- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
(*contributed equally to this work, § Technical contact) Published: Vol 15, Iss 19, Oct 5, 2025 DOI: 10.21769/BioProtoc.5467 Views: 1703
Reviewed by: Sérgio RibeiroAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples
Namrata Ghag [...] Nashwa Cheema
Jan 5, 2025 1917 Views

An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
Jeremy R. Pearson [...] Eduardo Rosa-Molinar
Apr 20, 2025 2500 Views

Improved Immunohistochemistry of Mouse Eye Sections Using Davidson's Fixative and Melanin Bleaching
Anne Nathalie Longakit [...] Catherine D. Van Raamsdonk
Nov 20, 2025 1565 Views
Abstract
Formalin-fixed paraffin-embedded (FFPE) slides are essential for histological and immunohistochemical analyses of organoids. Conventional preparation of FFPE slides from organoids embedded in basement membrane extract (BME) presents several challenges. During the fixation step, dehydration often causes collapse of the BME, which normally supports the three-dimensional architecture of organoids. As a result, organoids may lose their original morphology, particularly in the case of cystic or structurally delicate types, leading to distortion and reduced reliability in downstream histological evaluation. Here, we introduce a straightforward protocol that improves the reliability of FFPE slide preparation for BME-based organoids by enhancing sample integrity and sectioning quality. By using 2% agarose as a mold during the embedding process, organoids grown in BME were effectively stabilized, enabling reliable preservation of their morphology throughout FFPE slide preparation. This method effectively addresses the difficulties in processing structurally delicate organoids and allows robust preparation of diverse cancer organoid morphologies—such as cystic, dense, and grape-like structures—while maintaining their native three-dimensional architecture. Our approach simplified the technical process while ensuring reliable histopathological analysis, making it a valuable tool for cancer research and personalized medicine.
Key features
• FFPE preparation applicable to diverse cancer organoid morphologies, including cystic, dense, and grape-like, while preserving three-dimensional architecture.
• Agarose molding allows intact retrieval and fixation of BME domes, preventing collapse and maintaining organoid 3D architecture during FFPE preparation.
• Compatible with diagnostic IHC/IF markers (pan-CK, CK19, p63, Ki-67, p53) across cancer organoids.
Keywords: FFPEGraphical overview
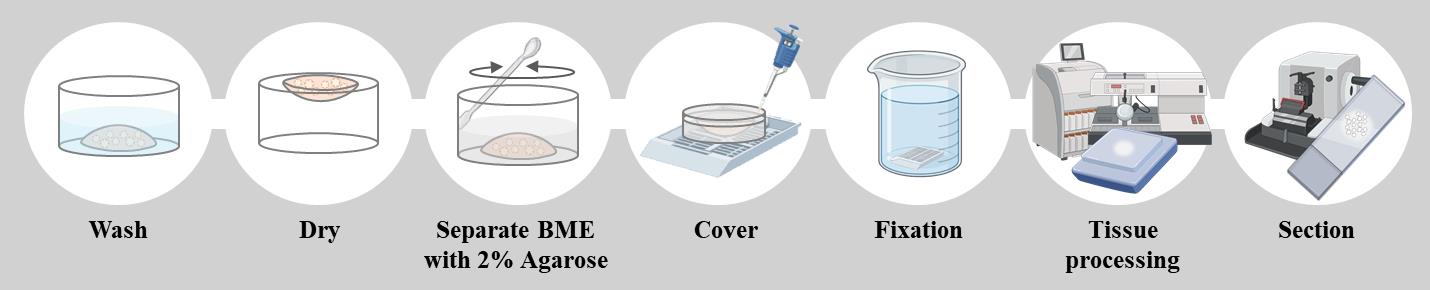
Schematic overview of the formalin-fixed paraffin-embedded (FFPE) organoid preparation protocol. Organoids cultured in BME are washed and partially dried before being embedded in 2% agarose. The agarose block is then fixed in formalin, processed for paraffin embedding, and sectioned for histopathological analysis. This method preserves organoid morphology and enables reliable staining for downstream applications such as H&E and immunostaining.
Background
Patient-derived organoids (PDOs) are regarded as reliable preclinical models for cancer research and personalized medicine [1]. They retain not only the structural complexity of the original tumor but also their biological behavior, including their responses to various therapeutic agents [2]. By faithfully recapitulating tumor-specific characteristics, PDOs offer a robust platform for testing novel therapeutics and developing individualized treatment strategies [3].
Importantly, PDOs display marked heterogeneity, and their morphology often reflects the underlying genetic and transcriptional features of the parental tumor [4–9]. For example, accumulation of mutations is frequently associated with dense or invasive organoids that can form grape-like structures, whereas organoids with lower mutational burden may maintain cystic or balloon-like morphologies resembling normal-like epithelial architecture. TP53 mutations, commonly present in head and neck, colorectal, and breast cancers, are associated with disorganized, compact structures lacking clear glandular architecture [10–12]. KRAS mutations, prevalent in pancreatic and colorectal cancers, often result in dense, multilayered organoids without lumen formation [13,14]. PIK3CA mutations lead to irregular, enlarged organoids in breast cancer [12], and CDH1 loss produces loosely cohesive or scattered organoids devoid of epithelial polarity in gastric cancer [4]. These genotype–phenotype relationships highlight the importance of preserving morphological integrity during sample preparation, as morphology serves as both a phenotypic marker and a functional readout of tumor biology.
Under laminin-rich basement membrane extract (BME), PDOs form distinct three-dimensional structures through enhanced self-renewal and self-organization, reinforcing their role in translational cancer research [15]. BME plays a critical role in maintaining polarity, lumen formation, and cystic morphology by providing a laminin- and collagen-rich extracellular scaffold [16]. However, during conventional FFPE processing, fixation often disrupts the BME, causing collapse of this structural support and leading to distortion or loss of normal-like organoid morphologies [17]. While dense organoids may partly preserve their structure through strong cell–cell adhesion, cystic or balloon-like morphologies are especially vulnerable once the BME is compromised [18].
This protocol was specifically designed to overcome the collapse of BME and distortion of fragile organoid morphologies that commonly occur during the dehydration steps of conventional FFPE processing. By functioning as a stabilizing matrix, the agarose mold immobilizes organoids within the BME, thereby preserving their native three-dimensional architecture. As a result, organoids maintain their original morphology, enabling accurate histological evaluation across a broad spectrum of organoid types, including cystic, dense, and grape-like structures.
The major strength of this approach lies in its ability to reliably preserve both structural integrity and histological detail, which are essential for downstream applications such as H&E staining, immunohistochemistry, and immunofluorescence. Although the method involves steps that may appear harsh—such as drying and the addition of warm agarose—and FFPE processing can impose certain limitations, molecular analyses that are highly sensitive to fixation stress may be restricted. Nevertheless, this protocol effectively supports morphological assessment and protein-level studies. In our validation, we confirmed the expression of cancer diagnostic markers such as CK19, pan-CK, and P53 and further demonstrated that marker expression relevant to subtype classification could be evaluated using tissue proteomics–based analysis [9,19–21]. Overall, this protocol provides a practical and robust method for generating high-quality FFPE organoid slides, making it well-suited for cancer research, biomarker evaluation, and translational applications in personalized medicine.
Materials and reagents
Biological materials
1. Patient-derived organoids from the tumor tissue of patients with pancreatic cancer
2. Patient-derived organoids from the tumor tissue of patients with gall bladder cancer
3. Patient-derived organoids from the tumor tissue of patients with bile duct cancer
4. Patient-derived organoids from the tumor tissue of patients with oral cancer
Reagents
1. DPBS (Hyclone, catalog number: SH30028.02)
2. Agarose (GenDEPOT, catalog number: A0100-010)
3. Surgipath Paraplast (Paraffin) (Leica, catalog number: 39601006)
4. Sterilized non-ionized water
5. 10% neutral buffered formalin (GDCHEM, catalog number: 010-1406-1010)
6. Xylene (Daejung, catalog number: 8587-4400)
7. Ethanol (DAEJUNG, catalog number: 4119-4410)
Solutions
1. 2% agarose solution (see Recipes)
2. 70% ethanol (see Recipes)
Recipes
1. 2% agarose solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Agarose | 2% (w/v) | 0.4 g |
| Deionized sterile water (DW) | n/a | 20 mL |
| Total | n/a | 20 mL |
2. 70% ethanol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol | 70% (w/v) | 210 mL |
| Deionized sterile water (DW) | n/a | 90 mL |
| Total | n/a | 300 mL |
Laboratory supplies
1. Embedding cassettes: Histosette I (SIMPORT, catalog number: HIS-M490-3)
2. Spatula (DAIHAN medical, catalog number: SL.Spa7011)
3. 50 mL beaker (DURAN, catalog number: BK1010-DU)
4. 10 mL disposable tips (Falcon, catalog number: 357551)
5. 200P tip (Axygen, catalog number: T-200-Y)
6. 1000P tip (Axygen, catalog number: T-1000-B)
7. 2 mL microcentrifuge tubes (Axygen, catalog number: MCT-200-C)
8. Glass jar (Lklab Korea, catalog number: H06-660-151)
9. Slide box (LABRYDAY, catalog number: HIP-1021B)
Equipment
1. Tissue processor (Leica, HistoCore, model: PEARL)
2. Heated paraffin embedding station (Lecia, HistoCore, model: Arcadia H)
3. Microtome (Leica, HistoCore, model: BIOCUT R)
4. -20 °C freezer
5. 4 °C refrigerator
6. Microwave oven (various suppliers)
7. Cold plate (Leica, model: EG1150 C)
8. Microscope (Leica, model: DM500)
9. Tissue float bath (JISICO, model: J-NBT)
10. ThermoE cooling and heating block (Bioer Technology, ThermoE, model: CHB-A4-2420)
11. Pipette-aid (DRUMMOND, model: HDR-4-000-201)
12. 200P and 1000P pipettes (GILSON, model: MyPIPETMAN)
Procedure
A. Preparation of 2% agarose solution
1. Preheat the heat block to 60 °C.
2. Insert 2 mL tubes into the heat block.
3. Add 0.4 g of agarose to 20 mL of distilled water in a 50 mL beaker (Figure 1A).
Tip: The minimum volume to make a 2% agarose gel is 20 mL. If considering multiple FFPE organoids, prepare sufficient amounts by calculating 1 mL of 2% agarose gel for each BME drop containing a growth organoid.
4. Melt the agarose sufficiently in a microwave oven until powder particles are not visible (Figure 1B).
5. Using a pipet-aid with a 10 mL disposable tip, dispense 1.5 mL of 2% agarose into 2 mL tubes placed on a prewarmed heat block.
6. Allow the agarose temperature to cool to 60 °C for 1 h in a heat block (Figure 1C).
Note: This section describes the process of dissolving agarose powder into a 2% solution for use as a mold to retrieve organoids embedded in BME. The agarose solution should be prepared before starting section B. To prevent solidification, maintain the agarose solution at 60 °C in a heat block until the completion of step B7.
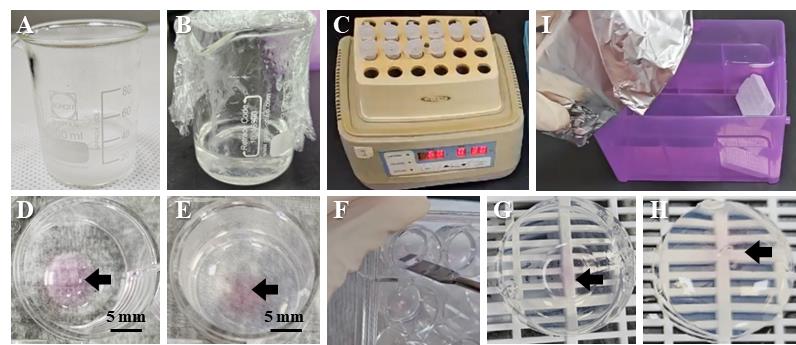
Figure 1. Harvest basement membrane extract (BME) with organoids using 2% agarose. (A) Agarose powder is prepared with water in a beaker to bring it completely into solution. (B) It is dissolved into a complete 2% agarose solution in the microwave. (C) The 2% agarose solution should be stored at 60 °C during the process. (D) BME (pink, black arrow) with embedded organoids after washing and drying. (E) The 2% agarose is solidified. (F) The 2% agarose is separated with BME using a spatula. (G) When the solidified 2% agarose is turned upside down, the bottom side of the BME is exposed to air. (H) Covering the bottom with 2% agarose solution completely envelops the BME. (I) The cassette is soaked to achieve fixation.
B. Embedding the organoid into the agarose mold
1. Wash the BME-containing organoids.
a. Remove the organoid culture media completely using a P1000 pipette.
b. Add 1 mL of 37 °C DPBS to each well.
c. Incubate for 1 min at room temperature (RT).
d. Repeat twice.
e. Remove as much DPBS as possible using a P1000 pipette (Figure 1D).
2. Dry the BME dome at RT.
a. Place the 24-well plate upside down at an angle while resting on the lid placed on a table, thus allowing air to pass through.
b. Dry at RT for 1 h.
3. Add 600 μL of 2% agarose to each well.
4. Solidify the agarose for 1 h at room temperature (Figure 1E).
Note: The agarose solution is initially clear but becomes visibly cloudy as gelation progresses, indicating successful solidification.
5. Separate the agarose by inserting a spatula into the gap between the plate walls along the edge (Figure 1F). The spatula must be gently moved in a circular motion along the edge of the well to evenly lift the agarose.
Note: Gently move the spatula in a circular motion along the edge of the well to lift the agarose evenly, thereby enabling efficient and smooth separation.
6. Place the agarose mold upside down on the tissue cassette (Figure 1G).
7. Cover the bottom of the agarose mold with 150 μL of 2% agarose (Figure 1H).
8. Solidify the agarose for 10 min at room temperature.
9. Close the lid of the cassette.
10. Soak the cassette in 10% formalin and fix overnight at 4 °C in a standard laboratory refrigerator (Figure 1I).
C. Tissue processing and embedding
1. After fixation, process the cassettes using the HistoCore PEARL tissue processor for 13 h, as detailed in Table 1.
2. Remove the cassettes from the tissue processor and transfer them into the molten paraffin of a heated paraffin-embedding station.
Table 1. Tissue processing program
| Step | Time | Temperature |
|---|---|---|
| 70% ethyl alcohol | 1 h | Room temperature |
| 80% ethyl alcohol | 1 h | Room temperature |
| 90% ethyl alcohol | 1 h | Room temperature |
| 95% ethyl alcohol | 1 h | Room temperature |
| 100% ethyl alcohol | 1 h | Room temperature |
| 100% ethyl alcohol | 1 h | Room temperature |
| 100% ethyl alcohol | 1 h | Room temperature |
| Xylene | 1 h | Room temperature |
| Xylene | 1 h | Room temperature |
| Xylene | 1 h | Room temperature |
| Paraffin | 1 h | 60 °C |
| Paraffin | 1 h | 60 °C |
| Paraffin | 1 h | 60 °C |
D. Making a slide
1. Place the FFPE organoid blocks in a -20 °C laboratory freezer for at least 1 h before sectioning to ensure thorough cooling. Keep each block on a cold plate until it is sectioned to maintain cooling and prevent softening.
2. Set the FFPE organoid on the microtome (Figure 2C).
Note: Blocks can be mounted either vertically or horizontally, depending on user preference.
3. Trim the FFPE organoid until the agarose layer appears (Figure 2A, B). The first layer is paraffin, which appears waxy (Figure 2B, D). When the block is further trimmed, the agarose layer is identified as a transparent area at the center (Figure 2B, E).
4. Trim the FFPE organoid until the BME layer appears.
Note: As the BME layer containing organoids may appear at any time after the agarose layer appears, check it after every 10 slices under a microscope (Figure 2B, F–H).
5. When organoids begin to appear, place the 4-μm slices in 70% ethanol and then place them in a tissue float bath warmed to 42 °C.
6. Lift the slice with the slide (Figure 2I).
7. Place the slides in a slide box and dry overnight at RT with the lid open.
8. Store the slides in a sealed slide box to prevent direct air exposure until they are used for staining.
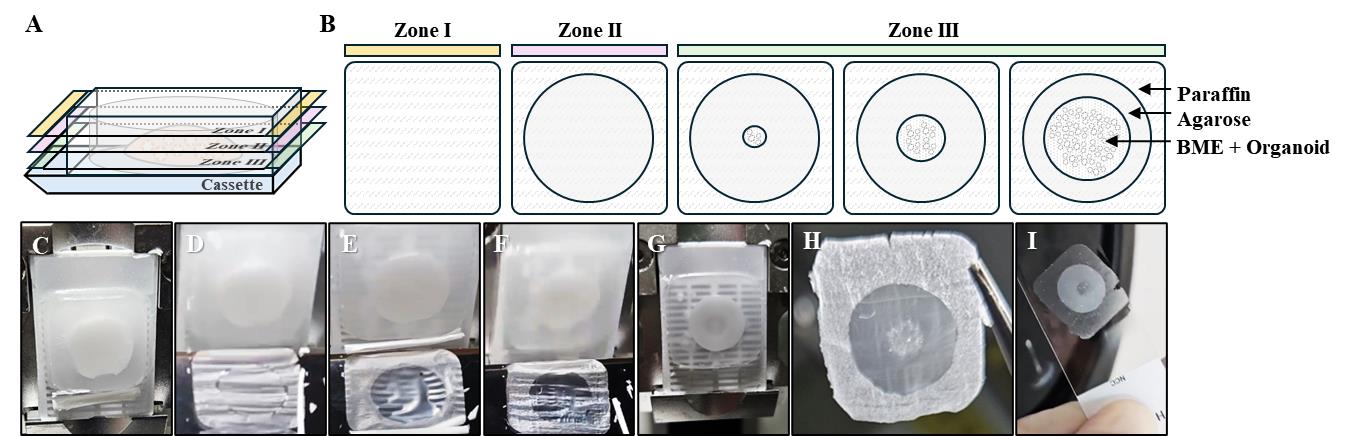
Figure 2. Preparation of a formalin-fixed paraffin-embedded (FFPE) organoid slide. (A, B) Sequential zones encountered during sectioning of FFPE organoid blocks. Zone I (yellow) corresponds to the paraffin-only layer. Zone II (pink) includes the paraffin plus agarose layer. Zone III (green) contains the organoid-embedded region. As sectioning progresses, organoids become visible within the slice. The paraffin layer is indicated by a diagonal pattern, the agarose layer is indicated by gray, and the BME layer is indicated by dots. (C) Set the FFPE organoid in the microtome. (D) Paraffin section of initially cut FFPE organoids. (E) Paraffin section with the entire agarose section exposed. (F) Paraffin section with organoids starting to appear. (G) Image of FFPE organoid indicating the exposed organoid layer. (H) Image of FFPE organoid section indicating the exposed organoid layer. (I) The cut section is left to float in water at 42 °C and placed on a slide.
Data analysis
This protocol addresses a common challenge encountered when generating paraffin blocks from organoids cultured in BME. When fixation is performed directly on organoids embedded in BME, the matrix undergoes dehydration during processing, leading to collapse or distortion of the organoid structures (Figure 3). While densely packed cancer organoids, maintained primarily by strong cell–cell adhesion, often retain their morphology, cystic or balloon-like organoids—representing normal-like or loosely cohesive tumor phenotypes—are particularly vulnerable. For these morphologies, structural integrity relies heavily on BME support; thus, dehydration of the matrix frequently results in the collapse of the organoid architecture.
As shown in Figure 4, when FFPE organoids were prepared using our improved method, organoids were distributed throughout the section while maintaining the original BME framework, and even normal-like organoids retained their cystic morphology without collapse. This demonstrates that the protocol effectively preserves delicate organoid structures and ensures reliable preparation of paraffin blocks.
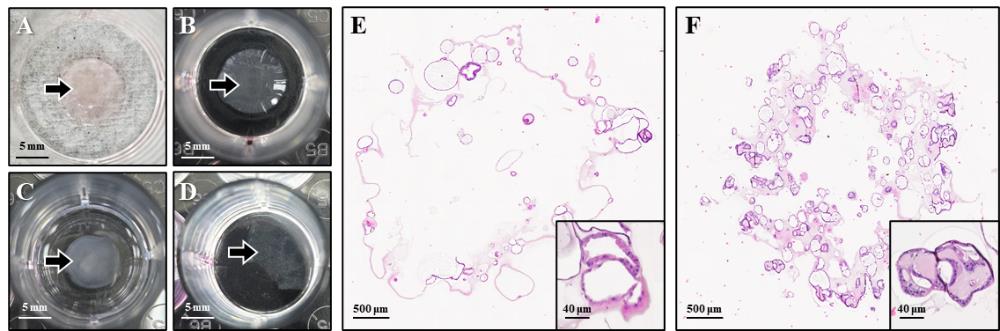
Figure 3. Limitations of the conventional formalin-fixed paraffin-embedded (FFPE) organoid preparation method. (A) Basement membrane extract (BME), indicated by black arrows, after the washing step. (B) BME collapse caused by dehydration following fixation. (C) Detached BME floating above the plate surface when fixation is followed by washing. (D) Complete loss of the 3D BME structure after washing. (E, F) Hematoxylin and eosin (H&E) staining of FFPE organoids prepared by the conventional method, showing loss of BME architecture and distortion of originally round, normal-like organoids that appear collapsed and misshapen. Stained slides were scanned using the Vectra PolarisTM Automated Quantitative Pathology Imaging System (Akoya Biosciences). The images were captured with the Phenochart software.
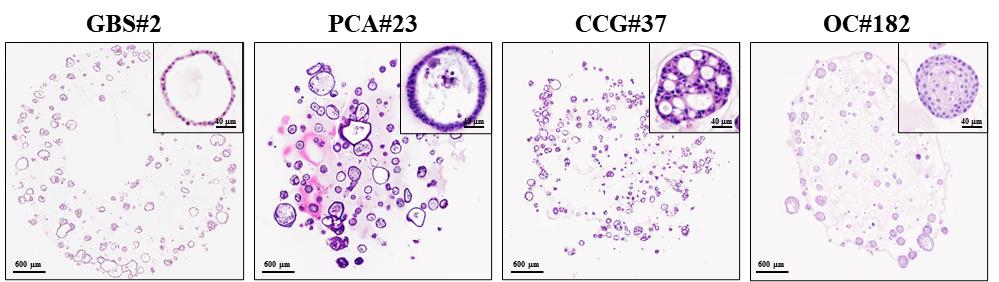
Figure 4. Improved preservation of organoid morphology using the optimized formalin-fixed paraffin-embedded (FFPE) organoid preparation protocol. Representative hematoxylin and eosin (H&E) images of organoids without morphological damage. Slides were scanned using the Vectra PolarisTM Automated Quantitative Pathology Imaging System (Akoya Biosciences). The images were captured with the Phenochart software. GBS, gallbladder cancer organoids derived from surgical tissue; PCA, pancreatic cancer organoids derived from ascites; CCG, cholangiocarcinoma organoids derived from percutaneous liver biopsy; OC, oral cancer organoids derived from surgical tissue.
Validation of protocol
This protocol was used to analyze the histological characteristics of more than 200 tumor organoids from nine cancers. It was verified that not only hematoxylin and eosin (H&E) but also immunohistochemistry (IHC) and immunofluorescence (IF) staining were possible without any change in organoid morphology due to the fixation solution. This protocol was validated in subsequent research articles focused on patient-derived organoids using pancreatic cancer, biliary tract cancer, and body fluids.
These paraffin-embedded organoid slides can subsequently be utilized for staining in the same way as conventional tissue slides, depending on the research purpose. Here, H&E and IHC were performed as representative examples, focusing on diagnostic markers such as CK19, Pan-CK, Ki-67, P63, and P53 (Figure 5).
By preserving the native 3D architecture formed within the BME dome, this protocol maintains the spatial organization of organoids, making it especially suitable for co-culture models where cell–cell interactions and tissue context are critical for biological interpretation (Figure 6).
• Cho et al. [19]. Refining the classification of cholangiocarcinoma subtypes through proteogenomic integration has revealed new therapeutic prospects. Gastroenterology (Figure 4G).
• Choi et al. [20]. Establishment of patient-derived organoids from cancer patients using ascitic or pleural fluid. Cancer Res Treat (Figure 1B, Supplementary Figures 4–6).
• Lee et al. [21]. Application of plasma circulating KRAS mutations as predictive biomarkers for targeted treatment of pancreatic cancer. Cancer Sci (Figure 3).
• Lee et al. [9]. Organoid morphology-guided classification of oral cancers reveals prognosis. Cell Rep Med (Figure 3)
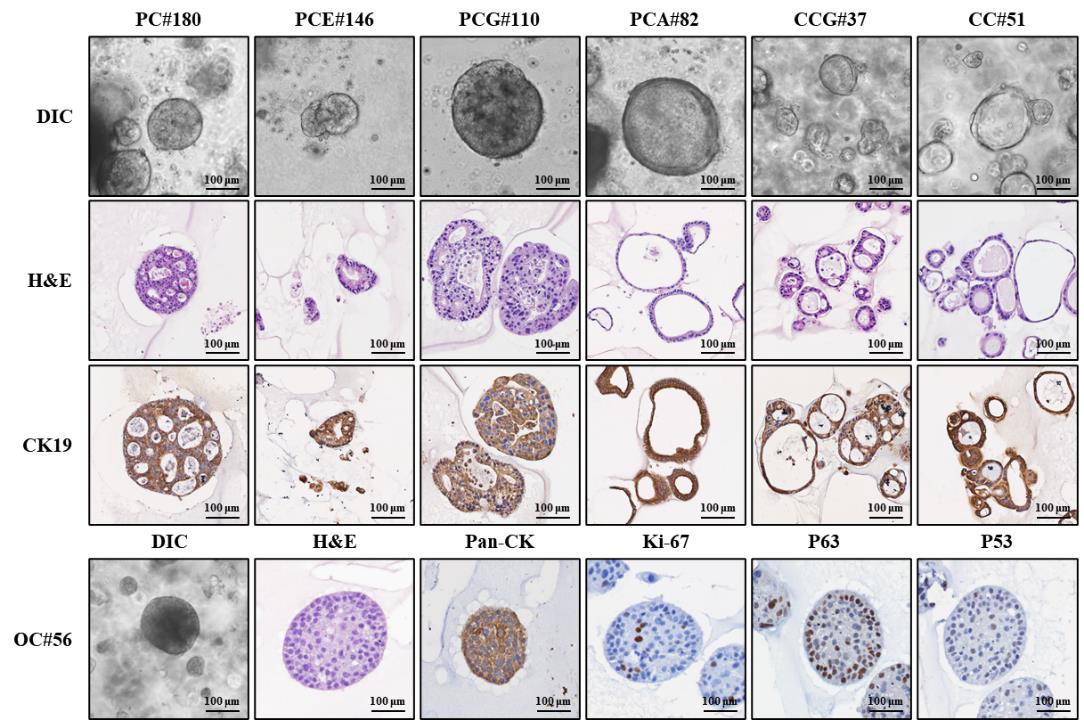
Figure 5. Histological and immunofluorescence characterization of formalin-fixed paraffin-embedded (FFPE) organoids. Representative images show brightfield morphology of patient-derived organoids (DIC) prior to fixation, hematoxylin and eosin (H&E), and immunohistochemistry (IHC) staining for cancer diagnostic markers, including CK19, Pan-CK, Ki-67, P63, and P53. DIC images were acquired using a Zeiss microscope with ZEN 3.7 software. Stained slides were scanned using the Vectra PolarisTM Automated Quantitative Pathology Imaging System (Akoya Biosciences). The images were captured with the Phenochart software. PC, pancreatic cancer organoid established from surgical tissue; PCE, pancreatic cancer organoid established from endoscopic ultrasound–guided fine needle biopsy; PCG, pancreatic cancer organoid established from percutaneous liver gun biopsy; PCA, pancreatic cancer organoid established from ascites; CCG, cholangiocarcinoma organoid established from percutaneous liver biopsy; CC, cholangiocarcinoma organoid established from surgical tissue; OC, oral cancer organoid established from surgical tissue.
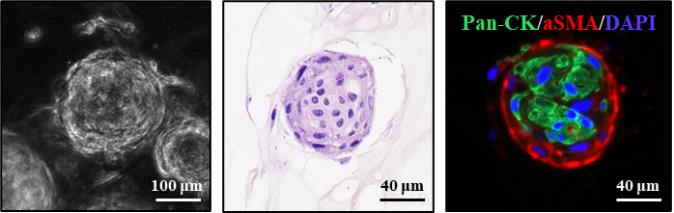
Figure 6. Characterization of oral cancer organoids co-cultured with cancer-associated fibroblasts (CAFs) using the formalin-fixed paraffin-embedded (FFPE) organoid preparation method. (Left) Brightfield morphology of oral cancer organoids after co-culture with CAFs. (Middle) H&E staining of FFPE organoid sections. (Right) Immunofluorescence staining shows oral cancer organoids stained with pan-CK (green), CAFs stained with α-SMA (red), and nuclei counterstained with DAPI (blue). Brightfield and IHC images were acquired using a Zeiss microscope with ZEN 3.7 software. Hematoxylin and eosin (H&E)-stained slides were scanned using the Vectra PolarisTM Automated Quantitative Pathology Imaging System (Akoya Biosciences), and images were captured with the Phenochart software.
General notes and troubleshooting
General notes
1. This method supports a wide range of organoid types—including oral, liver, pancreatic, bile duct, and gallbladder cancer—and is particularly effective for preserving delicate morphologies such as cystic, grape-like, or loosely cohesive organoids, which are often lost or distorted during standard processing. For FFPE preparation, any organoid culture method employing a BME such as Matrigel can be used, provided that an optimized culture medium is applied for each organoid type. If additional guidance on organoid establishment and culture prior to FFPE processing is needed, our recently published work may serve as a useful reference [22].
2. This protocol is optimized for tissue processing using an automated processor, following standard procedures typically applied to formalin-fixed tissue specimens. However, manual embedding workflows commonly used for tissue samples can also be applied without compromising the quality of FFPE organoid preparation.
3. The paraffin-embedded organoid blocks generated using this protocol can be sectioned and subsequently subjected to standard histological and immunostaining procedures in the same manner as conventional tissue samples.
4. This structural integrity enables robust downstream histological and molecular analyses such as H&E staining, immunohistochemistry (IHC), and immunofluorescence (IF). However, because the procedure involves relatively harsh processing steps, it may not be suitable for molecules or epitopes that are highly sensitive to stress, representing a limitation of the method.
Troubleshooting
Problem 1: A circular hole appears at the center of the 2% agarose mold after solidification of the agarose in step C4.
Possible cause: The volume of 2% agarose gel poured in step B3 was insufficient to fully cover the BME dome, resulting in incomplete embedding of the organoid.
Solution: A sufficient volume of 2% agarose gel needs to be added in step B3 to fully embed the BME dome. Typically, 600 μL of 2% agarose gel is sufficient to submerge a BME dome prepared with 40–50 μL of BME. However, the height of the dome may vary depending on the volume of BME used and whether the plate surface is coated. In these cases, the volume of agarose gel needs to be adjusted to ensure that the dome is fully embedded.
Problem 2: Upon lifting the agarose mold from the 24-well plate, the BME dome failed to remain embedded and was left behind on the surface of the well.
Possible cause: The BME was not sufficiently dried prior to the addition of 2% agarose gel, resulting in weak adhesion between the BME and the agarose mold.
Solution: After washing, the PBS needs to be thoroughly removed. If the BME remains visibly moist after drying at room temperature for 1 h, extend the drying period for an additional 30 min to 1 h. To avoid complete dehydration, BME should be sufficiently dried to promote adhesion to agarose while retaining its structural integrity.
Problem 3: The 2% agarose mold breaks easily.
Possible cause: The agarose concentration is too high.
Solution: When preparing a 2% agarose gel, minimize the evaporation of sterile water. When heating to dissolve the agarose powder, boil several times for short periods.
Problem 4: The density of organoids is too low in the sectioned paraffin slides.
Possible cause: A small number of organoids were seeded.
Solution: The number of seeded cells should be increased to allow for the observation of multiple organoids in a layer.
Problem 5: No organoids are visible in the FFPE section.
Possible cause: Organoids grew insufficiently.
Solution: Start generation of FFPE after the organoids have grown to a minimum size of approximately 100 μm.
Acknowledgments
This study was supported by grants from the NCC [grant numbers NCC-2410780 and 2510700]. This work was supported by the Research Core Center in NCC of Korea. This protocol was used in [9].
Competing interests
The authors declare no competing financial interests.
Ethical considerations
Written informed consent was obtained from all patients. The study protocol was approved by the Institutional Review Board of the National Cancer Center of Korea (approval numbers: NCC-2021-0282, NCC2020-0290, NCC2017-0122, NCC2020-0231, and NCC2021-0232).
References
- Yang, H., Sun, L., Liu, M. and Mao, Y. (2018). Patient-derived organoids: a promising model for personalized cancer treatment. Gastroenterol Rep (Oxf). 6(4): 243–245. https://doi.org/10.1093/gastro/goy040
- Zhao, Z., Chen, X., Dowbaj, A. M., Sljukic, A., Bratlie, K., Lin, L., Fong, E. L. S., Balachander, G. M., Chen, Z., Soragni, A. et al. (2022). Organoids. Nat Rev Methods Primers. 2. https://doi.org/10.1038/s43586-022-00174-y
- Tong, L., Cui, W., Zhang, B., Fonseca, P., Zhao, Q., Zhang, P., Xu, B., Zhang, Q., Li, Z., Seashore-Ludlow, B., et al. (2024). Patient-derived organoids in precision cancer medicine. Med. 5(11): 1351–1377. https://doi.org/10.1016/j.medj.2024.08.010
- Yan, H. H., Siu, H. C., Law, S., Ho, S. L., Yue, S. S., Tsui, W. Y., Chan, D., Chan, A. S., Ma, S., Lam, K. O., et al. (2018). A Comprehensive Human Gastric Cancer Organoid Biobank Captures Tumor Subtype Heterogeneity and Enables Therapeutic Screening. Cell Stem Cell. 23(6): 882–897.e11. https://doi.org/10.1016/j.stem.2018.09.016
- Geurts, M. H., Gandhi, S., Boretto, M. G., Akkerman, N., Derks, L. L. M., van Son, G., Celotti, M., Harshuk-Shabso, S., Peci, F., Begthel, H., et al. (2023). One-step generation of tumor models by base editor multiplexing in adult stem cell-derived organoids. Nat Commun. 14(1): e1038/s41467–023–40701–3. https://doi.org/10.1038/s41467-023-40701-3
- Seino, T., Kawasaki, S., Shimokawa, M., Tamagawa, H., Toshimitsu, K., Fujii, M., Ohta, Y., Matano, M., Nanki, K., Kawasaki, K., et al. (2018). Human Pancreatic Tumor Organoids Reveal Loss of Stem Cell Niche Factor Dependence during Disease Progression. Cell Stem Cell. 22(3): 454–467.e6. https://doi.org/10.1016/j.stem.2017.12.009
- Low, R. R. J., Fung, K. Y., Gao, H., Preaudet, A., Dagley, L. F., Yousef, J., Lee, B., Emery-Corbin, S. J., Nguyen, P. M., Larsen, R. H., et al. (2023). S100 family proteins are linked to organoid morphology and EMT in pancreatic cancer. Cell Death Differ. 30(5): 1155–1165. https://doi.org/10.1038/s41418-023-01126-z
- Wallaschek, N., Niklas, C., Pompaiah, M., Wiegering, A., Germer, C. T., Kircher, S., Brändlein, S., Maurus, K., Rosenwald, A., Yan, H. H., et al. (2019). Establishing Pure Cancer Organoid Cultures: Identification, Selection and Verification of Cancer Phenotypes and Genotypes. J Mol Biol. 431(15): 2884–2893. https://doi.org/10.1016/j.jmb.2019.05.031
- Lee, M. R., Kang, S., Lee, J., Kong, S. Y., Kim, Y., Lee, Y. S., Shon, H. W., Kang, G., Lee, J., Youn, S. M., et al. (2025). Organoid morphology-guided classification for oral cancer reveals prognosis. Cell Rep Med. 6(5): 102129. https://doi.org/10.1016/j.xcrm.2025.102129
- Fujii, M., Shimokawa, M., Date, S., Takano, A., Matano, M., Nanki, K., Ohta, Y., Toshimitsu, K., Nakazato, Y., Kawasaki, K., et al. (2016). A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell. 18(6): 827–838. https://doi.org/10.1016/j.stem.2016.04.003
- Driehuis, E., Kolders, S., Spelier, S., Lõhmussaar, K., Willems, S. M., Devriese, L. A., de Bree, R., de Ruiter, E. J., Korving, J., Begthel, H., et al. (2019). Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov. 9(7): 852–871. https://doi.org/10.1158/2159-8290.cd-18-1522
- Sachs, N., de Ligt, J., Kopper, O., Gogola, E., Bounova, G., Weeber, F., Balgobind, A. V., Wind, K., Gracanin, A., Begthel, H., et al. (2018). A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell. 172: 373–386.e10. https://doi.org/10.1016/j.cell.2017.11.010
- Tiriac, H., Belleau, P., Engle, D. D., Plenker, D., Deschênes, A., Somerville, T. D. D., Froeling, F. E. M., Burkhart, R. A., Denroche, R. E., Jang, G. H., et al. (2018). Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 8(9): 1112–1129. https://doi.org/10.1158/2159-8290.cd-18-0349
- Verissimo, C. S., Overmeer, R. M., Ponsioen, B., Drost, J., Mertens, S., Verlaan-Klink, I., Gerwen, B. v., van der Ven, M., Wetering, M. v. d., Egan, D. A., et al. (2016). Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. eLife. 5: e18489. https://doi.org/10.7554/elife.18489
- El Harane, S., Zidi, B., El Harane, N., Krause, K. H., Matthes, T. and Preynat-Seauve, O. (2023). Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells. 12(7): 1001. https://doi.org/10.3390/cells12071001
- Sato, T., Stange, D. E., Ferrante, M., Vries, R. G., van Es, J. H., van den Brink, S., van Houdt, W. J., Pronk, A., van Gorp, J., Siersema, P. D., et al. (2011). Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett's Epithelium. Gastroenterology. 141(5): 1762–1772. https://doi.org/10.1053/j.gastro.2011.07.050
- Havnar, C., Holokai, L., Ichikawa, R., Chen, W., Scherl, A. and Shamir, E. R. (2024). Histogel-based techniques for embedding organoids in paraffin blocks enable high throughput downstream histopathological analyses. J Histotechnol. 48(1): 46–57. https://doi.org/10.1080/01478885.2024.2398381
- Yoshimoto, S., Taguchi, M., Sumi, S., Oka, K. and Okamura, K. (2023). Establishment of a novel protocol for formalin-fixed paraffin-embedded organoids and spheroids. Biol Open. 12(5): e059882. https://doi.org/10.1242/bio.059882
- Cho, S. Y., Hwang, H., Kim, Y. H., Yoo, B. C., Han, N., Kong, S. Y., Baek, M. J., Kim, K. H., Lee, M. R., Park, J. G., et al. (2023). Refining Classification of Cholangiocarcinoma Subtypes via Proteogenomic Integration Reveals New Therapeutic Prospects. Gastroenterology. 164(7): 1293–1309. https://doi.org/10.1053/j.gastro.2023.02.045
- Choi, W., Kim, Y. H., Woo, S. M., Yu, Y., Lee, M. R., Lee, W. J., Chun, J. W., Sim, S. H., Chae, H., Shim, H., et al. (2023). Establishment of Patient-Derived Organoids Using Ascitic or Pleural Fluid from Cancer Patients. Cancer Res Treat. 55(4): 1077–1086. https://doi.org/10.4143/crt.2022.1630
- Lee, M. R., Woo, S. M., Kim, M. K., Han, S., Park, S., Lee, W. J., Lee, D., Choi, S. I., Choi, W., Yoon, K., et al. (2024). Application of plasma circulating KRAS mutations as a predictive biomarker for targeted treatment of pancreatic cancer. Cancer Sci. 115(4): 1283–1295. https://doi.org/10.1111/cas.16104
- Kang, S., Lee, M. R., Choi, W., Kong, S. Y. and Kim, Y. H. (2025). Protocol for generation and utilization of patient-derived organoids from multimodal specimen. STAR Protoc. 6(3): 104039. https://doi.org/10.1016/j.xpro.2025.104039
Article Information
Publication history
Received: Jul 10, 2025
Accepted: Sep 1, 2025
Available online: Sep 16, 2025
Published: Oct 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Lee, M. R., Kang, S., Jeon, A. R., Choi, S. W., Kong, S. Y. and Kim, Y. H. (2025). Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation. Bio-protocol 15(19): e5467. DOI: 10.21769/BioProtoc.5467.
Category
Cancer Biology > General technique > Cell biology assays
Cell Biology > Tissue analysis > Histomorphology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








