- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Practical Guide to In Vitro Clonal Propagation of Nicotiana benthamiana Using Axillary Shoot Induction
Published: Vol 15, Iss 18, Sep 20, 2025 DOI: 10.21769/BioProtoc.5443 Views: 1213
Reviewed by: Venkatasalam ShanmugabalajiAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Micrografting Technique of Hevea brasiliensis In Vitro Plantlets
Florence Dessailly [...] Julie Leclercq
Feb 20, 2025 1479 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1426 Views

Highly Efficient Agrobacterium-Mediated Transformation of Tomato cv Micro-Tom From Cotyledon Explants
Débora Pagliuso [...] Luciano Freschi
Dec 5, 2025 1460 Views
Abstract
This protocol outlines a reliable method for the micropropagation of Nicotiana benthamiana using axillary shoot branching. Axillary shoot induction involves stimulating the outgrowth of dormant buds located at the leaf axils, allowing for the development of genetically stable shoots without callus formation or the use of exogenous plant growth regulators. Nodal explants are cultured on MS medium supplemented with kinetin and indole-3-butyric acid (IBA) to induce shoot formation. Isolated shoots are then transferred to hormone-free MS medium for rooting. This method is simple, reproducible, and supports rapid plant multiplication for downstream applications such as agroinfiltration or transient protein expression.
Key features
• Axillary branching-based micropropagation enables efficient regeneration of N. benthamiana from nodal explants, supporting a cyclic system for continuous in vitro plant supply [1].
• Rooting occurs on hormone-free medium, with even shoots as small as 0.5 mm readily forming roots, simplifying and accelerating the propagation process.
• Root formation begins within 7 days, including in transgenic lines that are typically difficult to root using conventional methods.
• Fully developed, rooted plants are ready within 4 weeks, ideal for transient expression assays, recombinant protein production, and secondary metabolite extraction.
Keywords: MicropropagationGraphical overview
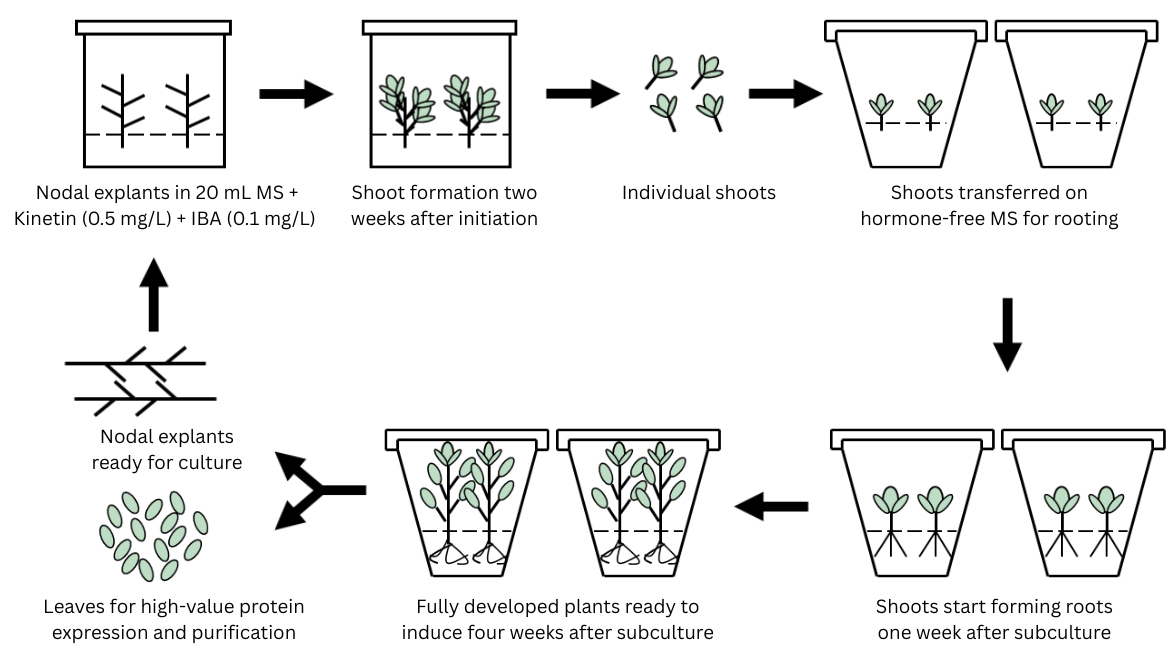
Schematic representation of the micropropagation workflow for N. benthamiana via axillary branching. The process includes nodal cutting culture, axillary shoot formation, shoot separation, rooting, and plantlet development for downstream use. This figure was created using Microsoft PowerPoint.
Background
Nicotiana benthamiana is a model plant widely used in plant molecular biology, particularly for transient gene expression, recombinant protein production, functional genomics research [2–4], and viral vector studies [5]. It is particularly amenable to Agrobacterium-mediated transformation and supports high levels of foreign gene expression. One of the most commonly used methods for transient gene expression in N. benthamiana is leaf infiltration. This technique involves introducing Agrobacterium tumefaciens carrying the gene of interest directly into the intercellular spaces of leaves. It allows for rapid and localized gene expression without the need for stable transformation. Leaf infiltration is simple, efficient, and highly reproducible, making it especially valuable for high-throughput functional genomics, protein localization, and recombinant protein production studies [4,6,7]. Its ease of use and adaptability make it a cornerstone method in plant biotechnology. One of its key advantages is the low level of interfering endogenous compounds, such as phenolics and proteases, which facilitates efficient recombinant protein purification and metabolite analysis [8,9].
To fully utilize N. benthamiana for high-throughput molecular studies or biomanufacturing applications, a reliable and efficient micropropagation system is essential. Ready access to uniform, healthy in vitro plants supports transient assays, stable transformation, and metabolite production. Although N. benthamiana is relatively easy to grow, propagation of transgenic lines and high-frequency regeneration systems remains a bottleneck. Efficient micropropagation techniques are essential for generating uniform plant material suitable for transformation and expression assays. Axillary branching offers a simple and hormone-responsive method for shoot multiplication, avoiding the complications associated with de novo regeneration. Continuous use of nodal segments from newly established in vitro plants enables a cyclic propagation system, ensuring a consistent and scalable supply of fully developed plants for downstream applications. Furthermore, since the rooting stage in this protocol does not require any auxins, it minimizes the risk of residual hormonal effects that could interfere with subsequent assays, such as transient gene expression. The following protocol is adapted from Deo et al. [1], enabling routine propagation of N. benthamiana on MS medium [10] under controlled conditions.
From practical experience, nodal cuttings from transgenic N. benthamiana are notoriously difficult to root, which limits shoot proliferation and leaf biomass, which are critical for recombinant protein extraction and purification. To address this limitation, the protocol described here utilizes a hormone-free micropropagation approach based on axillary shoot branching. Axillary shoot branching refers to the outgrowth of buds located at the junction between a leaf and stem (the axil), which can develop into new shoots under appropriate conditions. In tissue culture, these axillary buds can be stimulated to grow into genetically identical shoots without the need for dedifferentiation or callus formation, thereby reducing the risk of somaclonal variation and maintaining genetic stability. Efficient root induction from axillary shoots without the use of external auxins streamlines the micropropagation process. This rapid development supports a timely production of plants suitable for downstream applications such as transient gene expression and metabolite extraction. This system supports cyclic propagation, and using it, nearly 3 kg of in vitro leaves were harvested within six months, demonstrating scalability and robustness for molecular farming applications.
This protocol describes a simple, reproducible method for the mass propagation of N. benthamiana using axillary shoot branching, as illustrated in the Graphical overview. This method enables rapid multiplication and preparation of plants suitable for downstream applications such as agroinfiltration and recombinant protein expression.
Materials and reagents
Biological materials
1. N. benthamiana seeds (lab stock; originally obtained from the Centre for Tropical Crops and Biocommodities, now Centre for Agriculture and Bioeconomy, Queensland University of Technology). Researchers interested in acquiring seeds are advised to contact laboratories or research groups working with N. benthamiana for seed sharing, as commercial suppliers are limited
2. In vitro–cultured N. benthamiana plants (generated from lab stock seeds as described in the protocol)
Reagents
1. Murashige and Skoog (MS) basal medium powder (Sigma, catalog number: M5519)
2. Kinetin powder (Sigma, catalog number: K3378)
3. Indole-3-butyric acid (IBA) powder (Sigma, catalog number: I5386)
4. Sucrose (PhytoTech Labs, catalog number: S391)
5. Agar (or alternative gelling agent like phytagel) (Sigma, catalog number: A5054)
6. Commercial bleach (e.g., White KingTM 4% sodium hypochlorite) (from local supermarket)
7. Tween-20 (10%) (Sigma, catalog number: P9416)
8. Ethanol (70% and absolute) (Sigma, catalog number: 459844)
9. 1 M NaOH (Sigma, catalog number: 1091371000)
9. Sterile Milli-Q water for seed sterilization
10. Milli-Q water for hormone and media preparation
Solutions
1. IBA stock solution (see Recipes)
2. Kinetin stock solution (see Recipes)
3. MS basal medium without hormones (hormone-free MS) (see Recipes)
4. MS basal medium with hormones (MS1) (see Recipes)
5. Seed sterilization solution (see Recipes)
Recipes
Note: Be precise about the ingredients (e.g., buffer or media), quantities, and conditions established for your experiments. Note that the omission of minor details from recipes (e.g., type of water used or storage conditions) might lead to the failure of the experiment.
1. IBA stock solution (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| IBA powder | 1 mg/mL | 20 mg |
| Absolute ethanol | 70% | 14 mL |
| Milli-Q water | up to 20 mL | |
| Total | 20 mL |
Weigh 20 mg of IBA powder and transfer to a 50 mL Falcon tube. Dissolve in 14 mL of absolute ethanol. Make up the final volume to 20 mL with Milli-Q water. The final concentration of ethanol will be 70%. Filter sterilize using a 0.2 μm syringe filter. Aliquot 1 mL into sterile 1.5 mL Eppendorf tubes. Store at -20 °C. Use 100 μL per 1 L of MS medium for a final IBA concentration of 0.1 mg/L.
2. Kinetin stock solution (1 mg/mL)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kinetin powder | 1 mg/mL | 20 mg |
| 1 M NaOH | 100 μL | |
| Milli-Q water | up to 20 mL | |
| Total | 20 mL |
Weigh 20 mg of kinetin powder and transfer to a 50 mL Falcon tube. Dissolve in a few drops of 1 M NaOH and make up the final volume to 20 mL with Milli-Q water. Filter sterilize using a 0.2 μm syringe filter. Aliquot 1 mL into sterile 1.5 mL Eppendorf tubes. Store at -20 °C. Use 500 μL per 1 L of MS medium for a 0.5 mg/L final concentration.
3. MS basal medium without hormones (hormone-free MS)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder | 4 g/L | 4 g |
| Sucrose | 30 g/L | 30 g |
| Agar | 7 g/L | 7 g |
| Milli-Q water | n/a | up to 1,000 mL |
| Total | 1,000 mL |
Dissolve MS powder in 700 mL Milli-Q water. Add sucrose and mix well. Adjust pH to 5.8 using 1 M NaOH. Make up the volume to 1 L. Add 7 g of agar. Autoclave at 121 °C at 15 psi for 15 min. Cool to 40–50 °C and pour 70 mL per sterile 250 mL culture container in a laminar airflow hood.
Storage tip: Autoclaved hormone-free MS medium can be stored in the dark at room temperature (23–24 °C) for up to 3–4 weeks without compromising effectiveness.
4. MS basal medium with hormones (MS1)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS powder | 4 g/L | 4 g |
| Sucrose | 30 g/L | 30 g |
| Agar | 7 g/L | 7 g |
| Kinetin stock | 0.5 mg/L | 500 μL |
| IBA stock | 0.1 mg/L | 100 μL |
| Milli-Q water | up to 1,000 mL | |
| Total | 1,000 mL |
Follow the same steps as for the hormone-free MS solution. After autoclaving and cooling to 40–50 °C, add 500 μL of kinetin stock (1 mg/mL) and 100 μL of IBA stock (1 mg/mL). Mix gently and pour 20 mL per sterile 120 mL culture container in a laminar airflow hood.
Storage tip: Hormone-supplemented MS medium is best used fresh or within 1–2 weeks when stored at room temperature (23–24 °C), as prolonged storage may reduce hormone activity.
Time-saving tip: Prepare hormone-free MS medium, autoclave, cool, and store at room temperature (23–24 °C) in the dark for 3–4 weeks. When needed, the stored hormone-free MS medium can be reheated in a microwave to melt the agar and poured into culture vessels. If hormone supplementation is required (e.g., kinetin or IBA), the appropriate stock solutions can be added to the melted medium once it cools slightly (to approximately 50 °C) before pouring. This approach saves time by eliminating the need to prepare fresh medium from scratch.
5. Seed sterilization solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Commercial bleach (4%) | 1% sodium hypochlorite | 1.25 mL |
| Tween-20 (10%) | 0.05% | 25 μL |
| Sterile Milli-Q water | 3.75 mL | |
| Total | 5 mL |
Prepare fresh. Add 1.25 mL of 4% bleach to 3.75 mL of sterile Milli-Q water. Add 25 μL of 10% Tween-20 to the bleach solution and mix well. This solution contains 1% sodium hypochlorite (from 4% commercial bleach) with 0.05% Tween-20, suitable for mild seed surface sterilization. Use for the 5-min treatment of seeds during sterilization.
Laboratory supplies
1. Sterile forceps
2. Scalpel
3. Petri dishes 94 mm × 16 mm (Sigma, catalog number: BR452000)
4. 1.5 mL sterile Eppendorf tubes (Sigma, catalog number: HS4323)
5. Sterile Whatman filter paper (90 mm diameter) (Sigma, catalog number: WHA1004090)
6. Wide-bore pipette tips (P1000); this can be prepared by cutting the ends of standard P1000 tips to create a larger orifice, then autoclaving for sterilization (cost-effective alternative)
7. Plant culture vessels (e.g., jars, tubes)
8. 50 mL Falcon tube Corning® (Sigma, catalog number: CLS430829)
9. 0.2 μm syringe filter Corning® (Sigma, catalog number: CLS431224)
Equipment
1. Laminar flow hood
2. Growth chamber or culture room with controlled temperature (27 ± 1 °C) and photoperiod (16 h light/8 h dark)
3. Light source (cool white fluorescent or LED providing 30–50 μmol·m-2·s-1)
4. Orbital shaker (for seed sterilization)
5. Autoclave
6. pH meter
Procedure
A. Preparation of explants
Note: Work under sterile conditions in a laminar airflow (LAF) hood.
A1. From seeds
1. Seed sterilization and germination
Caution: N. benthamiana seeds are extremely small and lightweight, so handle them with care. They can be easily displaced by air currents, including forceful exhalation or blowing. See Figure 1 for freshly collected seeds of N. benthamiana below for reference.

Figure 1. Seeds of Nicotiana benthamiana prior to germination. These seeds can be surface-sterilized and germinated to generate plants for micropropagation. Image courtesy of Kylie Shand.
a. Prepare 5 mL of seed sterilization solution (see Recipes)
b. Place approximately 100–200 seeds into a 1.5 mL microcentrifuge tube (up to the 0.1 mL mark).
c. Add 1 mL of 70% ethanol and vortex for 30 s to 1 min.
d. Remove ethanol by aspirating out with a pipettor and add 1 mL of seed sterilization solution.
e. Shake on an orbital shaker at 50–60 rpm for 5 min (do not exceed to avoid reduced viability).
Caution: Do not exceed the bleach treatment beyond 5 min since this could reduce seed viability and lower germination.
f. Discard bleach solution by pipetting out with a pipettor. Add 1 mL of sterile water, vortex for 30 s, let seeds settle, then aspirate out with a pipettor.
g. Repeat the wash four more times.
h. Transfer seeds onto sterile filter paper and blot dry.
i. Using wide-bore pipette tips, transfer seeds onto hormone-free MS agar plates.
j. Seal plates and incubate at 27 ± 1 °C under a 16/8 h photoperiod.
k. After 2–3 days, viable seeds will swell (imbibe water).
l. After 10–14 days, when seedlings reach ~4–5 cm, excise nodal segments for micropropagation.
2. Explant preparation
Note: Before beginning explant dissection, familiarize yourself with the morphological parts of the plant. See the annotated image below (Figure 2) depicting the excision of nodal segments from N. benthamiana seedlings and established in vitro plants. These visuals serve as a guide for the precise excision of nodal segments from both young seedlings and established in vitro plants, supporting downstream applications such as micropropagation.
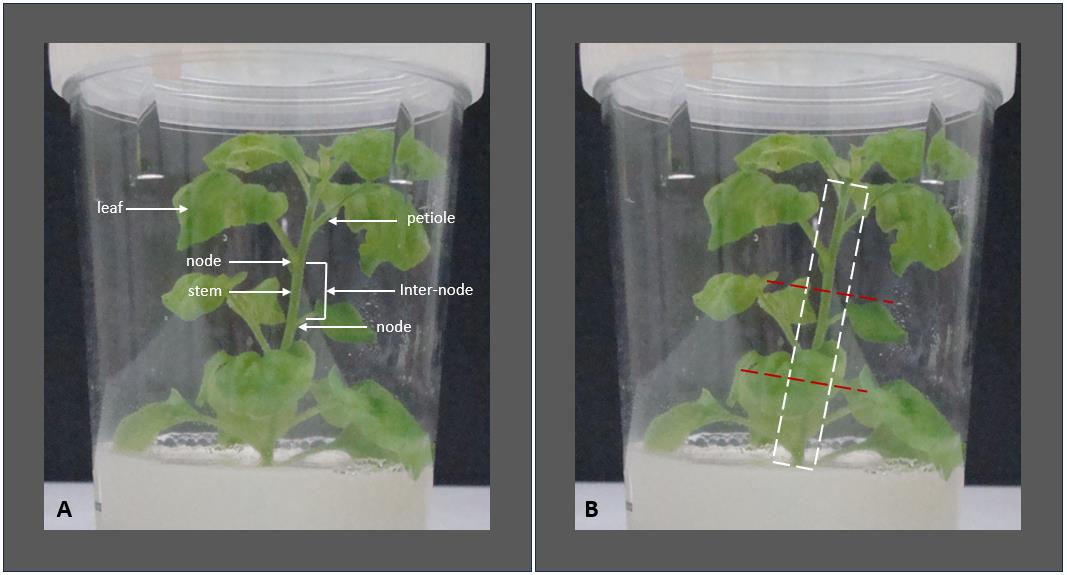
Figure 2. Morphological features and explant preparation of Nicotiana benthamiana plants. (A) Morphological features of an in vitro–grown N. benthamiana plantlet are labeled to identify key structures, including nodes, internodes, leaves, petioles, and the stem. Recognizing these features is essential for accurately selecting explant material. (B) Cutting points at the internodes—above and below each node—to generate 3–4 nodal explants (indicated with red dashed lines). The white dashed box marks the region where leaves and petioles should be removed. A small portion of the petiole base is left attached to the stem to avoid damaging the node.
a. Excise nodal segments from germinated seedlings.
b. Remove leaves, retaining petiole bases.
c. Cut into 4-node segments.
Caution:
1. Use sterile tools to cut across the internodes at the red dashed lines (Figure 2). Each segment should contain 3–4 nodes and a portion of internode above and below.
2. Do not cut too close to any node; this may damage the axillary bud, which is essential for shoot regeneration. Always leave a few millimeters of internodal tissue to protect the developing meristems.
A2. From donor plants
1. Excise nodal segments from healthy in vitro N. benthamiana donor plants.
2. Remove leaves but retain petiole bases (retain at least 5 mm of petiole base to protect the nodes from damage during explant preparation).
3. Cut into 3–4-node sections.
B. Culture initiation
1. Place nodal explants vertically onto MS1 medium (MS with hormones).
2. Incubate cultures under the following conditions: 16/8 h light/dark cycle; light intensity: 30–50 μmol·m-2·s-1; temperature: 27 ± 1 °C.
C. Shoot elongation and isolation
1. After approximately 2 weeks, axillary shoots begin to elongate from nodal explants cultured on MS1 medium.
2. Carefully isolate these axillary shoots using sterile forceps or a scalpel under aseptic conditions (even shoots as small as 5 mm can successfully root).
Notes:
1. This protocol does not involve repeated subculturing of isolated shoots on hormone-supplemented media for multiplication. Instead, each new cycle is initiated using fresh nodal segments from previously established in vitro plants to induce new axillary shoots.
2. Axillary shoots are preferred in this method because they root readily without exogenous auxins such as IBA, NAA, or IAA, simplifying the protocol and avoiding hormonal carryover effects.
3. For transgenic lines, this approach supports two downstream applications: leaves can be harvested for recombinant protein extraction and purification, and nodes can be reused for further micropropagation via axillary branching, ensuring cyclic, scalable propagation.
D. Rooting and plantlet development
1. Transfer elongated axillary shoots to hormone-free MS medium.
2. Maintain growth conditions as described in step B2.
Notes:
1. Roots form readily within 7 days due to the endogenous auxin sensitivity of axillary shoots.
2. Plantlets are usually fully developed and ready for acclimatization in approximately 4 weeks.
3. Gradually acclimatize rooted plantlets to ambient conditions before transferring to soil.
4. Always maintain some plants in vitro as mother plants, especially nodal segments, to serve as explants for the next cycle of axillary shoot induction.
Validation of protocol
This protocol was developed and optimized for the in vitro micropropagation of Nicotiana benthamiana via axillary shoots and was used routinely over a three-year period. After its development, the method was employed for the mass production of both transgenic and wild-type N. benthamiana plants. These plants were used for recombinant protein purification from in vitro–derived leaves. Across multiple experiments, 80%–90% of nodal explants consistently produced shoots within 14 days on MS1 medium. The regenerated plants were healthy and suitable for downstream applications, including leaf tissue sampling for protein extraction.
The experimental outcomes obtained using this method have been peer-reviewed and published in:
• Deo et al. [1]. In vitro micropropagation of Nicotiana benthamiana via axillary shoots. South Pacific Journal of Natural and Applied Sciences, 32, 55–60.
General notes and troubleshooting
General notes
1. Always include 3% sucrose and solidify media with 7–8 g/L agar unless otherwise specified.
2. Use ~20 mL of medium per culture vessel.
3. Petiole bases improve shoot induction.
4. Young seedlings are less prone to contamination.
5. Gelling agents other than agar (e.g., phytagel) may influence morphogenic responses.
6. This protocol is scalable for high-throughput micropropagation or transformation applications.
7. Minimum shoot length for transferring to rooting medium is 5 mm.
Troubleshooting
Problem 1: Poor seed germination after sterilization.
Possible cause: Seeds were overexposed to bleach or ethanol during sterilization.
Solution: Reduce exposure time to bleach (e.g., from 10 to 7 min), avoid excessive shaking, and rinse thoroughly with sterile water at least 4–5 times. Always use fresh sterilization solution.
Problem 2: Fungal or bacterial contamination during seed germination or tissue culture.
Possible cause: Incomplete sterilization or contaminated equipment/media.
Solution: Ensure that the seed sterilization solution was freshly prepared and mixed well. Autoclave all media and tools. Use proper aseptic technique in a laminar flow hood and sterilize tools between handling samples.
Problem 3: Poor or no axillary shoot formation from nodal explants.
Possible cause: Low-quality explants or improper hormone concentrations.
Solution: Use young, healthy nodal explants from in vitro–grown seedlings (4–5 cm tall). Confirm that the MS1 medium contains the correct concentrations of kinetin (0.5 mg/L) and IBA (0.1 mg/L), and that the pH was adjusted to 5.8 before autoclaving.
Problem 4: Isolated axillary shoots wilt or become necrotic.
Possible cause: Mechanical damage or delay in transferring shoots.
Solution: Use sharp sterile tools for shoot excision and minimize handling time. Transfer isolated shoots to fresh hormone-free MS medium immediately under sterile conditions to prevent desiccation or contamination.
Problem 5: Isolated shoots fail to root.
Possible cause: Shoots are too small or unhealthy.
Solution: Only use axillary shoots at least 5 mm in size and visibly healthy. Avoid excessive light exposure and maintain optimal growth conditions (27 °C, 16 h photoperiod, 30–50 μmol·m-2·s-1 light intensity).
Problem 6: Transgenic nodal cuttings fail to root.
Possible cause: Transgenic lines often show impaired rooting response when nodal cuttings are cultured directly.
Solution: Use axillary shoot-derived explants instead of nodal cuttings for rooting. Axillary shoots obtained via micropropagation root readily on hormone-free MS medium within 7 days, enabling robust plantlet regeneration and leaf development for downstream applications such as recombinant protein extraction.
Acknowledgments
This work was supported by funding from Leaf Energy Pty Ltd and conducted at Queensland University of Technology (QUT). The protocol was developed and the manuscript written solely by the author. Editorial assistance was provided by colleagues who are acknowledged through authorship in the original publication. This protocol was used in [1].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
This protocol involves the use of Nicotiana benthamiana plants and does not involve human participants or animal subjects. Therefore, no ethical approval or informed consent was required.
References
- Deo, P. C., Dugdale, B., Harding, R. M., Kato, M. and Dale, J. (2014). In vitro micro propagation of Nicotiana benthamiana via axillary shoots. South Pac J Nat Appl Sci. 32(2): 55–60. https://doi.org/10.1071/sp14009
- Bally, J., Jung, H., Mortimer, C., Naim, F., Philips, J. G., Hellens, R., Bombarely, A., Goodin, M. M. and Waterhouse, P. M. (2018). The Rise and Rise of Nicotiana benthamiana: A Plant for All Reasons. Annu Rev Phytopathol. 56(1): 405–426. https://doi.org/10.1146/annurev-phyto-080417-050141
- Xu, F., Copeland, C., Li, X. (2015). Protein immunoprecipitation using Nicotiana benthamiana transient expression system. Bio Protoc. 5(13): e1520. https://doi.org/10.21769/BioProtoc.1520
- Goodin, M. M., Zaitlin, D., Naidu, R. A. and Lommel, S. A. (2015). Nicotiana benthamiana: Its History and Future as a Model for Plant–Pathogen Interactions. Mol Plant Microbe Interact. 2015(1): 28–39. https://doi.org/10.1094/mpmi-00-00-1015-rev.testissue
- Matsuda, R., Abe, T., Fujiwara, K. (2017). Viral vector-based transient gene expression in Nicotiana benthamiana: effects of light source on leaf temperature and hemagglutinin content. Plant Cell Rep. 36(10): 1667–1669. https://doi.org/10.1007/s00299-017-2164-6
- Kapila, J., De Rycke, R., Van Montagu, M. and Angenon, G. (1997). An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 122(1): 101–108. https://doi.org/10.1016/s0168-9452(96)04541-4
- Sparkes, I. A., Runions, J., Kearns, A. and Hawes, C. (2006). Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc. 1(4): 2019–2025. https://doi.org/10.1038/nprot.2006.286
- Gleba, Y., Klimyuk, V. and Marillonnet, S. (2005). Magnifection—a new platform for expressing recombinant vaccines in plants. Vaccine. 23: 2042–2048. https://doi.org/10.1016/j.vaccine.2005.01.006
- Chen, Q., He, J., Phoolcharoen, W. and Mason, H. S. (2011). Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum Vaccin. 7(3): 331–338. https://doi.org/10.4161/hv.7.3.14262
- Murashige, T. and Skoog, F. (1962). A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol Plant. 15(3): 473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Article Information
Publication history
Received: Jun 20, 2025
Accepted: Aug 11, 2025
Available online: Aug 28, 2025
Published: Sep 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Deo, P. C. (2025). Practical Guide to In Vitro Clonal Propagation of Nicotiana benthamiana Using Axillary Shoot Induction. Bio-protocol 15(18): e5443. DOI: 10.21769/BioProtoc.5443.
Category
Plant Science > Plant cell biology > Tissue isolation and culture
Plant Science > Plant breeding > Micropropagation
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








