- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Characterization of Cervical Cancer-Associated Mesenchymal Stem Cells From Primary Tumors Using Explant Culture
(*contributed equally to this work) Published: Vol 15, Iss 12, Jun 20, 2025 DOI: 10.21769/BioProtoc.5358 Views: 2789
Reviewed by: Elena A. OstrakhovitchMithun Santra

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Monitoring Intestinal Organoid–Derived Monolayer Barrier Functions with Electric Cell–Substrate Impedance Sensing (ECIS)
Sarah Ouahoud [...] Vanesa Muncan
Mar 5, 2024 2297 Views

Primary Neuronal Culture and Transient Transfection
Shun-Cheng Tseng [...] Eric Hwang
Jan 20, 2025 2769 Views

Generation of Intestinal Epithelial Monolayers From Single-Cell Dissociated Organoids
Neta Felsenthal and Danijela Matic Vignjevic
Oct 5, 2025 2345 Views
Abstract
Cancer-associated mesenchymal stem cells (Ca-MSCs), an integral part of the tumor microenvironment, play a major role in modulating tumor progression; they have been reported to progress as well as inhibit various cancers, including cervical cancer. To understand the exact role of Ca-MSCs in tumor modulation, it is necessary to have an optimized protocol for Ca-MSCs isolation. This work demonstrates the isolation and expansion of a primary culture of cervical cancer–associated MSCs (CCa-MSCs) from the biopsy sample of cervical cancer patients using the explant culture technique. The isolated cells were characterized according to International Society for Cellular Therapy (ISCT) guidelines. Morphological analysis revealed that cells were adherent to the plastic surface and possessed spindle-shaped morphology. Flow cytometry analysis of the cells showed high expression (~98%) for MSC-specific cell surface markers (CD90, CD73, and CD105), negative expression (<0.5%) for endothelial cell marker (CD34) and hematopoietic cell marker (CD45), and negligible expression for HLA-DR, as recommended by ISCT. Further, trilineage differentiation potential analysis of the cells showed their osteogenic and chondrogenic potential and adipogenic differentiation. This standardized protocol will assist in the cultivation of CCa-MSCs and the study of their interactions with tumor cells and other components of the tumor microenvironment. This protocol may be utilized in the establishment of Ca-MSCs from other types of cancers as well.
Key features
• Isolation and expansion of cervical cancer–associated mesenchymal stem cells (CCa-MSCs) from patient biopsy sample.
• Characterization of isolated CCa-MSCs for the presence of MSC-specific cell surface markers and trilineage differentiation potential.
• CCa-MSCs can be employed to study the interactions with the tumor cells in the tumor microenvironment.
Keywords: Mesenchymal stem cellsGraphical overview
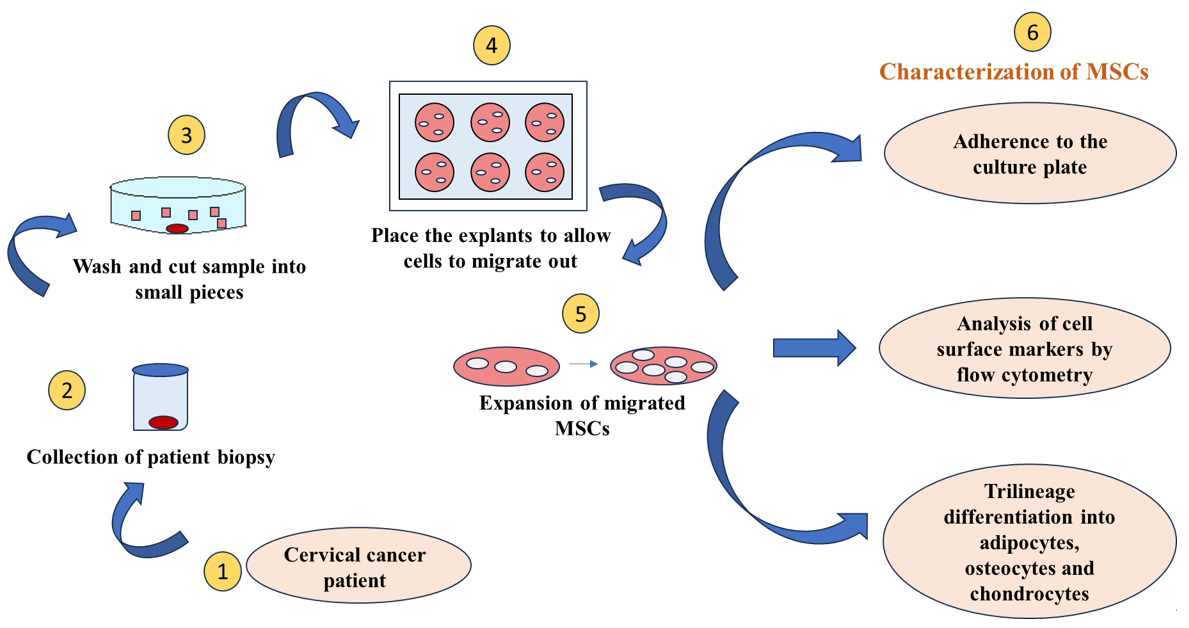
Background
A tumor is a micro-niche in which the cancer cells interact with their neighboring non-tumorigenic host cells. These intercellular communications are critical to the pathophysiology of this disease, and hence, they have gained much attention among researchers over the past couple of decades. The tumor niche, popularly termed tumor microenvironment (TME), comprises endothelial cells, fibroblasts, immune cells, and mesenchymal stem cells (MSCs), among others. It also consists of extracellular components like extracellular matrix, cytokines, or growth factors [1,2]. All these components are present in the vicinity of cancer cells and are further supported by a dense vascular network. This complex TME can modulate tumor initiation, progression, and metastasis and even alter the response of these cancer cells to therapy [3].
Currently, there is limited understanding of the interactions that occur within the TME. One such interaction that modulates tumor growth is one with MSCs, which, over the years, have been reported to act as a double-edged sword for participating both in tumor progression and inhibition [4]. MSCs are spindle-shaped cells that are present in almost all tissues of the body; with their high migratory abilities, they move to the site of the tumor. Numerous studies have been conducted to gain insight into the role of these MSCs in tumor progression, mainly using bone marrow–derived MSCs or adipose-derived MSCs (ADSCs) [5,6]. However, recent studies suggest that cancer-associated MSCs (Ca-MSCs) possess stronger pro-tumorigenic, immunosuppressive, and angiogenic properties than parental MSCs [1,7,8]. Studies suggest that MSCs migrated toward tumor sites differentiate into tumor-associated phenotypes to form Ca-MSCs. Even within the TME, Ca-MSCs have been reported to be educated by tumor cells, and they, in turn, secrete growth factors and chemokines that support tumor growth [9]. This indicates that MSCs isolated from tumor lesions truly represent the characteristics of TME rather than MSCs isolated from tumor-free tissues [10,11]. While the role of Ca-MSCs has been investigated in ovarian, gastric, hepatocellular, breast, and lung cancers, their role in cervical cancer remains largely unexplored [12–15].
Resistance to chemotherapy and radiotherapy remains a significant challenge, leading to tumor relapse in cervical cancer patients. This resistance is largely attributed to cancer stem cells within the TME, a small but highly potent subpopulation with strong self-renewal, differentiation, and tumorigenic potential [16]. Research aims to understand their resistance mechanisms and develop targeted therapies, necessitating an optimized protocol for isolating pure cancer stem cells. While Ca-MSCs have been isolated from various cancers [9], their characterization in cervical cancer remains limited. To address this, we focused on isolating and characterizing cervical cancer–associated mesenchymal stem cells (CCa-MSCs) using an optimized explant culture method.
Previously, Garcia et al. reported the isolation of CCa-MSCs using enzymatic digestion alone [17]. However, this method may compromise cell viability due to cellular damage. Research suggests that explant culture yields a more proliferative, homogenous cell population compared to enzymatic digestion [18]. Accordingly, in the present study, we have successfully demonstrated the isolation and characterization of CCa-MSCs by the explant culture method which generates a homogenous cell population free from contamination of other cell types [17–20].
Materials and reagents
Biological materials
1. Biopsy samples were collected from three treatment-naive cervical cancer patients diagnosed with squamous cell carcinoma or adenocarcinoma (Table 1). The sample was collected by the consulting gynecologist in the Department of Obstetrics and Gynecology, PGIMER Chandigarh, after obtaining proper informed consent and approval by the Institute Ethics Committee.
Table 1. Clinical details of the cervical cancer patients
| Patient ID | Age of the patient | Cervical cancer subtype |
|---|---|---|
| P1 | 54 | Adenocarcinoma |
| P2 | 65 | Moderately differentiated squamous cell carcinoma |
| P3 | 49 | Moderately differentiated squamous cell carcinoma |
Reagents
1. Alizarin red S stain (Sigma-Aldrich, catalog number: TMS-008)
2. Antibiotic antimycotic cocktail (Himedia, catalog number: A002-5X50ML)
3. Ascorbic acid (Himedia, catalog number: TC094-25G)
4. Dexamethasone 21-phosphate sodium (Sigma-Aldrich, catalog number: D1159-100MG)
5. DMEM low glucose (Gibco, catalog number: 11885-084)
6. Dulbecco’s phosphate-buffered saline (PBS) (Himedia, catalog number: TS1006-5L)
7. EZ Stain Chondrocyte Staining kit (Alcian blue stain) (Himedia, catalog number: CCK029)
8. Fetal bovine serum (FBS) (Gibco, catalog number: 16000044)
9. Human TGF-β3 (Peprotech, catalog number: 100-36E-10U)
10. Insulin-Transferrin-Selenous acid premix (Sigma-Aldrich, catalog number: I3146)
11. Monopotassium phosphate (Sigma-Aldrich, catalog number: 15655-100G)
12. Oil red O stain (Sigma-Aldrich, catalog number: 01391-250Ml)
13. Sodium pyruvate solution (Sigma-Aldrich, catalog number: S8636-100ML)
14. StemPro Adipogenesis Differentiation kit (Thermo Fisher Scientific, catalog number: A1007001)
15. TrypLE Express enzyme (Gibco, catalog number: 12604021)
16. β-glycerophosphate disodium salt hydrate (Sigma-Aldrich, catalog number: G9422-50G)
17. 10% neutral buffered formalin solution (Sigma-Aldrich, catalog number: HT501128-4L)
Antibodies
1. PE Mouse anti-human CD105 antibody (1:50) (BD-Pharmingen, catalog number: 560839)
2. FITC anti-human CD34 antibody (1:50) (BioLegend, catalog number: 343604)
3. FITC anti-human CD45 antibody (1:50) (BioLegend, catalog number: 304006)
4. FITC anti-human CD73 antibody (1:50) (BioLegend, catalog number: 344016)
5. FITC anti-human CD90 antibody (1:50) (BioLegend, catalog number: 328107)
6. PE anti-human HLA-DRHLA-DR (1:50) (BioLegend, catalog number: 307065)
Solutions
1. CCa-MSCs culture media (see Recipes)
2. PBS (see Recipes)
3. Chondrogenic differentiation media (see Recipes)
4. Osteogenic differentiation media (see Recipes)
5. Osteocyte staining solution (Alizarin Red S stain) (see Recipes)
6. Adipocyte staining solution (Oil Red O stain) (see Recipes)
Recipes
1. CCa-MSCs culture media
Supplement DMEM (1 g/L) with 10% FBS and 1× antibiotic antimycotic solution (100 U penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B).
2. PBS
Dissolve 9.6 g of PBS in sterile water and autoclave. Add 1× antibiotic antimycotic solution (100 U penicillin, 100 μg of streptomycin, and 0.25 μg of amphotericin B). Filter the PBS solution.
3. Chondrogenic differentiation media
| Reagent | Stock concentration | Final concentration | Volume |
|---|---|---|---|
| Ascorbic acid | 200 M | 0.2 M | 50 μL |
| Sodium pyruvate | 100 mM | 1 mM | 500 μL |
| Human TGF-β3 | 250 mM | 10 μM | 2 μL |
| Dexamethasone 21-phosphate sodium | 10 M | 1 mM | 5 μL |
| Insulin-Transferrin-Selenous acid premix | 1,000× | 1× | 50 μL |
| DMEM low glucose | n/a | n/a | 50 mL |
| Antibiotic-antimycotic solution | 100× | 1× | 500 μL |
| Total | n/a | 50 mL |
Note: Filter the differentiation media using a 0.22 μm syringe filter after mixing all the components. Prepare the stock solutions in sterile water. Individual components can be stored at -20 °C. Check for any precipitates after freeze thaw.
4. Osteogenic differentiation media
| Reagent | Stock concentration | Final concentration | Volume |
|---|---|---|---|
| β-glycerophosphate disodium salt hydrate | 2.5 M | 5 mM | 100 μL |
| Monopotassium phosphate | 0.5 M | 1.8 mM | 100 μL |
| Dexamethasone 21-phosphate sodium | 1 mM | 0.01 mM | 0.25 μL |
| DMEM low glucose | n/a | n/a | 50 mL |
| Antibiotic-antimycotic solution | 100× | 1× | 500 μL |
| Total | n/a | 50 mL |
Note: Filter the differentiation media using a 0.22 μm syringe filter after mixing all the components. Prepare the stock solutions in sterile water. Individual components can be stored at -20 °C. Check for any precipitates after freeze thaw.
5. Osteocyte staining solution (Alizarin Red S stain)
Note: Adjust the pH of the staining solution to 4.1–4.3 with 0.1% ammonium hydroxide or 0.1% HCl. Make up the total volume to 100 mL after adjusting the pH of the solution. Filter the solution through Whatman filter paper to remove any undissolved components.
| Reagent | Final concentration | Quantity |
|---|---|---|
| Alizarin Red S | n/a | 2 g |
| dH2O | n/a | 100 mL |
| Total | n/a | 100 mL |
6. Adipocyte staining solution (Oil Red O stain)
Note: Filter the solution through Whatman filter paper to remove any undissolved components.
| Reagent | Final concentration | Volume |
|---|---|---|
| Oil Red O stain | n/a | 30 mL |
| dH2O | n/a | 60 mL |
| Total | n/a | 90 mL |
Laboratory supplies
1. 6-well cell culture plate (Corning, catalog number: 3516)
2. T-25 flasks (Corning, catalog number: 430639)
3. T-75 flasks (Corning, catalog number: 430641U)
4. Petri dish (Tarson, catalog number: 460090-90MM)
5. 0.22 μm Whatman filter paper (Merck, catalog number: WHA1001325)
Equipment
1. EVOS LED microscope (Invitrogen)
2. FACS Canto flow cytometer (BD Biosciences)
3. Inverted microscope (Leica)
4. Centrifuge (ifuge L400P, model: LC01-3020 EU/AUS/IND)
5. Biosafety cabinet (Nuaire, model: NU-437-4009)
6. Surgical tools (scalpel and forceps)
7. CO2 incubator (Nuaire)
8. Hemocytometer (depth 0.1 mm) (Perfect Improved Neubauer)
9. -20 °C freezer (Vest Frost Solutions)
10. -80 °C freezer (Thermo Scientific)
11. Refrigerator (Samsung, model: RT28M3022S8/HL/2018)
12. 0.5–10 μL single channel variable pipette (Eppendorf, model: Research® plus, catalog number: 3125000028)
13. 10–100 μL single channel variable pipette (Eppendorf, model: Research® plus, catalog number: 3123000047)
14. 100–1,000 μL single channel variable pipette (Eppendorf, model: Research® plus, catalog number: 3123000063)
15. Autoclave (Equitron)
16. Mr. FrostyTM freezing container (Thermo Scientific, catalog number: 5100-0001)
17. Weighing balance (Wensar)
Software and datasets
1. BD FACSDiva 8.0.3
Procedure
A. Collection and transportation of biopsy samples to the tissue culture laboratory
1. Collect cervical cancer tissue from patients, making sure that the sample is collected under aseptic conditions by a qualified physician.
2. Collect the tissue in a sterile specimen container containing cold 1× sterile PBS, seal the specimen container with parafilm, and immediately transport it to the laboratory in an ice box (Figure 1i).
3. Process the sample within 1 h of collection to establish the primary culture.
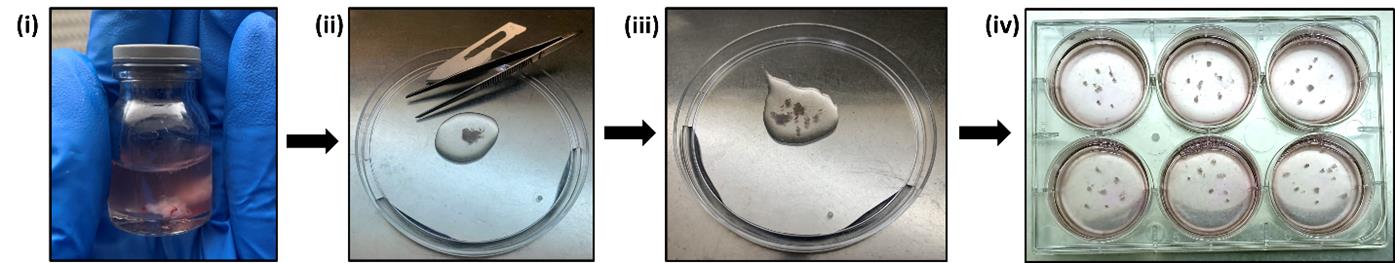
Figure 1. Stepwise protocol for establishing explant culture. (i) A biopsy sample was brought to the laboratory in PBS, (ii) washed with ice-cold PBS 3–4 times, and (iii) dissected into small pieces. (iv) 5–6 pieces were placed in each well of a 6-well culture plate.
B. Establishment of primary culture of CCa-MSCs from patient-derived cervical cancer biopsy
Note: Once the tissue has been carefully transported to the tissue culture laboratory, perform the subsequent steps in a biosafety cabinet level 2.
1. Using a pair of clean forceps, carefully place the tissue in a sterile Petri dish (100 mm × 20 mm) and rinse the tissue at least two to three times with 1× sterile PBS to get rid of any blood or any clots from the tissue (Figure 1ii).
Note: Since the cervical cancer tissue is highly vascularized and has an abundant blood supply, removing the blood from the tissue may require numerous rinsings with cold 1× PBS (see General note 1).
2. Transfer the tissue to another Petri plate containing sterile 1× PBS and dissect the tissue into small fragments (1–2 mm) using a pair of forceps and a sterile surgical blade (Figure 1iii).
3. Once the fragments have been cut, add 900–1,000 μL of DMEM to a 6-well culture plate.
4. Place 6–8 tissue fragments into each well of the 6-well cell culture plate using a pair of forceps and blades. Let the plate stay in the biosafety cabinet for 4–5 min to allow the explants to adhere well to the surface of the cell culture plate (Figure 1iv).
5. Carefully transport the plate to a humidified incubator maintained at 37 °C and 5% CO2 without disturbing the explants.
6. Next day, slightly tilt the plate, aspirate the media from all the wells, and add 900 μL of fresh CCa-MSCs culture media in each well gently along the sides of the wall, making sure not to disturb the explants at any step. Thereafter, change the media every 2 days.
7. Monitor the explants under an inverted microscope on a regular basis and observe the migration of spindle-like cells out of the explant (Figure 2).
Note: At this stage, CCa-MSCs can be identified based on their ability to adhere to the plastic surface of the cell culture dish and their spindle-shaped morphology.
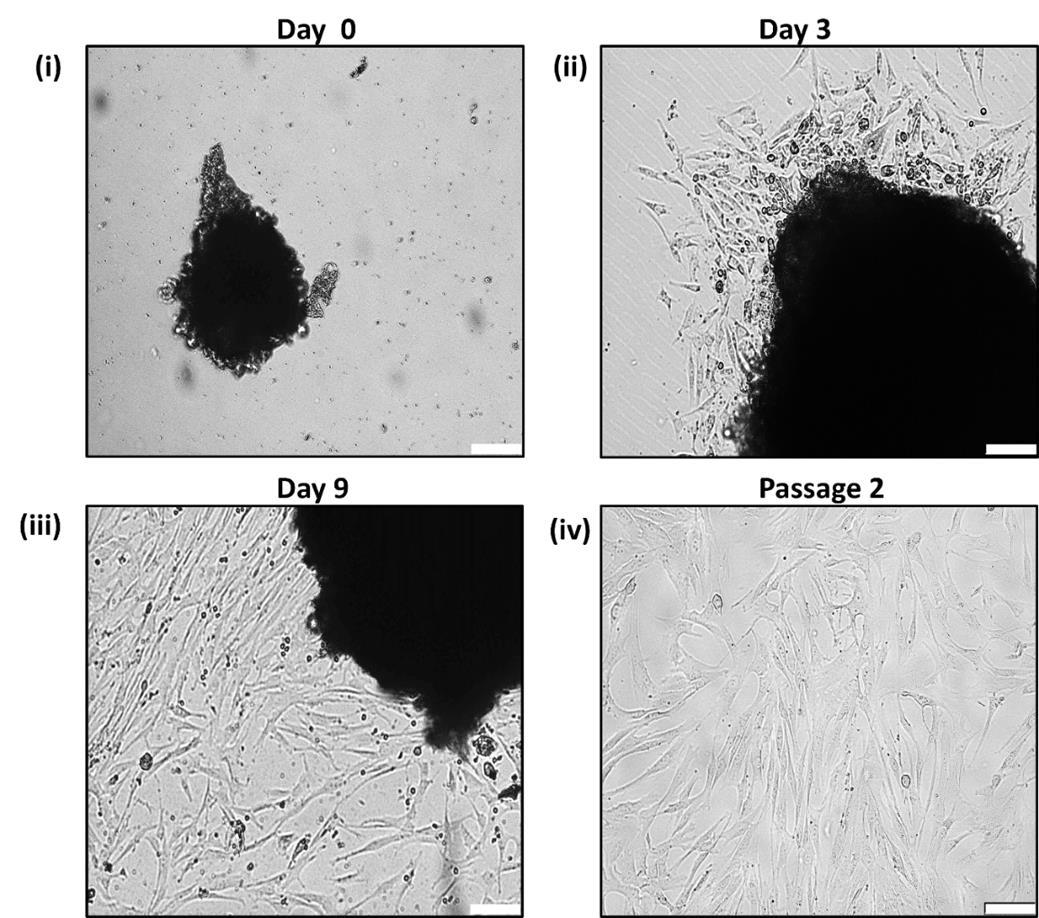
Figure 2. Primary culture of cervical cancer–associated MSCs (CCa-MSCs). (i) A single explant attached to the surface of the culture plate. (ii) Adherent spindle-shaped cells migrating out of the explants on Day 3. (iii) The explant surrounded by more adherent cells by Day 9. (iv) Spindle-shaped morphology of CCa-MSCs at passage 2. Scale bar = 100 μm.
8. Once a sufficient number of cells migrate out of the explants, remove the explants and allow the cells to proliferate and reach 70%–80% confluency for subculture as well as characterization (Figure 2).
9. For subculturing, decant the media from all wells of the cell culture plate and wash the cells with 1 mL of 1× PBS. Add 500 μL of 1× TrypLE Express enzyme to cover the entire surface of the cell culture dish.
10. Transfer the cell culture plate to the incubator and keep the flask at 37 °C for 1–2 min. Then, observe the cells under the microscope to check if they have detached from the surface of the plate.
Note: CCa-MSCs detach from the surface of the cell culture plate within 1–2 min after the addition of TrypLE. In case they do not detach, gently tap the plate from the sides and incubate it for an additional 1–2 min.
11. Add 1 mL of 1× PBS to all the wells of the cell culture dish and transfer the cell suspension to a fresh 15 mL conical centrifuge tube. Centrifuge the cell suspension at 570× g for 5 min at room temperature in a swinging bucket rotor to get a cell pellet at the bottom of the tube.
12. Decant the supernatant carefully without disturbing the pellet and add 5 mL of fresh CCa-MSCs culture media to the pellet.
13. Resuspend the pellet carefully to get a single cell suspension, transfer it to a T25 flask and keep it in the incubator at 37 °C and 5% CO2.
14. The next day, replace the media with fresh CCa-MSCs culture media and thereafter change the media every 2 days.
C. Expansion of CCa-MSCs culture
1. Decant the cell culture media from a confluent CCa-MSCs T25 cell culture flask and rinse it with 5 mL of 1× PBS.
2. Pipette 1 mL of 1× TrypLE Express enzyme into the flask and slowly move it in a figure-of-eight motion so that TrypLE evenly covers the surface of the flask.
3. Transfer the flask back to the incubator and keep it at 37 °C for 1–2 min. Then, observe the cells under the microscope to check if they have detached from the surface of the flask.
4. Add 2 mL of 1× PBS to the flask and transfer the cell suspension to a fresh and sterile 15 mL conical centrifuge tube. Add 2 mL of 1× PBS again to every well and transfer it again to the centrifuge tube to remove all cells from the flask.
5. Centrifuge the cell suspension at 570× g for 5 min at room temperature. Decant the supernatant carefully and add 1 mL of fresh CCa-MSCs culture media to the tube. Resuspend the cell pellet gently using a pipette to get a single-cell suspension.
6. Seed 6 × 105 cells/mL into T75 flasks containing 10 mL of CCa-MSCs culture media and keep the flasks in the incubator at 37 °C and 5% CO2.
7. Replace the media with fresh CCa-MSCs culture media on the subsequent day to remove any dead cells. Thereafter, change the media every 2 days.
D. Characterization of CCa-MSCs
The characterization of CCa-MSCs for all samples was done at passages 3 and 4.
D1. Phenotypic characterization of CCa-MSCs
1. Collect cells from a T75 flask (70%–80% confluent) containing approximately 20 × 105 cells at passage 4.
2. Resuspend the cell pellet in 1 mL of 1× PBS by pipetting gently and obtain a single-cell suspension.
3. Count the cells using a hemocytometer and divide the cell suspension into 5 microcentrifuge tubes containing approximately 105 cells per tube. Add 2 μL of fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated antibodies for cluster of differentiation (CD) markers CD90, CD73, CD105, CD34, CD45, and HLA-DR and incubate for 30 min in the dark at room temperature [20].
Note: Unstained cells and isotype controls must be used while preparing cells for acquisition to get rid of any background fluorescence.
4. Centrifuge at 570× g for 5 min to pellet down the cells and discard the supernatant. Add 1 mL of PBS to all tubes and centrifuge again to remove excess antibodies.
5. Resuspend the labeled cell pellet in 200 μL of 1× PBS and transfer the cell suspension to flow cytometry tubes for acquisition. The percentages of cells positive and negative for the above-mentioned markers can be assessed.
D2. Trilineage differentiation of CCa-MSCs
To analyze the differentiation potential of CCa-MSCs, cells between passages 3 and 5 should be used, and a control group containing only media should be cultured along with them.
• To set up the trilineage differentiation experiment, detach cells from a 70%–80% confluent flask as previously described and resuspend the cells in complete DMEM low-glucose media.
• Count the cells and seed 4 × 104 cells per well of a 24-well cell culture plate containing 500 μL of media and allow them to proliferate for further induction of differentiation.
1. Adipogenic differentiation
a. Once cells reach 40%–50% confluency, remove the media from all wells and add 400 μL of adipogenic differentiation media (from StemPro Adipogenesis Differentiation kit) to each well. For control wells, add only DMEM low glucose media containing no inductive factors (as per the manufacturer’s protocol). Change the media every 2 days and culture the cells for 18 days.
b. Following the end of the induction period, remove the media from the induced and control wells and wash the cells thrice with 1× PBS.
c. Fix the cells with 10% neutral buffered formalin solution for 20 min at room temperature.
d. Wash the fixed cells thrice with 1× PBS, add 400 μL of oil red O stain to each well, and incubate at room temperature for 40–45 min.
e. Remove the stain and rinse all wells with ddH2O at least 3–4 times to remove the excess stain. Observe the cells under a brightfield microscope for the formation of lipid droplets at 20× and 40×.
2. Osteogenic differentiation of CCa-MSCs
a. Once the CCa-MSCs monolayer reaches 40%–50% confluency, remove the media from all wells with the help of a pipette and then add osteogenic differentiation media. Simultaneously, in the control wells, add 400 μL of only DMEM low glucose media [21,22].
b. Change the media every 2 days and culture the cells for 18 days.
c. After 18 days of differentiation, remove the media from all wells and rinse the cells thrice with 1× PBS.
d. Fix the cells with 10% neutral buffered formalin solution for 20 min at room temperature and proceed with Alizarin red S staining that binds to calcium in osteocytes.
e. For staining, wash the fixed cells thrice with 1× PBS. Add 400 μL of the prepared stain solution and incubate at room temperature for 40–45 min.
f. Remove the stain and wash the cells at least 3–4 times with ddH2O to remove any excess stain. Observe the differentiated cells under the brightfield microscope at 4× and 10× magnification.
3. Chondrogenic differentiation of CCa-MSCs
a. Once the CCa-MSCs monolayer reaches 40%–50% confluency, remove the media from all wells with the help of a pipette and then add chondrogenic differentiation media.
b. Simultaneously, in the control wells, add 400 μL of only DMEM low glucose media without any chondrogenic induction components.
c. Change the media every 2 days and culture the cells for 18 days.
d. After 18 days of differentiation, remove the media from all wells and rinse the cells thrice with 1× PBS.
e. Fix the cells with 10% neutral buffered formalin solution for 20 min at room temperature.
f. Wash the cells thrice with 1× PBS and add EZ stain chondrocyte staining solution (alcian blue stain) that strongly binds to sulphated glycosaminoglycans and glycoproteins in cartilage. Incubate the cells at room temperature for 40–45 min.
g. Remove the stain and wash the cells at least 3–4 times with ddH2O to remove any excess stain. Observe the cells under the brightfield microscope at 4× and 10× magnification.
Data analysis
To characterize MSC surface antigens using antibodies against specific markers, at least 10,000 events were recorded on a BD FACScanto flow cytometer. BD FACSDiva 8.0.3 software was used to analyze flow cytometry data.
Validation of protocol
The protocol for isolation and characterization of CCa-MSCs has been validated on three different patient samples. In all three patient samples, the cells that migrated out of the explants showed adherence to the plastic surface with spindle-shaped morphology. Characterization of the isolated cells for cell surface markers showed high expression (>94%) for mesenchymal markers CD73, CD105, and CD90 and negligible expression (<1%) for hematopoietic marker CD45, endothelial marker CD34, and HLA-DR (Table 2, Figure 3). Further, isolated cells showed the ability to differentiate into osteogenic, chondrogenic, and adipogenic lineages when stimulated with respective differentiation media (Figure 4). These observations confirm that the isolated cells are mesenchymal stem cells. Since the cells were isolated from confirmed cases of cervical cancer, these can be called cervical cancer–associated mesenchymal stem cells.
Table 2. Phenotypic characterization of MSC-specific cell surface markers through flow cytometry
| Cell surface markers | P1 | P2 | P3 |
|---|---|---|---|
| Unstained | 99.55 ± 0.05 | 99.90 ± 0.0 | 99.5 ± 0.0 |
| CD73 | 99.55 ± 0.05 | 94.50 ± 0.40 | 98.5 ± 0.0 |
| CD105 | 97.35 ± 0.25 | 86.55 ± 0.05 | 99.15 ± 0.05 |
| CD90 | 98.35 ± 0.35 | 98.55 ± 0.15 | 99.25 ± 0.15 |
| CD45 | 0.2 ± 0.0 | 0.0 ± 0.0 | 0.30 ± 0.0 |
| CD34 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| HLA-DR | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
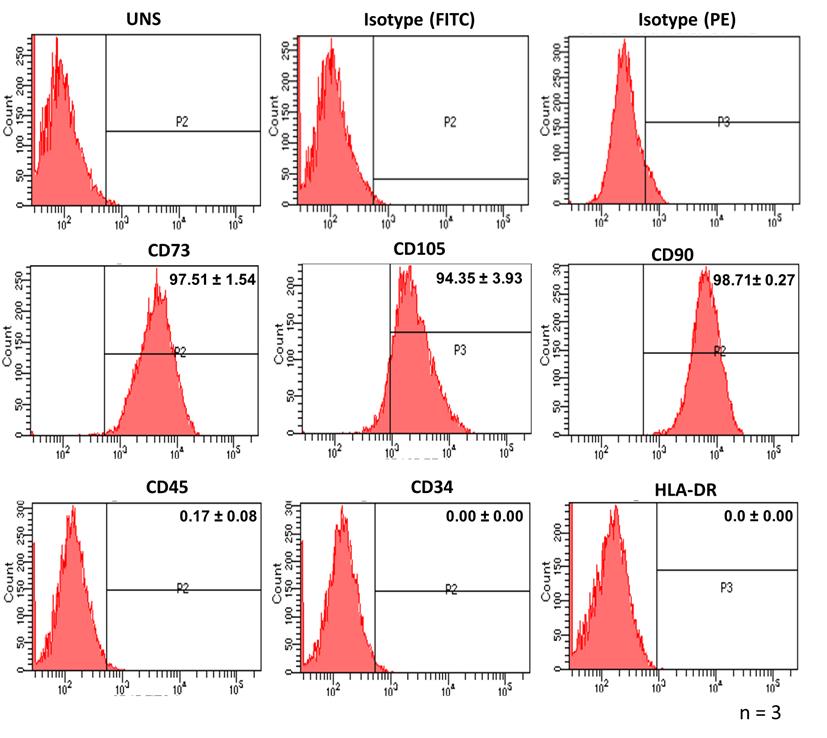
Figure 3. Characterization of cervical cancer–associated MSCs (CCa-MSCs) for cell surface markers. Characterization of cell surface markers by flow cytometry shows positive expression of MSC-specific surface markers (CD73, CD105, and CD90) and negative expression for hematopoietic cell marker (CD45), endothelial cell marker (CD34), and HLA-DR. The experiment was done in duplicates on three different patient samples.
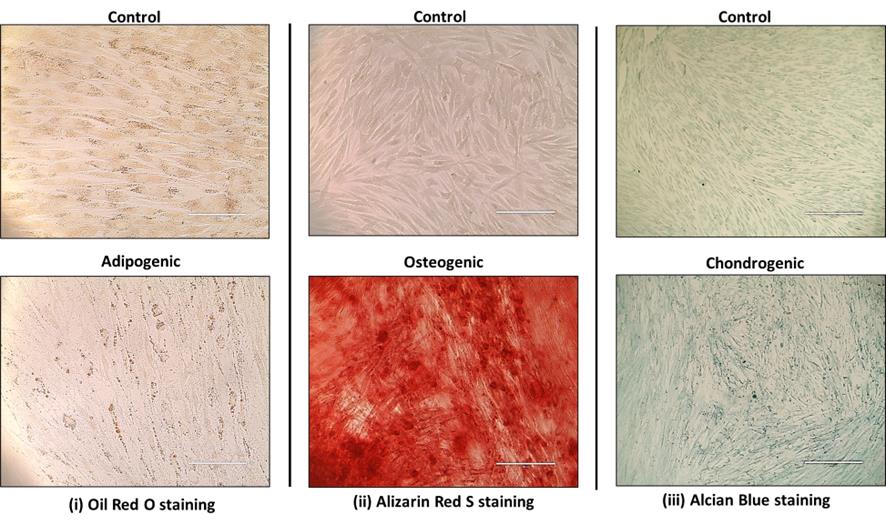
Figure 4. Trilineage differentiation of cervical cancer–associated MSCs (CCa-MSCs). Brightfield microscopic images showing the trilineage differentiation of isolated CCa-MSCs as depicted by uptake of (i) Oil red O, (ii) alizarin red S, and (iii) alcian blue stain by adipocytes, osteocytes, and chondrocytes, respectively. Scale bar = 100 μm (i), 200 μm (ii), and 400 μm (iii). Each experiment was done in triplicate on three different patient samples.
General notes and troubleshooting
General notes
1. Cervical cancer patients are mostly immunocompromised and are more prone to bacterial or fungal infection of the cervical region. Thus, there are increased chances of cervical cancer patient samples being contaminated with bacteria or fungi. Therefore, the explants should be washed at least 3 times with PBS before seeding.
Troubleshooting
Problem 1: It may be difficult to remove RBCs from the sample.
Possible cause: The cervix sample is highly vascularized.
Solution: Wash the sample at least 3 times with 1× ice-cold PBS. If RBCs persist even after PBS wash, the tissue can be treated with ACK lysis buffer for 5–10 min.
Problem 2: Improper acquisition of the cells in the flow cytometer.
Possible cause: Cells might have formed aggregates in the cell suspension.
Solution: Resuspend the cells properly to form a single-cell suspension.
Problem 3: Cells may not be stained by Alizarin Red S.
Possible cause: The pH of the stain is not appropriate.
Solution: Prepare fresh Alizarin red S staining solution with a pH 4.1–4.3.
Problem 4: Cells may not be stained using the EZ Stain Chondrocyte Staining kit.
Possible cause: The pH of the stain is not appropriate.
Solution: Make sure that the chondrocyte staining solution has pH 2.5.
Acknowledgments
The authors gratefully acknowledge the extramural funding received from ICMR, New Delhi (ID-2020-4349). S.S. gratefully acknowledges the Council of Scientific and Industrial Research (CSIR) for providing a research fellowship. S.K. gratefully acknowledges the University Grants Commission (UGC) for providing a research fellowship. The authors are grateful to the CSIC facility and PGIMER for assistance in conducting the experiments.
Competing interests
The authors declare that there are no competing interests.
Ethical considerations
All the procedures mentioned in this study were approved by the Institute Ethics Committee (IEC no. PGI/IEC/2020/000365) of P.G.I.M.E.R., Chandigarh. Biopsy samples were collected from patients diagnosed with different subtypes of cervical cancer after obtaining written informed consent from the patients as per the guidelines of the ethics committee of P.G.I.M.E.R., Chandigarh. Biopsy sample collection was done by a consulting gynecologist, and cytological analysis was done by a pathologist at the institute.
References
- Li, W., Yang, J., Zheng, P., Li, H. and Zhao, S. (2021). The Origins and Generation of Cancer-Associated Mesenchymal Stromal Cells: An Innovative Therapeutic Target for Solid Tumors. Front Oncol. 11: e723707. https://doi.org/10.3389/fonc.2021.723707
- Aravindhan, S., Ejam, S. S., Lafta, M. H., Markov, A., Yumashev, A. V. and Ahmadi, M. (2021). Mesenchymal stem cells and cancer therapy: insights into targeting the tumour vasculature. Cancer Cell Int. 21(1): 158. https://doi.org/10.1186/s12935-021-01836-9
- Shi, Y., Du, L., Lin, L. and Wang, Y. (2016). Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discovery. 16(1): 35–52. https://doi.org/10.1038/nrd.2016.193
- Li, X., Fan, Q., Peng, X., Yang, S., Wei, S., Liu, J., Yang, L. and Li, H. (2022). Mesenchymal/stromal stem cells: necessary factors in tumour progression. Cell Death Discovery. 8(1): 333. https://doi.org/10.1038/s41420-022-01107-0
- Scioli, M. G., Storti, G., D’Amico, F., Gentile, P., Kim, B. S., Cervelli, V. and Orlandi, A. (2019). Adipose-Derived Stem Cells in Cancer Progression: New Perspectives and Opportunities. Int J Mol Sci. 20(13): 3296. https://doi.org/10.3390/ijms20133296
- Nishikawa, G., Kawada, K., Nakagawa, J., Toda, K., Ogawa, R., Inamoto, S., Mizuno, R., Itatani, Y. and Sakai, Y. (2019). Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 10(4): 264. https://doi.org/10.1038/s41419-019-1508-2
- Frisbie, L., Buckanovich, R. J. and Coffman, L. (2022). Carcinoma-Associated Mesenchymal Stem/Stromal Cells: Architects of the Pro-tumorigenic Tumor Microenvironment. Stem Cells. 40(8): 705–715. https://doi.org/10.1093/stmcls/sxac036
- Antoon, R., Overdevest, N., Saleh, A. H. and Keating, A. (2024). Mesenchymal stromal cells as cancer promoters. Oncogene. 43(49): 3545–3555. https://doi.org/10.1038/s41388-024-03183-1
- Di Fiore, R., Suleiman, S., Drago-Ferrante, R., Subbannayya, Y., Pentimalli, F., Giordano, A. and Calleja-Agius, J. (2022). Cancer Stem Cells and Their Possible Implications in Cervical Cancer: A Short Review. Int J Mol Sci. 23(9): 5167. https://doi.org/10.3390/ijms23095167
- Wang, K. H. and Ding, D. C. (2022). Role of cancer-associated mesenchymal stem cells in the tumor microenvironment: A review. Tzu Chi Med J. 35(1): 24–30. https://doi.org/10.4103/tcmj.tcmj_138_22
- McLean, K., Gong, Y., Choi, Y., Deng, N., Yang, K., Bai, S., Cabrera, L., Keller, E., McCauley, L., Cho, K. R., et al. (2011). Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest. 121(8): 3206–3219. https://doi.org/10.1172/jci45273
- Korkaya, H., Liu, S. and Wicha, M. S. (2011). Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest. 121(10): 3804–3809. https://doi.org/10.1172/jci57099
- Yan, X. L., Jia, Y. L., Chen, L., Zeng, Q., Zhou, J. N., Fu, C. J., Chen, H. X., Yuan, H. F., Li, Z. W., Shi, L., et al. (2013). Hepatocellular carcinoma-associated mesenchymal stem cells promote hepatocarcinoma progression: Role of the S100A4-miR155-SOCS1-MMP9 axis. Hepatology. 57(6): 2274–2286. https://doi.org/10.1002/hep.26257
- Coffman, L. G., Pearson, A. T., Frisbie, L. G., Freeman, Z., Christie, E., Bowtell, D. D. and Buckanovich, R. J. (2018). Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma. Stem Cells. 37(2): 257–269. https://doi.org/10.1002/stem.2932
- Yan, C., Chang, J., Song, X., Qi, Y., Ji, Z., Liu, T., Yu, W., Wei, F., Yang, L. and Ren, X.(2021). Lung cancer-associated mesenchymal stem cells promote tumor metastasis and tumorigenesis by induction of epithelial–mesenchymal transition and stem-like reprogram. Aging. 13(7): 9780. https://doi.org/10.18632/aging.202732
- Wang, M., Zhao, C., Shi, H., Zhang, B., Zhang, L., Zhang, X., Wang, S., Wu, X., Yang, T., Huang, F., et al. (2014). Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 110(5): 1199–1210. https://doi.org/10.1038/bjc.2014.14
- de Lourdes Mora-García, M., García-Rocha, R., Morales-Ramírez, O., Montesinos, J. J., Weiss-Steider, B., Hernández-Montes, J., Ávila-Ibarra, L. R., Don-López, C. A., Velasco-Velázquez, M. A., Gutiérrez-Serrano, V., et al. (2016). Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 14(1): 302. https://doi.org/10.1186/s12967-016-1057-8
- Hilkens, P., Gervois, P., Fanton, Y., Vanormelingen, J., Martens, W., Struys, T., Politis, C., Lambrichts, I. and Bronckaers, A. (2013). Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 353(1): 65–78. https://doi.org/10.1007/s00441-013-1630-x
- Hendijani, F. (2017). Explant culture: An advantageous method for isolation of mesenchymal stem cells from human tissues. Cell Proliferation. 50(2): e12334. https://doi.org/10.1111/cpr.12334
- Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, D., Horwitz, E., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8(4): 315–317. https://doi.org/10.1080/14653240600855905
- Raik, S., Kumar, A., Rattan, V., Seth, S., Kaur, A. and Bhatta charyya, S. (2019). Assessment of Post-thaw Quality of Dental Mesenchymal Stromal Cells After Long-Term Cryopreservation by Uncontrolled Freezing. Appl Biochem Biotechnol. 191(2): 728–743. https://doi.org/10.1007/s12010-019-03216-6
- Kumar, A., Bhattacharyya, S. and Rattan, V. (2015). Effect of uncontrolled freezing on biological characteristics of human dental pulp stem cells. Cell Tissue Bank. 16(4): 513–522. https://doi.org/10.1007/s10561-015-9498-5
Article Information
Publication history
Received: Dec 15, 2024
Accepted: May 18, 2025
Available online: Jun 12, 2025
Published: Jun 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Singla, S., Khurana, S., Bagga, R., Srinivasan, R. and Bhattacharyya, S. (2025). Isolation and Characterization of Cervical Cancer-Associated Mesenchymal Stem Cells From Primary Tumors Using Explant Culture. Bio-protocol 15(12): e5358. DOI: 10.21769/BioProtoc.5358.
Category
Cancer Biology > Cancer stem cell > Tumor microenvironment
Cell Biology > Cell isolation and culture > Monolayer culture
Stem Cell > Adult stem cell > Cancer stem cell
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








