- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Combining Microwave Ablation With CAR-T-Cell Therapy in Tumor-Bearing Mouse Models
(*contributed equally to this work) Published: Vol 15, Iss 20, Oct 20, 2025 DOI: 10.21769/BioProtoc.5470 Views: 2324
Reviewed by: Pilar Villacampa AlcubierreAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Optical Modulation of the Blood-Brain Barrier for Glioblastoma Treatment
Qi Cai [...] Zhenpeng Qin
Jan 20, 2024 2373 Views

A Flow Cytometry–Based Method for Assessing CAR Cell Binding Kinetics Using Stable CAR Jurkat Cells
Alex Shepherd [...] Scott McComb
Jun 20, 2024 3173 Views

NanoPDLIM2-Based Combination Therapy for Lung Cancer Treatment in Mouse Preclinical Studies
Thi Hoa Le [...] Zhaoxia Qu
Sep 5, 2025 2235 Views
Abstract
Microwave ablation (MWA) is a thermal ablation technique widely used for local tumor control that has the added potential to stimulate systemic anti-tumor immunity. Although MWA alone rarely eliminates recurrent or metastatic disease, its ability to remodel the tumor microenvironment makes it a promising partner for adoptive cell therapies such as chimeric antigen receptor (CAR)-T cells. However, reproducible protocols for combining these approaches remain limited. This protocol describes the integration of MWA with CAR-T therapy in tumor-bearing mouse models. Human hepatocellular carcinoma cell lines (Hep3B and SK-HEP-1) are inoculated subcutaneously into NOG mice to establish tumors. Localized MWA is performed at adjustable power and duration to induce partial or complete ablation. At defined intervals following MWA, CAR-T cells derived from healthy donor T cells and transduced with a lentiviral vector are injected intravenously. This experimental design uniquely separates MWA and CAR-T delivery, enabling precise evaluation of thermal preconditioning effects on the tumor microenvironment and subsequent CAR-T activity. By combining localized ablation with adoptive immunotherapy, the protocol provides a translationally relevant platform to optimize treatment timing, enhance CAR-T efficacy in solid tumors, and address key barriers in tumor immunology and cancer therapy.
Key features
• Practical protocol to investigate MWA and CAR-T-cell combination therapy in mouse tumor models.
• Includes detailed procedures for tumor cell preparation, subcutaneous inoculation, MWA treatment, and CAR-T-cell administration.
• Novel protocol design separates MWA and CAR-T delivery, allowing rigorous analysis of preconditioning effects on the tumor microenvironment and therapy response.
• Offers translational relevance for improving CAR-T therapy in solid tumors, addressing a critical barrier to clinical application.
Keywords: Microwave ablationGraphical overview
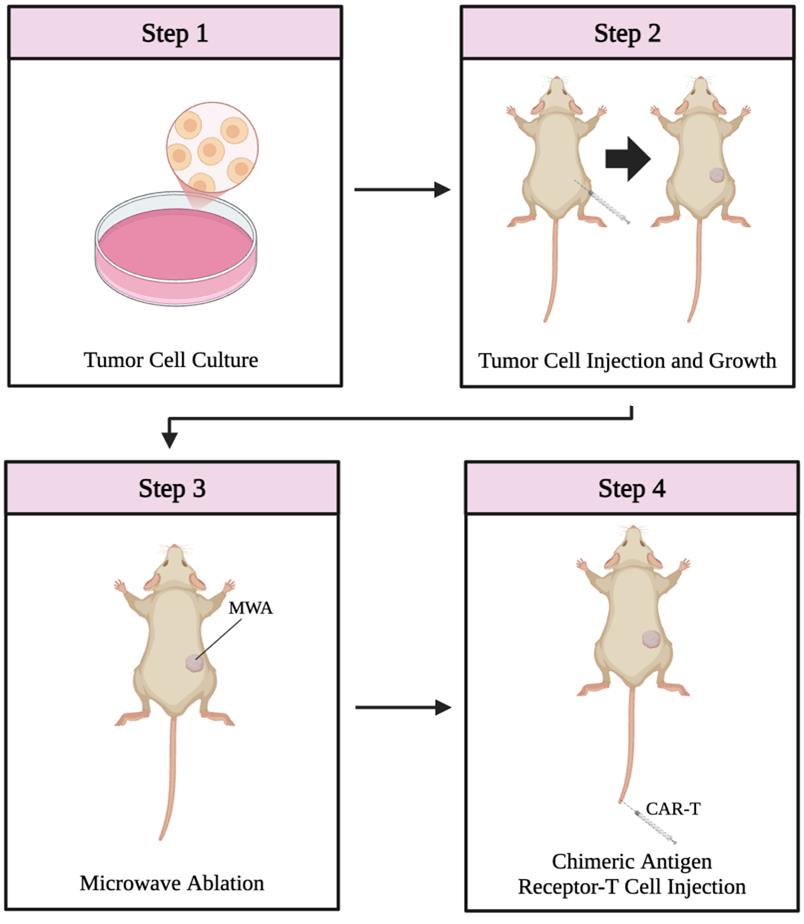
Overview of the combination therapy protocol. The protocol consists of four main steps: tumor cell culture (step 1, day 0), subcutaneous tumor establishment in mice (step 2, day 8), microwave ablation (step 3, days 15–24), and CAR-T-cell administration (step 4).
Background
Microwave ablation (MWA) is a thermal ablation technique for the treatment of tumors. In this procedure, a needle delivers microwave radiation to a tumor site, causing localized heating [1,2]. This process induces necrosis of surrounding tissues, resulting in cell damage at the membrane and subcellular levels and leading to decreased tumor burden [1]. Studies using animal models have demonstrated that MWA holds promise for treating various solid tumors, including liver, lung, bone, and renal masses [3]. In addition to its effectiveness, MWA is considerably less invasive than surgical approaches, typically requiring only a needle puncture rather than a full incision in most patients [4]. This minimally invasive nature results in shorter hospital stays, faster recovery times, and lower infection-related mortality rates [5]. As a result, MWA is increasingly being adopted as a viable clinical treatment for local tumor control [6,7].
Beyond its local cytotoxic effect, MWA has been shown to elicit an immune-mediated systemic response known as the abscopal effect. This phenomenon describes the regression of metastatic tumors at sites distant from the ablation, driven by immune activation resulting from post-MWA release of cytokines, chemokines, and neoantigens [8–13]. However, the abscopal effect alone is generally insufficient to eliminate secondary tumor sites completely [1,14]. Additionally, the high rate of tumor recurrence following MWA suggests that it may not be adequate as a standalone therapy for metastatic disease. Recent studies have shown that the immunostimulatory effects of MWA can complement immunotherapies such as chimeric antigen receptor (CAR)-T-cell therapy [15,16]. In particular, xenograft models of non-small cell lung cancer have demonstrated enhanced tumor regression when MWA is combined with CAR-T treatment [15,16]. This combination strategy has the potential to address both local and distant tumor sites more effectively than either modality alone.
Animal models, particularly mouse models, provide a valuable platform for investigating the synergistic potential of MWA and CAR-T-cell therapy before translation into clinical settings. However, protocols detailing the implementation of this combined approach remain limited. In this study, we present a methodology encompassing tumor cell culture, tumor inoculation, MWA administration, CAR-T cell infusion, and post-treatment monitoring in murine models, with the goal of establishing a standardized protocol for this promising therapeutic strategy.
Materials and reagents
Biological materials
1. Hep3B cells (ATCC, catalog number: HB-8064)
2. SK-HEP-1 cells (ATCC, catalog number: HTB-52)
3. CAR-T cells (generated in-house [16])
4. NOG (NOD/Shi-scid IL2rγnull) mice (Vital River, catalog number: 408)
Reagents
1. Trypsin (Corning, catalog number: MT25053CI)
2. Trypan blue (Corning, catalog number: MT25900CI)
3. Phosphate-buffered saline (PBS) (Invitrogen, catalog number: AM9624)
4. 0.25% trypsin-EDTA (Gibco, catalog number: 25200072)
5. Phenobarbital anesthesia (Covetrus, catalog number: 42494041503)
6. DMEM (Gibco, catalog number: 11885084)
7. RPMI-1640 (Gibco, catalog number: 61870036)
8. Fetal bovine serum (FBS) (Gibco, catalog number: A5670701)
9. Human recombinant IL-2 (PeproTech, catalog number: 200-02-50UG)
10. Penicillin-streptomycin (Gibco, catalog number:15140122)
11. Povidone iodine (Sigma-Aldrich, catalog number: 25655-41-8)
12. Ethanol (HYNAUT, catalog number: ZD119)
13. Carprofen (Yeasen, catalog number: 53634ES70)
14. Ophthalmic ointment (Fisher, catalog number: 50-218-8442)
15. T-Cell Isolation kit (StemCell Technologies, catalog number: 17951)
16. ImmunoCultTM human CD3/CD28 T cell activator (StemCell Technologies, catalog number: 17951)
Solutions
1. Tumor cell culture medium (see Recipes)
2. CAR-T-cell culture medium (see Recipes)
Recipes
1. Tumor cell culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM | n/a | 445 mL |
| FBS | 10% | 50 mL |
| Penicillin-streptomycin | 1% | 5 mL |
| Total | n/a | 500 mL |
Culture tumor cell lines, including Hep3B and SK-HEP-1, in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin. Store prepared medium at 4 °C.
2. CAR-T-cell culture medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| RPMI-1640 | n/a | 445 mL |
| FBS | 10% | 50 mL |
| Recombinant IL-2 | 10 ng/mL | n/a |
| Penicillin-streptomycin | 1% | 5 mL |
| Total | n/a | 500 mL |
Culture CAR-T cells in RPMI-1640 supplemented with FBS, IL-2, and penicillin-streptomycin. Store prepared medium at 4 °C.
Laboratory supplies
1. Cell culture dish (Greiner Bio-One, catalog number: 628160)
2. Injection syringe with needle (23–25 G) (KDL, catalog number: 60017031)
3. Scalpel (Fisherbrand, catalog number: 08-925)
4. Tissue glue (Vetbond, catalog number: NC0735004)
5. Caliper (Fisherbrand, catalog number: 06-664-16)
6. Tissue adhesive (3M Vetbond, catalog number: 6606-65-1)
7. Hemocytometer (Bright-LineTM, catalog number: Z359629)
8. 15 mL conical tubes (Corning, catalog number: CLS430052)
9. Cryogenic vials (Corning, catalog number: CLS430659-500EA)
10. Cotton swabs (McKesson, catalog number: 13262-2S)
11. Heating pad (Braintree Scientific, catalog number: 50-195-4000)
12. Forceps (Fisherbrand, catalog number: 08-875)
13. Scissors (Fisherbrand, catalog number: 08-940)
14. Insulin syringe (BD, catalog number: 14-829-1D)
Note: All surgical instruments should be autoclaved prior to use.
Equipment
1. Centrifuge (Eppendorf, catalog number: 05-413-113)
2. Microwave ablation device (Vision-China Medical Devices R&D center, catalog number: MTC-3C)
3. Water bath (37 °C) (Thermo Scientific, catalog number: 1184L86)
4. Liquid nitrogen tank (Thermo Scientific, catalog number: 31588)
5. Refrigerator (2–8 °C) (Haier, catalog number: HLR-310F)
6. Freezer (-20 °C) (Media, catalog number: MDRS712FIE61W)
7. CO2 incubator (37 °C) (Thermo Scientific, catalog number: 51033555)
8. Biological safety cabinet (Thermo Scientific, catalog number: 1337)
9. Inverted microscope (Olympus, catalog number: IX85P1ZF)
Procedure
A. Tumor cell culture
1. Retrieve frozen tumor cells from the liquid nitrogen tank and thaw the cryovial in a 37 °C water bath until just thawed (~1–2 min).
Note: Avoid over-thawing, as prolonged exposure to residual DMSO and heat may compromise cell viability.
2. Add 5 mL of prewarmed tumor cell culture medium (see Recipe 1) to a 15 mL conical centrifuge tube.
3. Transfer the thawed contents of the cryovial into the centrifuge tube.
4. Centrifuge at 400× g for 5 min at room temperature.
5. Carefully aspirate and discard the supernatant without disturbing the cell pellet.
6. Resuspend the cell pellet in 1 mL of fresh, prewarmed tumor cell culture medium by gentle pipetting to achieve a uniform single-cell suspension.
7. Transfer the cell suspension (2 × 105 cells) to a 100 mm tissue culture dish containing 10 mL of tumor cell culture medium.
8. Gently rock the dish back and forth and side to side to evenly distribute the cells across the surface.
9. Incubate the dish at 37 °C in a humidified incubator with 5% CO2.
10. Monitor cell confluency daily under a microscope. When the culture reaches approximately 85%–90% confluency, proceed to cell passaging.
11. Aspirate the culture medium and wash the cells once with 5 mL of sterile 1× PBS to remove residual medium.
12. Add 2 mL of 0.25% trypsin-EDTA solution to the dish and gently rock to ensure even coverage.
13. Incubate at 37 °C for 2–5 min to allow cells to detach.
Note: Avoid over-trypsinizing, as prolonged exposure may compromise cell viability and health.
14. Add 5 mL of tumor cell growth medium to neutralize the trypsin and pipette gently to dislodge any remaining adherent cells.
15. Transfer the cell suspension to a 15 mL centrifuge tube and centrifuge at 400× g for 5 min.
16. Discard the supernatant and resuspend the cell pellet in 1 mL of fresh culture medium.
17. Plate the cells (2 × 105) in a new 100 mm culture dish containing 10 mL of fresh medium.
18. Incubate the cells at 37 °C with 5% CO2 until the next passage or experimental use.
Note: It is generally recommended that cells be used within a low-to-moderate passage range (typically ≤20 passages) to preserve genetic stability, tumorigenicity, and experimental reproducibility in vivo.
B. Tumor cell inoculation
1. Harvest tumor cells by trypsinization and prepare a single-cell suspension in culture medium.
Note: Avoid over-trypsinizing, as prolonged exposure may compromise cell viability and health.
2. Mix 10 μL of the cell suspension with 10 μL of 0.4% trypan blue solution and determine cell viability using a hemocytometer.
3. Prepare a suspension containing 2 × 106 viable tumor cells in 100 μL of sterile 1× PBS.
4. Obtain 6–8-week-old (15–20 g) female NOG mice for tumor cell inoculation in either the right or left flank on day 0.
Critical: Prior to injection, disinfect the injection site on the mouse flank by swabbing sequentially with povidone iodine and 70% ethanol using sterile cotton swabs.
5. Using a 23–25 G needle, inject 100 μL of the tumor cell suspension subcutaneously into the right flank. For bilateral tumor models (e.g., to study the abscopal effect), inject 100 μL into both flanks (designated as local and distant tumors).
6. Slowly withdraw the needle to minimize backflow and ensure proper cell delivery.
7. Monitor tumor growth daily and allow tumors to establish over 7–16 days or until reaching a volume of approximately 200 mm3, as measured by digital calipers.
Note: Please refer to Figure 3a–c in our previous publication [15] for representative data on tumor growth progression over days.
C. Microwave ablation
1. Pre-ablation preparation
a. Prepare the surgical area by placing the mouse on a heating pad to maintain body temperature during the procedure.
b. Administer a subcutaneous injection of 100 μL (5 mg/kg) Carprofen (1 mg/mL, store at 4 °C) for a 20 g mouse as analgesia.
c. Induce anesthesia with an intraperitoneal injection of 100 μL of phenobarbital (10 mg/mL; 50 mg/kg for a 20 g mouse, stored at 4 °C) (Figure 1).
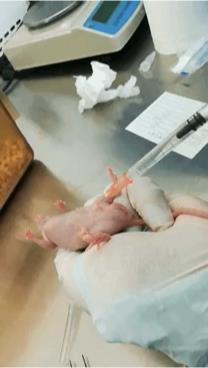
Figure 1. Anesthesia administration. Delivery of phenobarbital anesthesia via syringe prior to microwave ablation.
Critical: Confirm the depth of anesthesia by checking toe pinch reflexes.
d. Apply ophthalmic ointment to both eyes to prevent corneal drying during the procedure.
2. Tumor exposure and ablation
a. Activate and prime the cooling system of the MWA device per the manufacturer's instructions.
b. Sterilize the tumor area using povidone iodine followed by 70% ethanol with sterile cotton swabs.
c. Make a small (~1 mm) incision in the skin over the tumor on the right flank using forceps and scissors to expose the tumor mass (Figure 2).
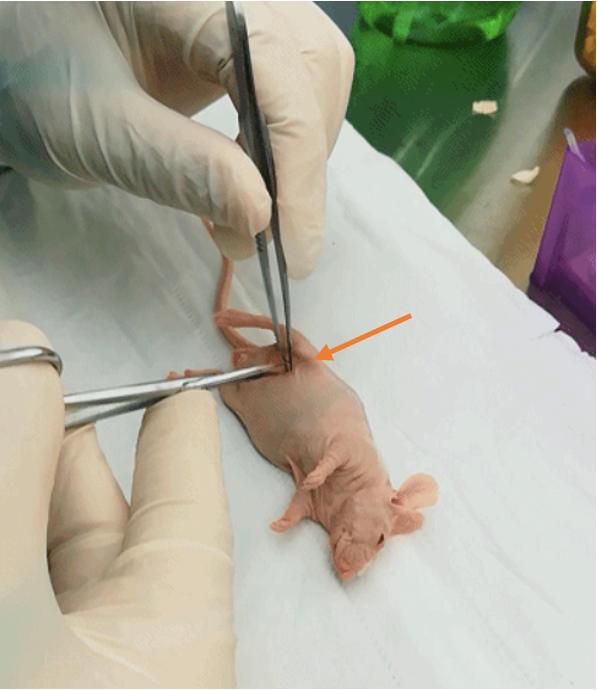
Figure 2. Skin incision. Use of forceps and scissors to make a small incision in the skin, exposing the tumor site for microwave ablation.
d. Set the MWA system to 10 W on the control panel.
e. Select the ablation duration based on the experimental design.
Note: Partial ablation: 35–40 s; complete ablation of a ~200 mm3 tumor: 120 s (Figure 3).

Figure 3. Microwave ablation (MWA) system. MWA control unit and needle configuration. Power is set to 10 W, with ablation duration adjusted according to the experimental group.
f. Insert the MWA needle directly into the exposed tumor, positioning the needle tip at the center of the tumor, and initiate the ablation procedure (Figure 4).
Note: The needle is inserted from the top of the tumor and then advanced to the bottom to ensure proper placement within the tumor mass.
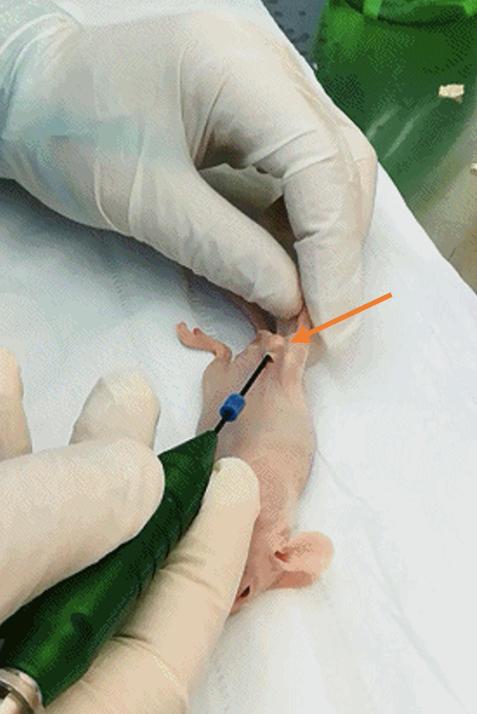
Figure 4. Microwave ablation (MWA) administration. Insertion of the MWA needle into the tumor site for localized thermal ablation.
3. Post-ablation care
a. Upon completion of ablation, carefully remove and properly dispose of the ablation needle.
b. Sterilize the incision site with povidone iodine.
c. Close the incision using tissue adhesive and gently press the skin edges together until sealed (Figure 5).
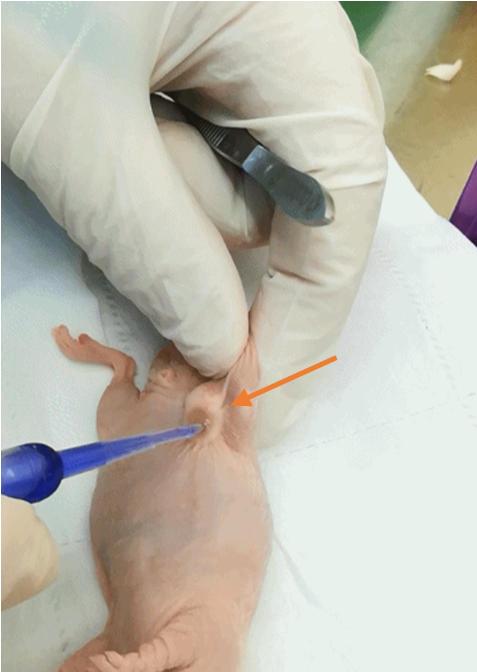
Figure 5. Closure of the microwave ablation (MWA) site. Demonstration of wound closure at the ablation site using tissue adhesive.
d. Monitor mice until full recovery from anesthesia on a heating pad.
D. CAR-T-cell preparation and injection
1. Culture CAR-T cells in CAR-T-cell culture medium (see Recipe 2) in a CO2 incubator until the next passage or experimental use.
Note: The generation and characterization of CAR-T cells have been described in detail in our previous work [16]. Briefly, T cells were positively selected from peripheral blood mononuclear cells (PBMCs) of healthy donors using the T-Cell Isolation kit and activated with human CD3/CD28 T-cell activator for 24–72 h. T cells were then transduced with CAR lentivirus. Informed consent was obtained from all healthy PBMC donors.
2. Prepare a suspension of 2.5–5 × 106 CAR-T cells in 100 μL of sterile 1× PBS.
Note: Fresh T cells are preferred, as cryopreservation can reduce cell viability. Adjust the cell dose according to the experimental design.
3. Keep the prepared CAR-T-cell suspension on ice until the time of injection.
4. Following MWA, intravenously inject 100 μL of the 5 × 106 CAR-T cell suspension (viability confirmed by trypan blue staining) into the tail vein of each mouse using a sterile insulin syringe.
Critical: Perform the injection gently and steadily to avoid tail vein rupture or cell loss. For detailed guidance on tail vein injection, readers may refer to previously published protocols [17].
Note: Tips to increase the success rate of tail vein injections: (1) Use an insulin syringe, which has a smaller diameter than a standard 1 mL syringe. (2) Warm the tail beforehand to dilate the vein. (3) Ensure proper restraint and steady positioning of the mouse. (4) If available, use a tail vein injection apparatus (Biofargo, https://biofargo.com/products/tail-vein-injection-apparatus?variant=44894937940149), which enhances visualization of the tail vein.
E. Post-operative monitoring
1. Following MWA and CAR-T cell treatment, monitor mice daily for at least 24 days to assess treatment response and overall health status.
2. Evaluate clinical signs by observing body temperature, physical activity, posture, grooming, and feeding behavior.
3. Measure tumor dimensions using digital calipers every 2–3 days.
Note: Calculate tumor volume using the following formula: Tumor volume (mm3) = 0.5 × length × (width)2.
4. Record tumor volumes throughout the observation period to monitor progression or regression in response to treatment.
5. To analyze the tumor microenvironment after MWA, euthanize 3–5 additional mice following MWA and CAR-T-cell injection. Residual tumors should be harvested and assessed for CAR-T-cell infiltration by flow cytometry or histological staining.
6. Track survival outcomes across experimental groups and plot Kaplan–Meier survival curves as needed.
Note: In mice bearing bilateral tumors (local and distant sites), measure and document the growth of distant (non-ablated) tumors to assess potential abscopal effects of the combination therapy. Humane endpoints include tumor volume exceeding 2000 mm3, body weight loss greater than 20%, or impaired mobility, at which point mice were euthanized.
Data analysis
Data are presented as mean ± standard deviation. Group differences were assessed using Student’s t-test or one/two-way ANOVA with Tukey’s multiple comparisons test. Survival data were analyzed with Kaplan–Meier curves and compared using the log-rank test. Statistical analyses were performed with GraphPad Prism, and p < 0.05 was considered statistically significant.
Validation of protocol
This protocol has been used and validated in the following research article: Cao et al. [16]. CV1-secreting sCAR-T cells potentiate the abscopal effect of microwave ablation in heterogeneous tumors. Cell Rep Med. (Figures 2, 4, 6, and 7)
General notes and troubleshooting
General notes
1. This study establishes a practical and reproducible methodology for investigating combination therapy using MWA and CAR-T cells in mouse models. The outlined approach integrates key preparatory steps, including tumor cell culture, with precise protocols for tumor cell inoculation, MWA, and CAR-T-cell administration. Collectively, these methods allow for controlled exploration of how local thermal ablation may synergize with adoptive cell therapy to improve tumor control.
2. A major technical advantage of MWA lies in its tunable energy delivery, enabling researchers to adjust the extent of tumor destruction based on power and ablation time [18]. Our procedure allows for both partial and complete ablation of subcutaneous tumors, with time settings of 35–40 s and 120 s, respectively, being effective for ~200 mm3 tumors. While partial ablation models are useful for investigating tumor recurrence and immune-mediated control, they also reflect clinical scenarios where full ablation is not feasible due to tumor location or proximity to critical structures. Notably, partial ablation has been associated with higher recurrence rates and ethical concerns in human therapy [19]. These findings reinforce the need to identify optimal ablation parameters that balance tumor cytoreduction with preservation of immunogenic tumor debris for CAR-T engagement.
3. The ability of MWA to stimulate systemic immune responses, including the abscopal effect, offers a rationale for its combination with CAR-T cells. This model enables investigation of how different ablation durations and CAR-T-cell doses influence both local tumor regression and potential systemic anti-tumor immunity. Following treatment, tumor progression is monitored using standardized caliper measurements and volume calculations, allowing for the generation of tumor growth curves over time. These data provide a quantitative foundation for assessing therapeutic synergy.
4. Importantly, this combination therapy has shown promising anti-tumor effects in previous studies without observable systemic toxicity in mouse models [15]. The minimally invasive nature of MWA, delivered via a needle rather than surgical incision, also presents clinical advantages such as reduced recovery time, lower infection risk, and increased patient comfort [3]. When coupled with the tumor-targeting specificity of CAR-T cells, this approach has the potential to effectively suppress solid tumors while minimizing off-target effects [16].
5. The protocol presented here offers a foundational platform for evaluating the mechanistic and therapeutic potential of combining MWA with CAR-T-cell therapy. It provides a path forward for refining treatment parameters, understanding immune interactions, and ultimately translating this strategy into a novel clinical option for solid tumor treatment.
Troubleshooting
Problem 1: Variable tumor regression outcomes following MWA and CAR-T therapy.
Possible cause: Intrinsic differences in tumor cell lines and tumor types can affect their sensitivity to ablation and immune-mediated killing.
Solution: Perform tumor-specific optimization of experimental parameters, including ablation energy, CAR-T construct design, dosing, and treatment schedule to ensure reproducible outcomes.
Problem 2: Immunological variability compromises CAR-T-cell function.
Possible cause: Differences in host immune status, immune cell infiltration, or tumor immunogenicity may limit CAR-T expansion or activity.
Solution: Use well-characterized, immunocompetent, or humanized mouse models to better reflect immune-tumor interactions and evaluate CAR-T-cell persistence, trafficking, and function under controlled immune contexts.
Acknowledgments
All authors contributed to the writing and preparation of the manuscript. The graphical abstract was created using BioRender.com. This protocol is adapted from the original work by Cao et al. [16], and funding support for Bihui Cao and Qi Zhao is detailed in reference [16].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
All protocols involving human subjects were approved by the Institutional Review Board of the Second Affiliated Hospital of Guangzhou Medical University. Written informed consent was obtained from all participants, including healthy PBMC donors and tumor patients. All animal experiments were performed in accordance with the relevant guidelines and regulations and were approved by the Experimental Animal Care Commission of Guangzhou Medical University.
References
- Chu, K. F. and Dupuy, D. E. (2014). Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 14(3): 199–208. https://doi.org/10.1038/nrc3672
- Izzo, F., Granata, V., Grassi, R., Fusco, R., Palaia, R., Delrio, P., Carrafiello, G., Azoulay, D., Petrillo, A., Curley, S. A., et al. (2019). Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 24(10): e990–e1005. https://doi.org/10.1634/theoncologist.2018-0337
- Gala, K. B., Shetty, N. S., Patel, P. and Kulkarni, S. S. (2020). Microwave ablation: How we do it? Indian J Radiol Imaging. 30(2): 206–213. https://doi.org/10.4103/ijri.IJRI_240_19
- Wang, Z., Liu, M., Zhang, D., Wu, S., Hong, Z., He, G., Yang, H., Xiang, B., Li, X., Jiang, T., et al. (2022). Microwave ablation versus laparoscopic resection as first‐line therapy for solitary 3–5‐cm HCC. Hepatology. 76(1): 66–77. https://doi.org/10.1002/hep.32323
- Wicks, J. S., Dale, B. S., Ruffolo, L., Pack, L. J., Dunne, R., Laryea, M. A., Hernandez-Alejandro, R. and Sharma, A. K. (2023). Comparable and Complimentary Modalities for Treatment of Small-Sized HCC: Surgical Resection, Radiofrequency Ablation, and Microwave Ablation. J Clin Med. 12(15): 5006. https://doi.org/10.3390/jcm12155006
- Ni, Y., Peng, J., Yang, X., Wei, Z., Zhai, B., Chi, J., Li, X. and Ye, X. (2021). Multicentre study of microwave ablation for pulmonary oligorecurrence after radical resection of non-small-cell lung cancer. Br J Cancer. 125(5): 672–678. https://doi.org/10.1038/s41416-021-01404-y
- Qi, E., Zhang, S., Li, X., Cheng, Z., Han, Z., Yu, J., Liang, P. and Yu, X. (2022). Comparison of percutaneous microwave ablation and surgical resection for hepatocellular carcinoma in the caudate lobe. J Cancer Res Ther. 18(2): 378–383. https://doi.org/10.4103/jcrt.jcrt_1067_21
- Liao, C., Zhang, G., Huang, R., Zeng, L., Chen, B., Dai, H., Tang, K., Lin, R. and Huang, Y. (2023). Inducing the Abscopal Effect in Liver Cancer Treatment: The Impact of Microwave Ablation Power Levels and PD-1 Antibody Therapy. Pharmaceuticals. 16(12): 1672. https://doi.org/10.3390/ph16121672
- Yu, L., Xie, H., Wang, L., Cheng, M., Liu, J., Xu, J., Wei, Z., Ye, X., Xie, Q., Liang, J., et al. (2023). Microwave ablation induces abscopal effect via enhanced systemic antitumor immunity in colorectal cancer. Front Oncol. 13: e1174713. https://doi.org/10.3389/fonc.2023.1174713
- Wakefield, S., Srimathveeravalli, G., Yates, J., McIntosh, L. and Mittal, K. (2023). Abscopal Regression of a Lung Metastasis Induced by Microwave Ablation of Locally Recurrent Renal Cell Carcinoma. J Vasc Interv Radiol. 34(3): 495–496. https://doi.org/10.1016/j.jvir.2022.11.008
- Ma, F., Lin, Y., Ni, Z., Wang, S., Zhang, M., Wang, X., Zhang, Z., Luo, X. and Miao, X. (2024). Microwave ablation enhances the systemic immune response in patients with lung cancer. Oncol Lett. 27(3): e14239. https://doi.org/10.3892/ol.2024.14239
- Ma, F., Miao, X., Lin, Y., Luo, X., Chen, T., Ni, Z. and Wang, X. (2025). Microwave ablation combined with α-PD-L1 enhances abscopal effect and promotes CTL activation and intratumoral homing by a cytokine network involving IFN-γ and CXCL9. Int Immunopharmacol. 153: 114498. https://doi.org/10.1016/j.intimp.2025.114498
- Yu, M., Pan, H., Che, N., Li, L., Wang, C., Wang, Y., Ma, G., Qian, M., Liu, J., Zheng, M., et al. (2020). Microwave ablation of primary breast cancer inhibits metastatic progression in model mice via activation of natural killer cells. Cell Mol Immunol. 18(9): 2153–2164. https://doi.org/10.1038/s41423-020-0449-0
- Shao, C., Yang, M., Pan, Y., Xie, D., Chen, B., Ren, S. and Zhou, C. (2021). Case Report: Abscopal Effect of Microwave Ablation in a Patient With Advanced Squamous NSCLC and Resistance to Immunotherapy. Front Immunol. 12: e696749. https://doi.org/10.3389/fimmu.2021.696749
- Cao, B., Liu, M., Wang, L., Zhu, K., Cai, M., Chen, X., Feng, Y., Yang, S., Fu, S., Zhi, C., et al. (2022). Remodelling of tumour microenvironment by microwave ablation potentiates immunotherapy of AXL-specific CAR T cells against non-small cell lung cancer. Nat Commun. 13(1): e1038/s41467–022–33968–5. https://doi.org/10.1038/s41467-022-33968-5
- Cao, B., Liu, M., Xiao, Z., Leng, D., Zhou, Y., Zhang, Z., Wang, L., Huang, X., Ni, Q., Cheng, W., et al. (2025). CV1-secreting sCAR-T cells potentiate the abscopal effect of microwave ablation in heterogeneous tumors. Cell Rep Med. 6(2): 101965. https://doi.org/10.1016/j.xcrm.2025.101965
- Yano, J., Lilly, E. A., Noverr, M. C. and Fidel, P. L. (2020). A Contemporary Warming/Restraining Device for Efficient Tail Vein Injections in a Murine Fungal Sepsis Model. J Visualized Exp.: e3791/61961. https://doi.org/10.3791/61961
- Simon, C. J., Dupuy, D. E. and Mayo-Smith, W. W. (2005). Microwave Ablation: Principles and Applications. RadioGraphics. 25: S69–S83. https://doi.org/10.1148/rg.25si055501
- Shi, L., Wang, J., Ding, N., Zhang, Y., Zhu, Y., Dong, S., Wang, X., Peng, C., Zhou, C., Zhou, L., et al. (2019). Inflammation induced by incomplete radiofrequency ablation accelerates tumor progression and hinders PD-1 immunotherapy. Nat Commun. 10(1): e1038/s41467–019–13204–3. https://doi.org/10.1038/s41467-019-13204-3
Article Information
Publication history
Received: Jul 29, 2025
Accepted: Sep 9, 2025
Available online: Sep 17, 2025
Published: Oct 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Cao, B., Wheeler, G. L., Mast, J., Zhao, Q. and Shen, J. (2025). Combining Microwave Ablation With CAR-T-Cell Therapy in Tumor-Bearing Mouse Models. Bio-protocol 15(20): e5470. DOI: 10.21769/BioProtoc.5470.
Category
Cancer Biology > General technique > Cancer therapy
Cancer Biology > Tumor immunology > Cancer therapy
Immunology > Immunotherapy > CAR-T
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link