- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Evaluating In Vivo Translation Inhibition via Puromycin Labeling in Botrytis cinerea Germlings Treated With Antifungal Peptides
Published: Vol 15, Iss 6, Mar 20, 2025 DOI: 10.21769/BioProtoc.5250 Views: 2515
Reviewed by: Ritu GuptaYuko KuritaAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Reliable In Planta Inoculation and Antifungal Screening Protocol for Rhizoctonia solani-Induced Sheath Blight in Rice
Alinaj Yasin [...] Palash Deb Nath
Nov 5, 2025 1576 Views

In Silico Prediction and In Vitro Validation of Bacterial Interactions in the Plant Rhizosphere Using a Synthetic Bacterial Community
Arijit Mukherjee [...] Sanjay Swarup
Nov 5, 2025 1659 Views

Reproducible Emu-Based Workflow for High-Fidelity Soil and Plant Microbiome Profiling on HPC Clusters
Henrique M. Dias [...] Christopher Graham
Jan 20, 2026 421 Views
Abstract
Antimicrobial peptides are effective agents against various pathogens, often targeting essential processes like protein translation to exert their antimicrobial effects. Traditional methods such as puromycin labeling have been extensively used to measure protein synthesis in mammalian and yeast systems; however, protocols tailored for plant pathogenic filamentous fungi, particularly those investigating translation inhibition by antifungal peptides, are lacking. This protocol adapts puromycin labeling to quantify translation inhibition in Botrytis cinerea germlings treated with antifungal peptides. Optimizing the method specifically for fungal germlings provides a precise tool to investigate peptide effects on fungal protein synthesis, advancing our understanding of translation dynamics during pathogen–host interactions in filamentous fungi.
Key features
• This protocol is designed for in vivo experiments, enabling the estimation of protein translation inhibition by antifungal peptides in Botrytis cinerea.
• It is optimized for antifungal peptides capable of penetrating fungal cells and inhibiting translation, making it applicable to other filamentous fungal pathogens.
• The protocol is ideal for laboratories utilizing traditional western blotting techniques, ensuring accessibility and ease of implementation.
• This protocol for in vivo application in filamentous fungi is adapted from the SUnSET method [1].
Keywords: Puromycin labelingGraphical overview
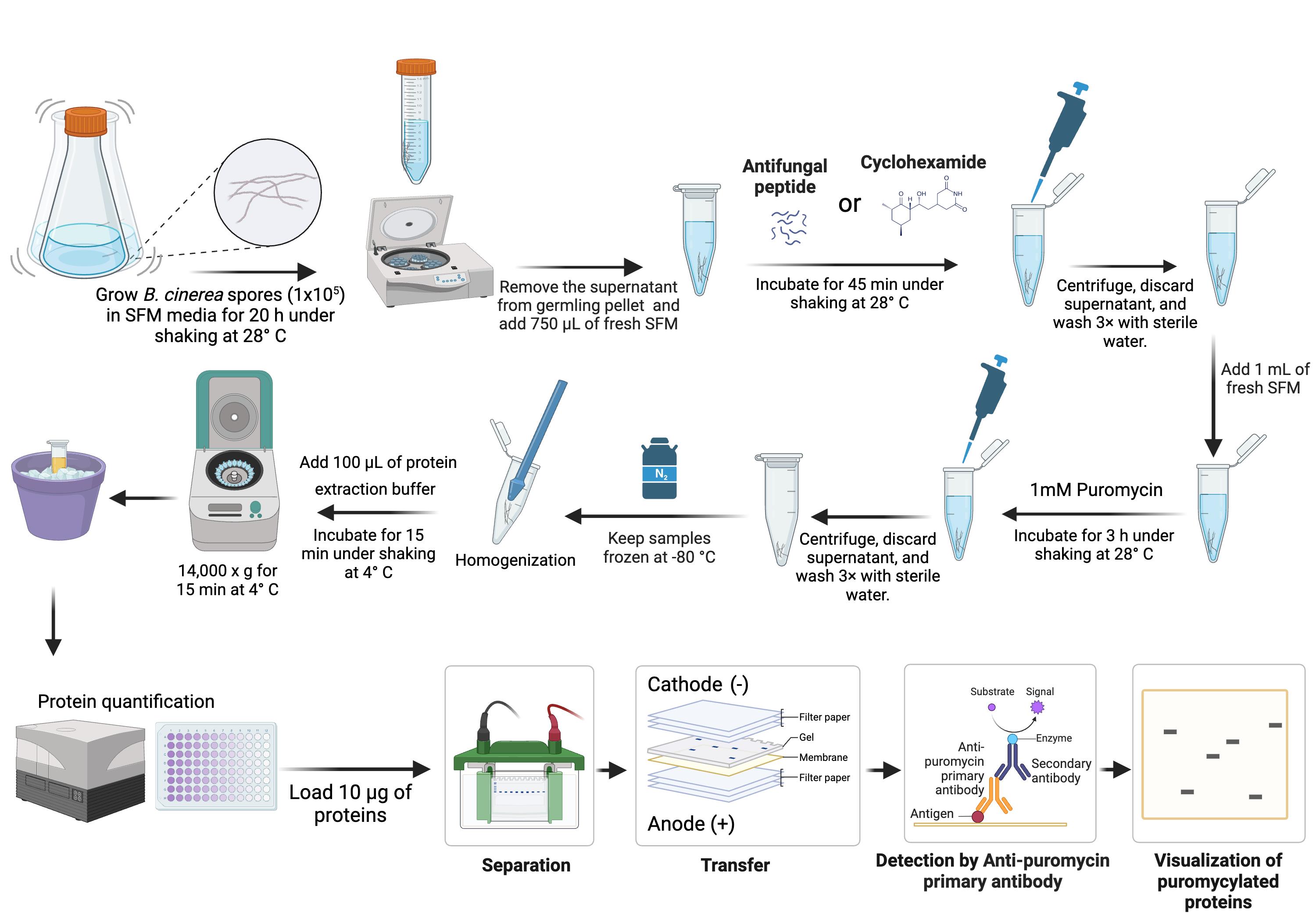
Background
Antifungal peptides have emerged as powerful agents in the fight against fungal pathogens [2]. These peptides are particularly promising in agriculture, where fungal pathogens cause significant crop losses [3]. Understanding their modes of action, such as their inhibitory effects on fungal protein synthesis, is critical for developing innovative and sustainable strategies to manage fungal diseases. Among existing methodologies, puromycin labeling has been extensively used in mammalian and yeast systems to measure protein synthesis. Puromycin, an aminoacyl-tRNA analog, is selectively incorporated into nascent polypeptides on actively elongating ribosomes [4]; puromycin labeling exploits puromycin’s ability to be incorporated into elongating polypeptide chains, providing a direct and reliable assessment of translation activity. However, protocols specifically designed to study protein translation inhibition in filamentous fungal pathogens, such as Botrytis cinerea, are lacking. Filamentous fungi present unique challenges due to their complex cellular structures, growth patterns, and physiological processes, which differ significantly from those of yeast and mammalian cells. As a result, traditional puromycin labeling protocols often require substantial adaptation to suit the biology of these organisms. By adapting puromycin labeling to B. cinerea, this protocol enables the precise quantification of protein translation inhibition following treatment with previously characterized antifungal peptide disulfide variants, such as NCR13_PFV1 and NCR13_PFV2 [5]. Prior work from our lab [5] has demonstrated that NCR13_PFV1 exhibits stronger translation inhibition than NCR13_PFV2 in a wheat germ in vitro translation system [6]. However, the in vivo translation-inhibitory effects of these peptides in B. cinerea remain to be determined. To address this, we have developed and optimized an in vivo protocol specifically tailored for B. cinerea germlings, enabling precise measurement of translation inhibition by antifungal peptides.
This protocol offers a few key advantages over existing methodologies. First, it is designed for the model fungus B. cinerea germlings, ensuring high sensitivity and reproducibility in measuring translation dynamics. Second, it provides a direct measure of protein translation inhibitory effects of antifungal peptides, allowing researchers to extend its use to other filamentous fungal pathogens. This protocol is also suitable for the identification of novel antifungal compounds that target protein translation in vivo. However, it also has limitations, including the need for further optimization for different fungal species or developmental stages and the potential variability in germling responses across isolates. By addressing a critical methodological gap, this protocol will allow us to explore novel antifungal mechanisms and contribute to the development of effective strategies for managing fungal diseases in agriculture and beyond.
Materials and reagents
Biological materials
1. Pathogen: B. cinerea strain T4 [5]. B. cinerea spores are preserved in 25% glycerol at -80 °C for long-term storage. B. cinerea can be obtained from the American Type Culture Collection (ATCC)
2. Peptides: Nodule-Specific Cysteine-Rich 13 (NCR13) peptides: NCR13_PFV1 and NCR13_PFV2. Details of peptide synthesis and characterization of NCR13_PFV1 and NCR13_PFV2 can be found in [5]. These peptides are stored at -20 °C, and the required peptide concentrations are prepared in sterile Milli-Q water
Reagents
1. Puromycin (Cell Signaling Technology, catalog number: 40939S)
2. 1 M HEPES (Thermo Scientific, catalog number: J61275)
3. EDTA (Sigma-Aldrich, catalog number: E5134)
4. ProBlock gold yeast/fungi protease inhibitor cocktail (GoldBio, catalog number: GB-333-1)
5. 4%–20% mini-PROTEAN TGX stain-free protein gel (Bio-Rad, catalog number: 4568094
6. Bovine serum albumin (BSA) (Goldbio, catalog number: A-421-100)
7. ProSieve QuadColor protein ladder (VWR, catalog number: 89125-526)
8. Cycloheximide (CHX) (Sigma-Aldrich, catalog number: 239763)
9. Mouse anti-puromycin antibody (clone 12D10) (Merck Millipore, catalog number: MABE343)
10. HRP-conjugated anti-mouse secondary antibody (Thermo Fisher Scientific, catalog number: G-21040)
11. Pierce BCA Protein Assay kit (Thermo Scientific, catalog number: 23227)
12. SuperSignal West PICO PLUS chemiluminescent substrate (Thermo Scientific, catalog number: 34580)
13. Triton X-100 (Sigma-Aldrich, catalog number: T8787)
14. V8 vegetable juice (Campbell Soup)
15. Calcium carbonate (Sigma-Aldrich, catalog number: C6763)
16. Potassium hydrogen phosphate (K2HPO) (G-Biosciences, catalog number: RC-081)
17. Magnesium sulfate (MgSO4) (GoldBio, catalog number: 10034-99-8)
18. Ferrous sulfate (FeSO4) (Sigma-Aldrich, catalog number: 12354)
19. Cobalt(II) chloride (CoCl2) (Acros Organics, catalog number: 423571000)
20. Copper(II) sulfate (CuSO4) (Sigma-Aldrich, catalog number: C3036)
21. Sodium molybdate (NaMoO4) (Sigma-Aldrich, catalog number: 243655)
22. Boric acid (HBO) (Sigma-Aldrich, catalog number: B6768)
23. Potassium iodide (KI) (Sigma-Aldrich, catalog number: 746428)
24. Zinc sulfate (ZnSO4) (Sigma-Aldrich, catalog number: Z0501)
25. Manganese (II) sulfate (MnSO4) (Thermo Scientific, catalog number: 033341-36)
26. L-Methionine (Sigma-Aldrich, catalog number: M5308)
27. myo-Inositol (Sigma-Aldrich, catalog number: I7508)
28. Biotin (Sigma-Aldrich, catalog number: B4501)
29. Thiamine hydrochloride (Thermo Scientific, catalog number: 148991000)
30. Pyridoxine hydrochloride (Sigma-Aldrich, catalog number: P8666)
31. D-Glucose (Research Products International, catalog number: G32040)
32. L-Asparagine (Bioworld, catalog number: 40100280)
33. Glycerol (Thermo Scientific, catalog number: 158922500)
34. Bacto agar (Fisher Scientific, catalog number: DF0140-01-0)
35. 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250)
36. Tris base (Sigma-Aldrich, catalog number: 648310)
37. Glycine (Amresco, catalog number: M103)
38. Methanol (VWR, catalog number: 0323-4L)
39. Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 567530)
40. Phosphate buffered saline (PBS), 10× (Thermo Scientific, catalog number: BP39920)
41. Tween 20 (Sigma-Aldrich, catalog number: P1379)
42. Laemmli protein sample buffer 4× (Bio-Rad, catalog number: 1610747)
Solutions
1. 2× Synthetic fungal media (SFM) (see recipes)
2. Protein extraction buffer (see recipes)
3. PBST buffer (see recipes)
4. Western blot (WB) transfer buffer (see recipes)
5. Puromycin stock preparation (see recipes)
6. Cycloheximide stock preparation (see recipes)
7. V8 media (see recipes)
8. 0.5 M EDTA solution (see recipes)
Recipes
1. 2× ynthetic fungal media (SFM) (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Glucose | 20 g/L | 10 g |
| Asparagine | 2 g/L | 1 g |
Dissolve glucose and asparagine in the appropriate volume (474.62 mL) of distilled water and autoclave this solution at 121 °C for 20 min.
After cooling, add the following stock solutions of vitamins, trace elements, and other supplements to the autoclaved solution.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M K2HPO4 | 5 mM | 2.5 mL |
| 0.5 M MgSO4 | 100 μM | 100 μL |
| 0.05 M FeSO4 | 10 μM | 10 μL |
| 0.01 M CoCl2 | 0.2 μM | 10 μL |
| 0.01 M CuSO4 | 0.2 μM | 10 μL |
| 0.05 M Na2MoO4 | 4 μM | 10 μL |
| 0.05 M H3BO3 | 1 μM | 10 μL |
| 0.01 M KI | 0.2 μM | 10 μL |
| 0.05 M ZnSO4 | 1 μM | 10 μL |
| 0.01 M MnSO4 | 0.2 μM | 10 μL |
| 2 g/L Methionine | 40 mg/L | 10 mL |
| 2 g/L Myo-inositol | 4 mg/L | 1 mL |
| 2 g/L Biotin | 0.4 mg/L | 100 μL |
| 2 g/L Thiamine HCl | 2 mg/L | 500 μL |
| 2 g/L Pyridoxine HCl | 0.4 mg/L | 100 μL |
After adding all components, filter sterilize the solution using a bottle-top vacuum filter (0.22 μM). Store the SFM medium at 4 °C until use. The SFM medium has a shelf life of up to 3 months. Dilute in sterile Milli-Q water as necessary.
2. Protein extraction buffer (1 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M HEPES buffer | 100 mM | 100 μL |
| 0.5 M EDTA | 5 mM | 10 μL |
| 100% Triton-X 100 | 0.1% | 1 μL |
| Protease inhibitor cocktail | 1× | 10 μL |
| Milli-Q water | Make up the volume to 1 mL | 880 μL |
Prepare fresh protein extraction buffer for each extraction. Store at 4 °C until use.
3. PBST buffer (200 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× PBS stock solution | 1× | 20 mL |
| Tween-20 | 0.1% (v/v) | 0.2 mL |
| Milli-Q water | Make up the volume to 200 mL | 179.8 mL |
4. Western blot (WB) transfer buffer (200 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 25 mM | 0.605 g |
| Glycine | 192 mM | 2.88 g |
| Methanol | 20% (v/v) | 40 mL |
| Milli-Q water | Make up the volume to 200 mL | 159.4 mL |
5. Puromycin stock preparation
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Puromycin | 50 mM | 27.5 mg in 1 mL |
Freshly prepare in sterile Milli-Q water and keep at -20 °C in aliquots.
6. Cycloheximide stock preparation
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Cycloheximide | 177.6 μM | 50 µg in 1 mL |
Freshly prepare in sterile Milli-Q water and keep at -20 °C in aliquots.
7. V8 media (1000 mL)
| Reagent | Quantity or Volume |
|---|---|
| V8 juice | 200 mL |
| Calcium carbonate | 3 g |
| Bacto agar | 15 g |
| Total | Make up the volume to 1000 mL |
8. 0.5 M EDTA solution (500 mL)
| Reagent | Quantity or Volume |
|---|---|
| EDTA | 93.05 g |
Dissolve in 350 mL of Milli-Q water. Adjust the pH to 8.0 with NaOH (~10 g of NaOH pellets). Make up the volume to 500 mL using Milli-Q water. Dispense into aliquots and sterilize by autoclaving.
Equipment
1. Mini-PROTEAN tetra vertical electrophoresis cell (Bio-Rad, catalog number: 1658004)
2. Trans-Blot Turbo transfer system (Bio-Rad, catalog number: 1704150)
3. ChemiDoc touch imaging system (Bio-Rad, catalog number: 12003153)
4. Pipettes and micropipettes (Gilson)
5. Centrifuge (Eppendorf, model: 5810R)
6. Cold room
7. Heat block (Fisher Scientific, catalog number: 117186)
8. Pestles (Kimble Chase, catalog number: G3956477)
9. Bottle-top vacuum filter (Corning, catalog number: 430521)
10. Improved Neubauer hemocytometer (Grid Optik, model: JSB348)
11. Nitrocellulose membrane (Cytiva, catalog number: 10600009)
12. Pipette aspirator (Biologix, catalog number: 01-2201)
13. Incubator Shaker (New Brunswick Scientific, model: I26)
14. Rotator (Labnet International, catalog number: H5500)
15. Orbital shaker (VWR, model: MP4)
16. Light microscope (Olympus, model: CKX31)
17. SterilGard III Advance Biosafety cabinet (Baker, model number: SG603A)
18. Autoclave (Beta Star)
Software and datasets
1. BioRender (https://www.biorender.com/). The following figures were created using BioRender: https://app.biorender.com/illustrations/66a3f08c8d303af25d573a57
Procedure
A. Spore inoculation
1. Inoculate 10 mL of SFM (see Recipes) with B. cinerea spores (1 × 10 spores/mL) in a 50 mL conical flask.
Note: B. cinerea spores are harvested from 2-week-old plates grown at 28 °C in V8 media (see Recipes). The spore concentration can be determined using a hemocytometer, as described in [7].
2. Incubate the culture overnight (20 h) with shaking (250 rpm) at 28 °C to allow spore germination.
Note: Ensure the spores are thoroughly mixed during addition and briefly vortex after addition to achieve uniform mixing. Otherwise, germlings may form larger clumps, which is not ideal for the experiment.
B. Peptide treatment
1. Centrifuge the fungal germlings (grown overnight) at 4,000× g for 20 min at room temperature (RT).
2. Discard the supernatant.
Caution: Carefully aspirate the supernatant using a pipette aspirator, as the pelleted germlings do not adhere strongly to the tubes and there is a risk of accidentally removing them.
3. Resuspend the fungal germlings in 750 μL of fresh SFM in a 2 mL tube.
4. Prepare 750 μL of each peptide solution (in our case, NCR13_PFV1 and NCR13_PFV2 at 2× concentration, e.g., for a final concentration of 1.5 μM, prepare the peptide at 3 μM) and add the peptide solution to the germlings for a final volume of 1.5 mL.
Critical: The concentration of the peptide is critical for determining its efficacy. Initial experiments should test a range of peptide concentrations: below, at, and above the minimum inhibitory concentration (MIC). The optimal duration of peptide incubation can be determined using fungal growth curve data under peptide treatment and supported by microscopy experiments, if available, to ensure peptide entry and accurate peptide treatment conditions.
5. For control samples, add 3 μL of translation inhibitor CHX and sterile Milli-Q water instead of peptides.
Caution: CHX is highly hazardous and toxic to the environment. Prevent its release into the environment. Adhere to institutional safety guidelines or consult your Environmental Health and Safety (EHS) department for appropriate waste disposal procedures.
6. Incubate the samples with shaking (200 rpm) in a microcentrifuge tube for 45 min at 28 °C.
7. After incubation, centrifuge the peptide-treated fungal germlings at 14,000× g for 3 min at RT. Wash the pellet three times with 1 mL of water to remove residual peptides or cycloheximide.
Note: Carefully aspirate the supernatant, as the germling pellets do not adhere strongly to the tubes, and there is a risk of accidentally removing them.
C. Puromycin treatment
1. Add 980 μL of SFM and 20 μL of 50 mM puromycin stock (see Recipes) to each sample pellet from step B7 to achieve a final concentration of 1 mM.
2. Incubate for 3 h with shaking (200 rpm) at 28 °C to allow puromycin incorporation into newly synthesized proteins.
3. After the 3 h incubation with puromycin, centrifuge at 14,000× g for 3 min at RT to pellet the germlings.
4. Carefully remove and discard the supernatant containing SFM and puromycin without disturbing the germling pellet.
5. Add 1 mL of sterile Milli-Q water to wash the pellet and resuspend it using a pipette.
6. Centrifuge the sample again at 14,000× g for 5 min at RT.
7. Repeat the resuspension two more times to ensure complete removal of residual puromycin and media.
8. After the final rinse, carefully remove as much liquid as possible from the germling pellet.
9. Flash-freeze the pellet in liquid nitrogen and store it at -80 °C for later use.
Pause point: Samples can be stored at -80 °C at this stage before further processing.
D. Protein extraction
1. Using a sterile pestle, grind the pellet in the tube thoroughly while keeping it cold by periodically submerging it in liquid nitrogen.
2. Ensure the pellet is fully ground to a fine powder.
Caution: Wear safety goggles and cold-resistant gloves when handling liquid nitrogen and grinding frozen samples.
3. Add 100 μL of cold protein extraction buffer (see Recipes) to the ground pellet.
4. Place the tube on a rotator in the cold room and incubate for 15 min to allow protein extraction.
5. Centrifuge the sample at 14,000× g for 15 min at 4 °C to pellet cellular debris.
6. Carefully transfer the clear supernatant to a new, pre-chilled microcentrifuge tube without disturbing the pellet.
7. Measure the protein concentration using the BCA Protein Assay kit according to the manufacturer’s instructions.
Note: Any reliable protein quantitation method can be followed based on availability.
8. Normalize protein concentration across all samples by adding the appropriate volume of 4× Laemmli sample buffer (see Recipes).
9. Heat the samples at 95 °C for 10 min to denature the proteins.
10. Briefly spin down the tubes.
11. Proceed with SDS-PAGE analysis.
E. Western blotting
1. Load 10 µg of protein per sample onto a 4%–20% TGX stain-free protein gel.
2. Add 5 μL of protein ladder to one lane to estimate molecular weights.
3. Run the gel at 150 V for approximately 45 min to 1 h or until protein separation is complete.
4. Once the gel run is complete, activate the stain-free gel using the ChemiDoc imaging system to confirm equal loading of proteins (Figure 1).
5. Soak a nitrocellulose membrane (pore size = 0.2 μM) in WB transfer buffer (see Recipes) for 5 min.
6. Soak the gel in the transfer buffer for 5 min.
7. Cut two pieces of thick blotting filter paper (2 mm thickness) and soak them in transfer buffer.
8. Assemble the transfer sandwich from the negative (cathode) side to the positive (anode) side in the following order (bottom to top): thick filter paper > nitrocellulose membrane > gel > thick filter paper.
Note: Carefully roll out air bubbles between the membrane and gel using a glass test tube or a roller pin.
9. Lock the sandwich into the cassette of the Bio-Rad Trans-Blot Turbo system and transfer the proteins at 25 V for 30 min.
10. Incubate the membrane in PBST (see Recipes) for 5 min with gentle agitation in an orbital shaker.
11. Block the membrane with 3% BSA prepared in PBST for 1 h at RT with gentle agitation.
12. Briefly rinse the membrane for 1 min with PBST and discard the buffer.
13. Incubate the membrane with anti-puromycin antibody (1:3,000) in 3% BSA in PBST overnight at 4 °C with gentle agitation.
Note: The primary antibody incubation can be shortened to 3 h at RT and still work effectively.
14. Wash the membrane three times for 5 min each in PBST with gentle agitation.
15. Incubate the membrane with anti-mouse HRP-conjugated secondary antibody (1:15,000) in 2% BSA for 1 h at RT with gentle agitation.
16. Wash the membrane four times for 5 min each in PBST.
17. Dry the membrane using paper towels.
18. Apply 1 mL of Thermo SuperSignal West PICO Substrate evenly over the membrane surface.
19. Detect chemiluminescence using a ChemiDoc imaging system (Figure 1).
20. Auto-expose the membrane for 10–15 s to capture the signal.
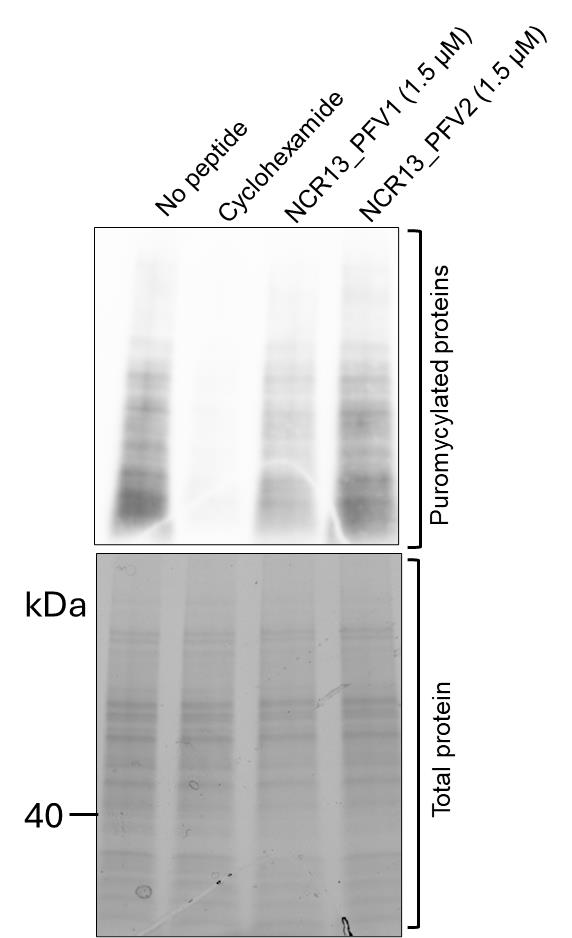
Figure 1. Assessment of protein translation in Botrytis cinerea germlings using puromycin labeling. Top panel: Western blot detection of puromycin-labeled nascent polypeptides in B. cinerea germlings treated with antifungal peptides. Protein translation was inhibited in germlings treated with peptide NCR13_PFV1 (Lane 3) and peptide NCR13_PFV2 (Lane 4) compared to the untreated control (Lane 1). Cycloheximide-treated samples (Lane 2) serve as a positive control for translation inhibition. Bottom panel: Stain-free gel image showing equal total protein loading across all lanes. This figure demonstrates the efficacy of NCR13_PFV1 in inhibiting translation in B. cinerea germlings, as shown by the reduced puromycin incorporation detected with an α-puromycin antibody, indicating decreased translation output. Cycloheximide is used as a positive control to achieve complete translation inhibition, resulting in minimal or no puromycin incorporation.
Validation of protocol
This protocol has been validated for reproducibility and robustness through multiple independent replicates and appropriate experimental controls. This protocol was adapted from the original article [1]. The SUnSET method was performed and validated for in vitro applications and later validated for in vivo protocols [8].
General notes and troubleshooting
General notes
1. This protocol is optimized for B. cinerea germlings but can be adapted for other filamentous fungal pathogens with similar growth characteristics. For species-specific adaptations, optimize spore inoculation densities, peptide concentrations, and puromycin incubation times.
2. To minimize variability, use germlings from the same batch of spores and maintain consistent experimental conditions, including temperature, shaking speed, and incubation times.
3. Include appropriate negative controls (e.g., untreated samples or samples treated with water) and positive controls (e.g., cycloheximide-treated samples) to ensure a reliable interpretation of results.
4. Some antifungal peptides may degrade or lose activity under certain storage conditions or during incubation. Ensure peptides are stored and prepared as per the guidelines.
5. Responses may vary depending on the fungal strain, culture conditions, and developmental stage. Conduct preliminary experiments to standardize conditions for consistent germling growth and peptide sensitivity.
6. The efficiency of puromycin labeling depends on its penetration into fungal cells. Consider testing higher puromycin concentrations for less permeable species.
7. For weak signals, consider loading higher protein amounts or increasing the exposure time during detection. Prolonged primary antibody incubation (e.g., overnight) enhances signal strength and consistency.
8. While this protocol is designed for antifungal peptides, the puromycin labeling method can be applied to study other inhibitors of protein synthesis in filamentous fungi with appropriate adjustments.
9. Cycloheximide and puromycin are hazardous. Always adhere to institutional safety guidelines for handling, use, and disposal. Consider alternative translation inhibitors if safety concerns arise.
10. Minor fluctuations in environmental conditions can impact fungal growth and germling sensitivity to peptides. Maintain standardized conditions across experiments.
Troubleshooting
Problem 1: No observable differences in puromycin labeling between peptide treatment and no-peptide control.
Possible cause: The peptide concentration or incubation duration may be insufficient for the peptide to enter fungal germlings and inhibit translation.
Solution: Use peptide concentrations below, at, and above their MIC values to determine the effective range. Utilize fluorophore-tagged peptide microscopy studies to assess peptide entry dynamics and internalization into fungal germlings, allowing evaluation of peptide treatment duration. Design experiments to evaluate peptide activity at multiple time points to identify the optimal duration for translation inhibition.
Problem 2: Weak or no signal in western blot.
Possible cause: Insufficient puromycin incorporation or poor protein extraction.
Solution: Ensure puromycin is used from a fresh batch and optimize the correct puromycin concentration for your experimental system. Optimize the incubation time to allow adequate incorporation and confirm that protein extraction is thorough. Use freshly prepared protein extraction buffer supplemented with protease inhibitors.
Problem 3: Inconsistent germling growth.
Possible cause: Uneven spore inoculation or contamination in culture.
Solution: Thoroughly mix spores before and after inoculation and verify the sterility of media and equipment. Standardize spore density and culture conditions.
Problem 4: Poor protein recovery.
Possible cause: Insufficient cell lysis.
Solution: Use additional mechanical disruption methods, such as bead-beating, to improve lysis or increase Triton-X 100 concentration. Keep samples cold throughout the extraction to prevent protein degradation.
Problem 5: Variability between replicates.
Possible cause: Minor variations in protein loading can lead to inconsistent or misleading results.
Solution: Quantify protein concentration accurately using a reliable method (e.g., BCA assay). Ensure equal protein loading by normalizing samples before SDS-PAGE and, after loading, use stain-free gels. After transfer, confirm equal loading with stain-free membrane imaging or Ponceau S staining.
Acknowledgments
Conceptualization, J.G. & D.M.S. Investigation, J.G. Writing—Original Draft, J.G. Writing—Review & Editing, J.G. & D.M.S. Funding acquisition, D.M.S. Supervision, D.M.S.
The work presented here was supported by the National Science Foundation grant IOS-2037981 and the National Institute of Food and Agriculture GRANT13515963.
This protocol was adapted from the original article [1]. The current protocol validates the in vitro translation inhibition assays in our published study [5]. We also thank Dr. Hani Zaher and Dr. Nanjaraj Urs Ankanahalli for their valuable suggestions and for providing an initial aliquot of the α-puromycin antibody, which was used in our preliminary experiments.
Competing interests
The authors declare no conflicts of interest.
References
- Schmidt, E. K., Clavarino, G., Ceppi, M. and Pierre, P. (2009). SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods. 6(4): 275–277. https://doi.org/10.1038/nmeth.1314
- Montesinos, E. (2007). Antimicrobial peptides and plant disease control. FEMS Microbiol Lett. 270(1): 1–11. https://doi.org/10.1111/j.1574-6968.2007.00683.x
- Chiu, T., Poucet, T. and Li, Y. (2022). The potential of plant proteins as antifungal agents for agricultural applications. Synth Syst Biotechnol. 7(4): 1075–1083. https://doi.org/10.1016/j.synbio.2022.06.009
- Yarmolinsky, M. B. and Haba, G. L. D. L. (1959). Inhibition by Puromycin of Amino Acid Incorporation into Protein. Proc Natl Acad Sci USA. 45(12): 1721–1729. https://doi.org/10.1073/pnas.45.12.1721
- Godwin, J., Djami-Tchatchou, A. T., Velivelli, S. L. S., Tetorya, M., Kalunke, R., Pokhrel, A., Zhou, M., Buchko, G. W., Czymmek, K. J., Shah, D. M., et al. (2024). Chickpea NCR13 disulfide cross-linking variants exhibit profound differences in antifungal activity and modes of action. PLoS Pathog. 20(12): e1012745. https://doi.org/10.1371/journal.ppat.1012745
- Hack, E., Hendrick, C. A., Al-Janabi, S. M., Crane, V. C. and Girton, L. E. (1994). Translation in a wheat germ cell-free system of RNA from mitochondria of the normal and Texas male-sterile cytoplasms of maize (Zea mays L.). Curr Genet. 25(1): 73–79. https://doi.org/10.1007/bf00712971
- Scholz, P., Chapman, K.D., Ischebeck, T., Guzha, A. Quantification of Botrytis cinerea Growth in Arabidopsis thaliana. Bio Protoc. 13(16): e4740. https://doi.org/10.21769/BioProtoc.4740
- Leipheimer, J., Bloom, A. L. M., Campomizzi, C. S., Salei, Y. and Panepinto, J. C. (2019). Translational Regulation Promotes Oxidative Stress Resistance in the Human Fungal Pathogen Cryptococcus neoformans. mBio. 10(6): e02143–19. https://doi.org/10.1128/mbio.02143-19
Article Information
Publication history
Received: Dec 23, 2024
Accepted: Feb 17, 2025
Available online: Mar 9, 2025
Published: Mar 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Godwin, J. and Shah, D. M. (2025). Evaluating In Vivo Translation Inhibition via Puromycin Labeling in Botrytis cinerea Germlings Treated With Antifungal Peptides. Bio-protocol 15(6): e5250. DOI: 10.21769/BioProtoc.5250.
Category
Microbiology > Antimicrobial assay > Antifungal assay
Plant Science > Plant immunity > Host-microbe interactions
Molecular Biology > Protein > Anti-microbial analysis
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









