respective T-cell fraction three times with medium same as B-cells
- Assess the purity of the various cell fractions by Flow cytometry analysis using a Flow cytometer. Stain cells with fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (clone 145-2C11 to detect total T-cells), phycoerythrin (PE)-conjugated anti-CD19 (clone 1D3 to detect B-cells), FITC-conjugated anti-CD4 (clone RM4-5 to detect CD4+ T-cells) and PE-conjugated anti-CD8 (clone 53-6.7 to detect CD8+ T-cells) and analyse for positive cells according to standard procedures.
Recipes
- Separation buffer
1x PBS with 5 mM EDTA and 0.5% BSA
Acknowledgments
The authors thank the German Research Foundation (DFG) and the University Erlangen-Nuremberg for funding. We thank A. von Berg and L. Sologub for excellent technical assistance.
References
- Miltenyi, S., Muller, W., Weichel, W. and Radbruch, A. (1990). High gradient magnetic cell separation with MACS. Cytometry 11(2): 231-238.
Article Information
Copyright
© 2013 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Weigmann, B. (2013). Cell Isolation of Spleen Mononuclear Cells. Bio-protocol 3(9): e689. DOI: 10.21769/BioProtoc.689.
Category
Immunology > Immune cell isolation > Lymphocyte
Cell Biology > Cell isolation and culture > Cell isolation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









