- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Simultaneous Non-Invasive Electrocardiogram and Respiration Rate Recordings in Head-Fixed Awake Mice
Published: Vol 16, Iss 1, Jan 5, 2026 DOI: 10.21769/BioProtoc.5556 Views: 641
Reviewed by: Rachael E. HokensonAnonymous reviewer(s)
Abstract
Autonomic regulation of heart and respiratory rates is essential for understanding brain–body interactions in health and disease. Preclinical cardiovascular recordings are often performed under anesthesia or via telemetry, both of which introduce physiological confounds such as stress or impaired recovery due to the need for acute or chronic implantation of sensors. Here, we present a minimally invasive protocol for simultaneous acquisition of high-quality electrocardiography and respiratory signals in awake mice. Using an in-house-modified physiological monitor in awake, head-fixed mice that were briefly habituated to experimental conditions, we ultimately enable stable, long-term physiological recordings alongside in vivo microscopy. This protocol provides a robust, low-stress method for acquiring physiological signals, enabling the simultaneous study of cardiovascular–cerebral dynamics in awake head-fixed mice, thereby enhancing the translational relevance of preclinical measurements.
Key features
• Minimally invasive electrocardiogram and respiration rate acquisition in awake, head-fixed mice suitable for long-term physiological recordings.
• Custom-built setup integrates physiological monitoring with in vivo imaging without surgical implantation or telemetry.
• Rapid habituation protocol ensures low-stress conditions and high-quality signal acquisition in conscious mice.
• Enables correlation of cardiovascular dynamics with brain activity and cerebrospinal fluid flow in translational neuroscience studies.
Keywords: Non-invasiveBackground
Heart and respiratory rates reflect dynamic responses of the autonomic nervous system to external and internal stimuli [1,2]. Cardiogenic pulsatility, frequently assessed via non-invasive electrocardiogram (ECG) measurements, plays a crucial role in driving various homeostatic processes in the brain, including the regulation of blood flow and cerebrospinal fluid (CSF) dynamics [3]. While ECG is a state-of-the-art method in clinics and preclinical settings, in small animal research models, it is predominantly performed under anesthesia to avoid the confounding effects of stress and motion [4]. Furthermore, simultaneous ECG and brain imaging in awake conditions can provide valuable correlative measures that allow a closer understanding of the relationship between brain function, cognitive processes, motor behavior, CSF transport, and the complex cardiovascular dynamics associated with autonomous nervous system activity [5].
With the advent of glymphatic system research [6], simultaneous in vivo ECG and microscopy recordings in awake animal models are crucial to underpin the influence of complex heart–brain interaction and to bring higher translational value to basic glymphatic research.
Existing methods for obtaining ECGs in fully awake mice typically involve restraint or telemetry devices, which often represent a suboptimal solution or introduce confounding effects into the acquired signals by inducing stress or altering recovery after implantation of the device [2,4,7,8]. To address these limitations, we designed a technique that enables rapid, minimally invasive ECG recordings in awake head-fixed mice. This approach enables long-term, simultaneous ECG and respiratory recordings alongside in vivo microscopy, which is highly desired in neuroscientific applications. Therefore, the proposed and validated setup allows a comprehensive platform for studying cardiovascular dynamics alongside cerebrovascular changes during full wakefulness. This integrated methodology surpasses historical constraints, offering a powerful tool for understanding the interplay between cardiovascular and neural activities during various physiological and pathological conditions.
Materials and reagents
Reagents
1. Ethanol 70% (Vol Technisolv, catalog number: C83801.360)
2. Distilled water (in-house, from reverse osmosis)
Laboratory supplies
1. Signagel electrode (Gel Parker Laboratories, catalog number: 15-60)
2. Dent Silicone-V (Shofu INC, catalog number: 072312)
3. MAG-1, simple head holder plate (Narishige, catalog number: MAG-1)
4. Tesa isolation tape (10 m × 15 m) or other electric insulation tape (Tesaflex, catalog number: 53988)
5. Surgical tape (1.25 cm) (3M, catalog number: 1530-1)
6. Copper tape with one-sided adhesive (0.25 mm thickness) (BilTema, catalog number: 29-2500)
7. Dental pad 30 × 45 cm or smaller (KRUUSE, catalog number: 161248)
8. Cardboard (Bio-Tunnels for mice) tubes (approximate length 7.5, diameter 5.02 cm; half-tubes height ~2.5 cm, thickness 0.35 cm) (Bio-Serv, catalog number: K3556)
9. Clean gauze 5 × 5 cm (Medshop.dk, catalog number: 754040)
10. Tissue paper (KIMTECH Science, Kimberly-Clark Professional, catalog number: 7558)
Equipment
1. Small animal physiological monitoring system (Harvard Apparatus, catalog number: 75-1500)
Software and datasets
1. Clampex Version 10.7.0.3 (Molecular Devices, LLC)
2. MATLAB ver. R2023a or later (MathWorks Inc.)
Procedure
A. Preparation of the physiological monitoring system: increasing the ECG electrode surface for measurements in awake and moving mice
1. Clean the monitoring platform
a. Wipe all surfaces with distilled water and dry tissue paper.
b. If necessary, use 70% ethanol, but avoid using isopropane as it may lead to plastic corrosion.
Pause point: Allow to air dry completely for 15 min (Figure 1A).
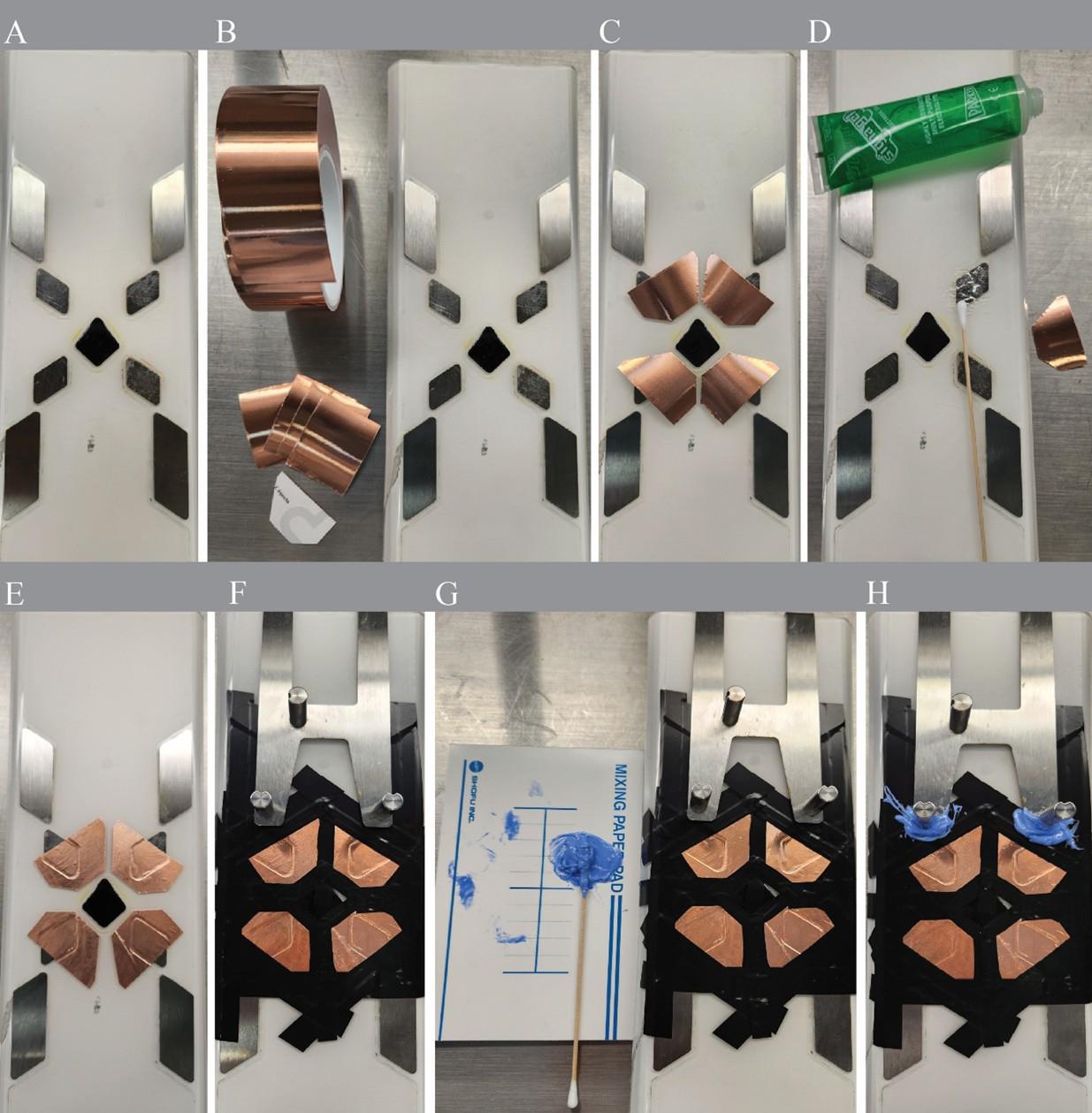
Figure 1. Step-by-step preparation and modification of the small animal physiological monitoring system for simultaneous electrocardiogram (ECG) and respiratory recordings in awake, head-fixed mice. (A) Cleaning the surface of the Harvard Apparatus ECG monitoring platform with distilled water and drying with tissue paper. (B, C) Cutting and preparing four copper tape electrodes (4.5 × 2.5 cm) with even dimensions. (D) Applying a thin layer of Signagel electrode gel to ensure conductivity with platform and copper tape electrodes. (E) After copper tape electrode attachment. (F) Applying Tesaflex electrical insulation tape to isolate the copper tape edges and prevent accidental bridging. (G, H) Sealing exposed conductive surfaces with Dent Silicone-V to minimize electrical noise during recording.
2. Prepare copper electrode pads
a. Cut copper tape (0.25 mm thickness, conductive adhesive) into four oval pads (approximately 4.5 × 2.5 cm) using scissors (Figure 1B).
b. To ensure uniform change in electrical impedance of the new electrode fields, cut the pads to similar sizes (Figure 1C). However, bear in mind that the contribution of the additional copper layer to the overall electrode impedance would be negligible when considering that of the skin–electrode interface.
c. Apply a thin, even layer of Signagel electrode gel (one drop, ~1 mm thick) to each copper pad (Figure 1D).
3. Attach copper pads to the ECG sensor surface
a. Attach each copper to the existing electrode fields to extend their surface (Figure 1E).
b. Maintain 2–3 mm distance between pads to prevent short circuits (Figure 1E).
c. Remove any excess gel with clean tissue paper.
4. Isolate conductive surfaces
a. Cover the edges of each copper pad using Tesaflex electrical tape (Figure 1F).
b. Apply Dent Silicone-V over exposed conductive regions to prevent interference (Figure 1G–H).
5. (Caution) Do not allow gel to contact insulation material, to prevent creating unintended conductive paths. Install absorbent pads.
a. Cut KRUUSE dental pads into strips (0.5 cm wide) to fit over the copper pads.
b. Fix strips in place using 3M Micropore surgical tape (1.25 cm width) to prevent movement. These strips absorb urine or excess gel during recording (Figure 2A).
Note: Replace absorbent pads between animals.

Figure 2. Final preparation of the physiological monitoring system before and after head-fixation of the mice for subsequent imaging, along with electrocardiogram (ECG) recordings. (A) Cutting and placing narrow strips of KRUUSE dental pads (0.5 cm wide) over each electrode area to absorb urine and excess gel, secured with 3M surgical tape. (B) Constructing and placing a custom half-tube cardboard tunnel (approx. 7.5 cm × 2.5 cm) to provide restraint and comfort.
6. Prepare the restraint tunnel
a. Cut a cylindrical cardboard tube (e.g., mailing tube or toilet roll) lengthwise to create a half-tube (approximately 7.5 cm × 2.5 cm, thickness 0.35 cm).
b. Cut the top opening for head-fixation access (Figure 2B).
c. (Optional) Cut a bottom opening for injection access during pharmacological studies.
7. Mount the tunnel
a. Position the half-tube over the platform and secure it using surgical tape.
b. Ensure stability so the animal remains comfortably supported (Figure 2B).
Note: Ensure the mouse can move its forepaws freely under the tunnel without obstruction.
B. Animal habituation to the setup
Note: Animal models were adult, male, C57BL/6J mice, 8–12 weeks old, group-housed (5 per cage) under a 12-h light/dark cycle, 22 ± 1 °C, with ad libitum food and water. All procedures were approved by the Danish Animal Experiments Inspectorate and University of Copenhagen, Denmark.
B1. Begin 5-day habituation protocol
1. Day 1
a. Head-fix mouse in the MAG-1 stage for 5–10 min per session, 2 sessions separated by ≥1 h. The home cage is used for habituation to reduce stress.
b. Return to the home cage and reward with a sunflower seed or a treat of your choice.
2. Day 2
a. First session: Gently head-fix the mice in the MAG-1 stage and place them in the home cage for 30 min.
b. Return the mice to the home cage for 1 h before the second session.
c. Second session: Gently head-fix the mice in the MAG-1 stage and place them in the home cage for 90 min.
d. Return the mice to the home cage and reward with sunflower seeds or a treat of your choice.
Day 3
a. Single session: Gently head-fix the mice in the MAG-1 stage and place them in the home cage for 90 min.
b. Return the mice to the home cage and reward with sunflower seeds or a treat of your choice.
Day 4
a. Single session: Gently head-fix the mice in the MAG-1 stage and place them in the home cage for 180 min.
b. Return the mice to the home cage and reward with sunflower seeds or a treat of your choice.
Day 5
a. Single session: Gently head-fix the mice in the MAG-1 stage and place them in the home cage for 180 min.
b. Return the mice to the home cage and reward with sunflower seeds or a treat of your choice.
Day 6
a. Ready for full recording. Gently head-fix the mice in the MAG-1 as shown in Figure 2 for imaging.
Note: If mice display stress or excessive motion, extend habituation by 1–2 days.
B2. Ensure safety and stress monitoring
1. Observe for signs of distress (e.g., escape behavior, vocalization, urination).
2. (Caution) Do not proceed to recording until stress levels are minimal.
C. Preparation for the recording
1. Secure the mouse in the MAG-1 stage
a. Gently head-fix the mouse to the physiological monitor using the headplate.
b. Adjust height and alignment to ensure stability without excessive pressure.
c. Confirm if the body fits under the tunnel comfortably.
2. Apply electrode gel
a. Place the mouse’s forepaws directly on the copper pads.
b. Apply ~0.05 mL of Signagel under each paw using a pipette tip.
c. Confirm stable, direct contact.
3. Cover the body: Place the cardboard tunnel over the mouse’s torso and tape it securely to the platform.
Note: This improves restraint and reduces motion artifacts.
4. Insulate the mouse: Gently place a 5 × 5 cm sterile gauze pad over the back to provide warmth and reduce evaporative heat loss.
5. Add electrical grounding: Connect the monitor’s ground wire to a known earth-grounded source (e.g., grounded outlet, metal lab bench).
Caution: Improper grounding may introduce 50/60 Hz electrical noise.
6. Allow acclimation
a. Leave the animal in position for 10 min before starting recordings.
b. Observe signals in Clampex to assess baseline quality.
D. Recordings
1. Start the Clampex software. Use Clampex v10.7.0.3 for live signal monitoring and acquisition.
2. Check signal quality
a. Confirm visibility of clear ECG peaks and respiration waveforms.
b. Adjust gel or paw position if signal-to-noise ratio is low.
3. Record data
a. Acquire continuous data for 30–180 min, depending on experimental needs.
b. Observe the animal throughout for signs of discomfort.
c. (Optional) Note behavioral events or interventions during recording.
4. End the session
a. Turn off recording and gently release the headplate.
b. Return the animal to its original home cage, with fresh bedding.
5. Clean the setup
a. Remove used pads and tape.
b. Wipe surfaces with distilled water or 70% ethanol.
c. Dispose of waste following institutional biosafety guidelines.
d. Ensure full drying before next session.
Data analysis
Raw ECG and respiration signals were acquired using Clampex software (v10.7.0.3) and saved in .atf (Axon Text File). These files were exported into MATLAB (R2023a or later) for preprocessing and analysis.
1. Preprocessing
a. ECG signals were subjected to low-pass Butterworth filtering (cutoff: 50 Hz) to remove high-frequency noise and electrical interference.
b. Respiration data were filtered with a low-pass filter at 10 Hz to retain slow-wave respiratory patterns.
c. Baseline drift was corrected using detrending functions.
2. Noise detection and correction
a. Signal quality was visually inspected, and segments with artifacts (e.g., due to motion or loose paw contact) were identified based on sudden signal amplitude jumps and flatlining or extreme deviations in R–R intervals.
3. These sections were manually removed or flagged and excluded from automated analysis using NaN masking. Filtering was validated by checking the signal-to-noise ratio and the presence of regular ECG morphology (P-QRS-T complex).
4. Signal analysis
a. R-peaks were identified using a custom MATLAB peak detection algorithm (findpeaks function), optimized for murine heart rate ranges (~300–800 bpm).
b. Respiratory peaks and troughs were extracted to calculate respiratory rate over time.
c. Heart rate variability (HRV) was calculated using SDNN (standard deviation of normal-to-normal intervals), RMSSD (root mean square of successive differences), and inter-beat intervals, which were exported as time series for each mouse.
5. Arrhythmia detection
a. ECG traces were analyzed for irregular patterns using R–R interval variability and visual inspection.
b. Events such as premature beats or skipped beats were flagged and quantified across recording sessions.
6. Data output: Results were plotted as time series of heart and respiration rates, histograms of R–R intervals, and summary bar graphs of HRV metrics per group or condition. Sample outputs are provided in Figure 3A–C.
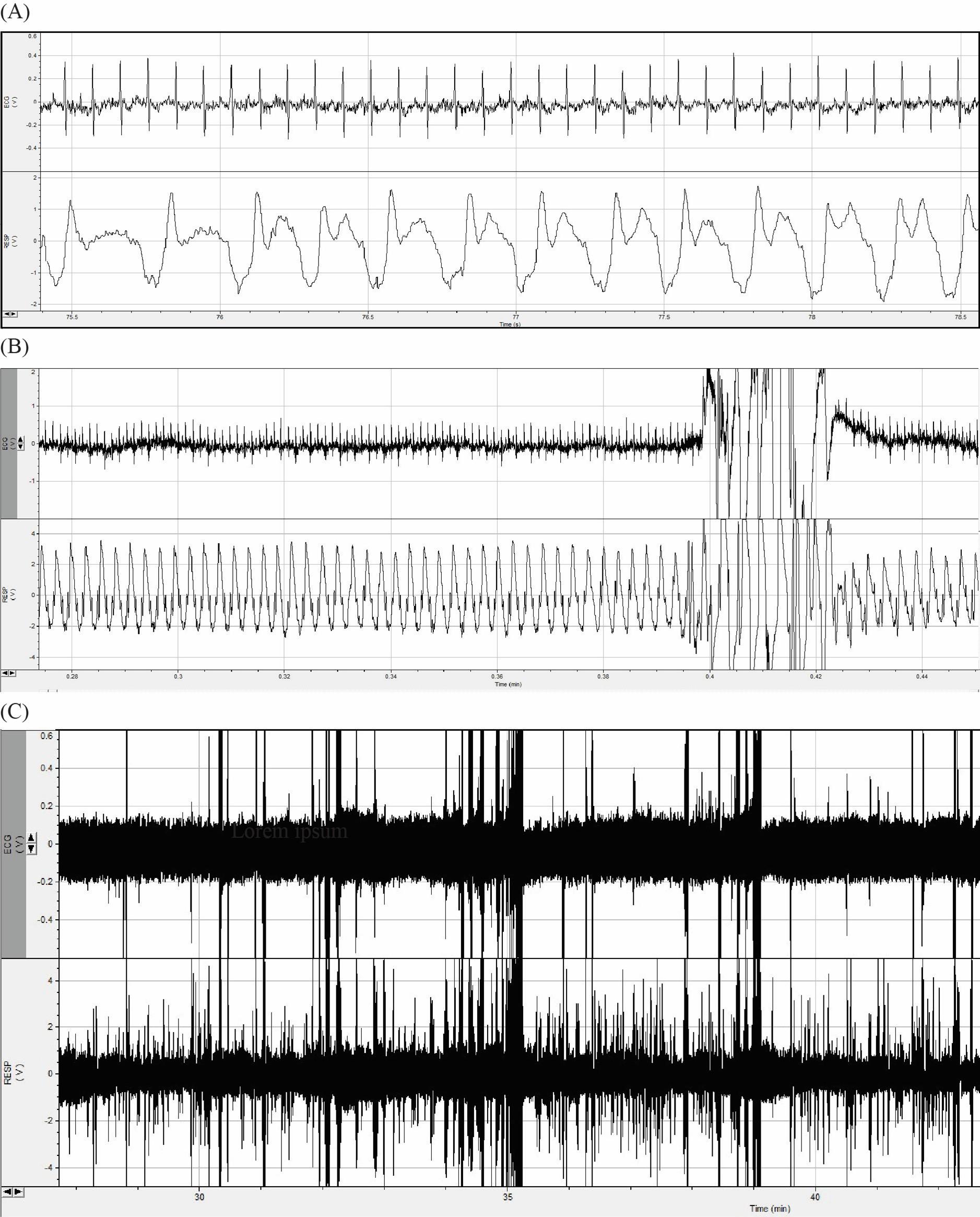
Figure 3. Electrocardiogram (ECG) and respiration rate recordings of three mice. (A) Artifact-free portion of ECG and respiration rate recordings. (B) Movement artifact at 0.4–0.42 min. (C) Full raw recordings over 3 h.
Validation of protocol
This protocol has been applied in numerous awake, head-fixed mice across multiple experimental series, demonstrating high reproducibility and robust physiological signal quality. The setup supports stable long-term recordings of ECG and respiration signals, with durations of up to 3 h following the described habituation protocol. Although motion-related artifacts may occur, they can be reliably identified and removed during data analysis using peak detection and filtering algorithms.
To illustrate signal stability and physiological relevance, summary data from seven representative mice are presented in Table 1, including sex, duration, and heart and respiration rate. While the full dataset is not yet published, it underpins ongoing investigations of neurocardiovascular coupling in migraine and sleep regulation models. Representative traces and analysis workflows are included in this manuscript (see Figure 3A–C).
Table 1. Representative summary of electrocardiogram (ECG) and respiration rates over 3 h of recordings in awake head-fixed mice
| Mouse ID | Sex | Duration (min) | Heart rate (bpm) | Respiration rate (bpm) |
|---|---|---|---|---|
| 1 | Male | 243 | 572 | 195 |
| 2 | Female | 181 | 610 | 192 |
| 3 | Female | 180 | 628 | 185 |
| 4 | Female | 180 | 636 | 197 |
| 5 | Male | 182 | 539 | 195 |
| 6 | Male | 187 | 607 | 201 |
| 7 | Female | 191 | 602 | 190 |
General notes and troubleshooting
Problem 1: Poor ECG signal quality or excessive noise.
Possible causes: Inadequate electrode contacts or residual gel bridging the copper tapes.
Solutions: Ensure even application of conductive gel, maintain sufficient separation of copper electrodes, and avoid gel overflow.
Problem 2: Animal shows stress or excessive movement during recording.
Possible causes: Insufficient habituation or unstable head-fixation.
Solutions: Extend the habituation period by 1–2 additional days. Recheck headplate fit and tunnel stability.
Problem 3: Short circuits or signal dropout.
Possible causes: Electroconductive gel leaking onto adjacent electrodes or setup wiring.
Solutions: Apply absorbent dental pad strips more thoroughly and double-check insulation with electrical tape and silicone sealant.
Acknowledgments
H.G. is supported by a Neuroscience Research Training Scholarship Funded by the American Academy of Neurology.
Competing interests
We declare no competing interests.
Ethical considerations
All experiments were approved by the Danish Animal Experiments Inspectorate and were overseen by the University of Copenhagen Institutional Animal Care and Use Committee, in compliance with the European Communities Council Directive of 22 September 2010 (2010/63/EU) legislation governing the protection of animals used for scientific purposes.
References
- Appel, M. L., Berger, R. D., Saul, J., Smith, J. M. and Cohen, R. J. (1989). Beat to beat variability in cardiovascular variables: Noise or music?. J Am Coll Cardiol. 14(5): 1139–1148. https://doi.org/10.1016/0735-1097(89)90408-7
- Hsueh, B., Chen, R., Jo, Y., Tang, D., Raffiee, M., Kim, Y. S., Inoue, M., Randles, S., Ramakrishnan, C., Patel, S., et al. (2023). Cardiogenic control of affective behavioural state. Nature. 615(7951): 292–299. https://doi.org/10.1038/s41586-023-05748-8
- Mestre, H., Tithof, J., Du, T., Song, W., Peng, W., Sweeney, A. M., Olveda, G., Thomas, J. H., Nedergaard, M., Kelley, D. H., et al. (2018). Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. 9(1): e1038/s41467–018–07318–3. https://doi.org/10.1038/s41467-018-07318-3
- Ho, D., Zhao, X., Gao, S., Hong, C., Vatner, D. E. and Vatner, S. F. (2011). Heart Rate and Electrocardiography Monitoring in Mice. Curr Protoc Mouse Biol. 1(1): 123–139. https://doi.org/10.1002/9780470942390.mo100159
- Levy, M. N. (1971). Brief Reviews. Circ Res. 29(5): 437–445. https://doi.org/10.1161/01.RES.29.5.437
- Hablitz, L. M. and Nedergaard, M. (2021). The Glymphatic System: A Novel Component of Fundamental Neurobiology. J Neurosci. 41(37): 7698–7711. https://doi.org/10.1523/jneurosci.0619-21.2021
- Chu, V., Otero, J. M., Lopez, O., Morgan, J. P., Amende, I. and Hampton, T. G. (2001). Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 1(1): 6. https://doi.org/10.1186/1472-6793-1-6
- Sato, M., Matsumoto, N., Noguchi, A., Okonogi, T., Sasaki, T. and Ikegaya, Y. (2018). Simultaneous monitoring of mouse respiratory and cardiac rates through a single precordial electrode. J Pharmacol Sci. 137(2): 177–186. https://doi.org/10.1016/j.jphs.2018.06.009
Article Information
Publication history
Received: Jul 28, 2025
Accepted: Nov 23, 2025
Available online: Dec 4, 2025
Published: Jan 5, 2026
Copyright
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Ghanizada, H., Gomolka, R. S. and Nedergaard, M. (2026). Simultaneous Non-Invasive Electrocardiogram and Respiration Rate Recordings in Head-Fixed Awake Mice. Bio-protocol 16(1): e5556. DOI: 10.21769/BioProtoc.5556.
Category
Neuroscience > Basic technology
Neuroscience > Behavioral neuroscience
Biophysics > Electrophysiology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








