- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Micrografting Technique of Hevea brasiliensis In Vitro Plantlets
Published: Vol 15, Iss 4, Feb 20, 2025 DOI: 10.21769/BioProtoc.5215 Views: 1481
Reviewed by: Samik BhattacharyaPreeti SinghAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Live Imaging of the Shoot Apical Meristem of Intact, Soil-Grown, Flowering Arabidopsis Plants
Gabriele Bradamante
Jun 20, 2024 2275 Views

Practical Guide to In Vitro Clonal Propagation of Nicotiana benthamiana Using Axillary Shoot Induction
Pradeep Chand Deo
Sep 20, 2025 1214 Views

Direct Plant Regeneration From Immature Male Inflorescence of Banana (Musa spp.)
Pradeep Chand Deo
Oct 20, 2025 1426 Views
Abstract
To prepare Hevea brasiliensis plantations, selected planting material is propagated by grafting using illegitimate seedlings as rootstocks, whose paternal genotype is unknown. Recent advances in rubber tree in vitro cloning propagation open the possibility of using these techniques to supply new planting material. Micrografting is a promising technique to speed up the preparation of plant material for rootstock–scion interaction studies. This article describes the implementation of an efficient micrografting technique from Hevea in vitro plants from clone PB 260. The procedure combines several conditions to preserve the root system and the grafted scion and to prevent any breakage of rootstock buds. This technique paves the way for clonal propagation and holds potential for further development on other rubber clones for further studies on the interaction between rootstock and scion.
Key features
• This protocol requires rejuvenated in vitro plantlets.
• Requires between 40 and 60 days before scion bud break.
• Sterile working conditions.
Keywords: BuddingBackground
Hevea brasiliensis is the main source of natural rubber, which is harvested by regular tapping of the trunk, and accounts for 42% of world rubber consumption. Like many perennial species, the rubber tree is an allogamous species that requires grafting to clone selected material. In contrast to fruit tree species in which the scion and the rootstock are the selected planting material, rubber tree propagation has been carried out by grafting, using scion clonal material and illegitimate seedlings as rootstocks, whose paternal genotypes are unknown. This planting material has resulted in heterogeneity in rubber plantations due to the non-clonal rootstock component. Microcutting and somatic embryogenesis have been introduced to overcome this heterogeneity problem [1,2]. The use of clonal rootstock material could be an alternative to improve the productivity of rubber plantations [3]. In fruit trees like chestnut [4], micrografting is also used to obtain disease-free material for early detection of scion/rootstock incompatibility. A first attempt at micrografting has been made in Hevea to rejuvenate clonal lines for micropropagation [5]. Perrin and collaborators micrografted rubber bud tips onto one-month-old sterile seedlings used as rootstocks. Microcutting lines were then established from this micrografted material. Because of the low multiplication rate and the time required for rejuvenation in the microcutting procedure, somatic embryogenesis emerged as an alternative. Over more than a decade, somatic embryogenesis has evolved into a short-term process using compact callus and a long-term propagation method using friable embryogenic callus lines [2,6]. The latter has been widely and successfully used for the implementation of genetic modifications [7–9] and functional characterization of genes of interest [10–12]. However, even though these techniques are robust and well-established, they are still restricted to some rubber clones. On the other hand, mastery of micrografting is still insufficient to support studies such as the analysis of rootstock/scion interactions using rejuvenated and clonal material. The aim of this research is to propose an efficient micrografting procedure in rubber using in vitro–rejuvenated plantlets from somatic embryogenesis.
Materials and reagents
Biological materials
Here, 2.5-months-old vitroplants were used, after germination of the somatic embryos, having reached between 1 and 2 leaf stages with root development equivalent in size to the one of the aerial part.
1. Hevea in vitro plantlets of interest for scion sampling. Plantlets should have the same diameter as the rootstock to make it easier to cut the scion
2. Hevea in vitro–rooted plantlets of interest to be grafted (serving as a rootstock)
Materials
1. Sterile round filter paper 85 mm diameter as culture support (Whatman, catalog number: 1001085)
Reagents
1. Agar agar (Sigma, catalog number: A1296)
2. NH4NO3 (Labover, catalog number: A0501-1000)
3. KNO3 (Sigma, catalog number: 1050611000)
4. NaH2PO4·H2O (Labover, catalog number: 13429448)
5. CaCl2·2H2O (Sigma, catalog number: 1420021000)
6. MgSO4·7H2O (Sigma, catalog number: 23039)
7. H3BO3 (Sigma, catalog number: B6768)
8. MnSO4·H2O (Sigma, catalog number: M7899)
9. ZnSO4·7H2O (Sigma, catalog number: Z0251)
10. CuSO4·5H2O (Sigma, catalog number: C8027)
11. NaMoO4·2H2O (Labover, catalog number: S0525-25)
12. KI (Sigma, catalog number: P8166)
13. CoCl2·6H2O (Sigma, catalog number: C8661)
14. Myo inositol (Sigma, catalog number: I7508)
15. Nicotinic acid (Labover, catalog number: N0611.0100)
16. Pyridoxin HCl (Labover, catalog number: P0612.0050)
17. Thiamin HCl (Labover, catalog number: T0614.0025)
18. D(+) Biotin (Labover, catalog number: B0603.1000)
19. Calcium pantothenate (Sigma, catalog number: C0400000)
20. Ascorbic acid (Labover, catalog number: A0602.0100)
21. Choline chloride (Sigma, catalog number: C1879)
22. Cysteine HCl (Labover, catalog number: C0706.0100)
23. Glycine (Labover, catalog number: G0709.1000)
24. Riboflavin (Labover, catalog number: R0613.0025)
25. FeSO4·7H2O (Sigma, catalog number: 1039630001)
26. Na2EDTA·2H2O (Sigma, catalog number: E6635)
27. Sucrose (Labover, catalog number: S0809.9025)
28. Osmosis water
Solutions
1. MACRO MS 10× stock solution (see Recipes)
2. MICRO MH 100× stock solution (see Recipes)
3. Vitamins 1,000× stock solution (see Recipes)
4. Fe-EDTA 100× stock solution (see Recipes)
5. Germination culture (GER) medium (see Recipes)
Recipes
For each stock solution, mix the components, in the order listed, with osmosis water. These stock solutions can be kept for one month in the refrigerator or for up to one year in the freezer at -20 °C.
1. MACRO MS 10× stock solution
| Reagent | Final concentration |
|---|---|
| NH4NO3 | 400 μM |
| KNO3 | 400 μM |
| NaH2PO4·H2O | 40 μM |
| CaCl2·2H2O | 30 μM |
| MgSO4·7H2O | 60 μM |
2. MICRO MH 100× stock solution
| Reagent | Final concentration |
|---|---|
| H3BO3 | 15,008 μM |
| MnSO4·H2O | 10,000 μM |
| ZnSO4·7H2O | 4,000 μM |
| CuSO4·5H2O | 148 μM |
| NaMoO4·2H2O | 99 μM |
| KI | 500 μM |
| CoCl2·6H2O | 101 μM |
3. Vitamins 1,000× stock solution
| Reagent | Final concentration |
|---|---|
| Myo inositol | 300 μM |
| Nicotinic acid | 20 μM |
| Pyridoxin HCl | 3 μM |
| Thiamin HCl | 2 μM |
| Biotin | 0.2 μM |
| Calcium pantothenate | 1 μM |
| Ascorbic acid | 1 μM |
| Choline chloride | 1 μM |
| Cysteine HCl | 60 μM |
| Glycine | 5 μM |
| Riboflavin | 1 μM |
4. Fe-EDTA 100× stock solution
| Reagent | Final concentration |
|---|---|
| FeSO4·7H2O | 10 μM |
| Na2EDTA·2H2O | 10 μM |
5. Germination culture (GER) medium
| Reagent | Final concentration |
|---|---|
| Macro MS 10× | 0.5× |
| Micro MH 100× | 1× |
| Fe-EDTA 100× | 1× |
| Vitamins 1,000× | 1× |
| Sucrose | 234 mM |
| Agar | 7 g/L |
Mix all medium components in osmosis water. Adjust pH to 5.8. Autoclave for 20 min at 120 °C. Dispense 5 mL of liquid medium into sterile tubes and the semi-solid medium in Petri dishes. Media can be stored for 1–2 weeks at room temperature in the dark.
Equipment
1. Laminar flow hood
2. Magnifying glass
3. Sterile fines clamp
4. Sterile large clamp (for plantlet manipulation)
5. Sterile scalpel
6. Sterile razor blade
7. Sterile blade holder
8. Food-grade plastic film
Software and datasets
1. XLSTAT software (for data analysis) (Addinsoft, version 2023.1.1., Lumivero [13])
Procedure
The entire protocol, except for media preparation, must be carried out under sterile conditions using sterile equipment.
A. Material preparation
1. Prepare GER medium (see Recipes).
2. Sterilize a circle of filter paper 85 mm in diameter in a capped glass tube (used as support for the grafted plant) as described in Figure 1.
3. After autoclaving GER medium, pour it into the glass tube.
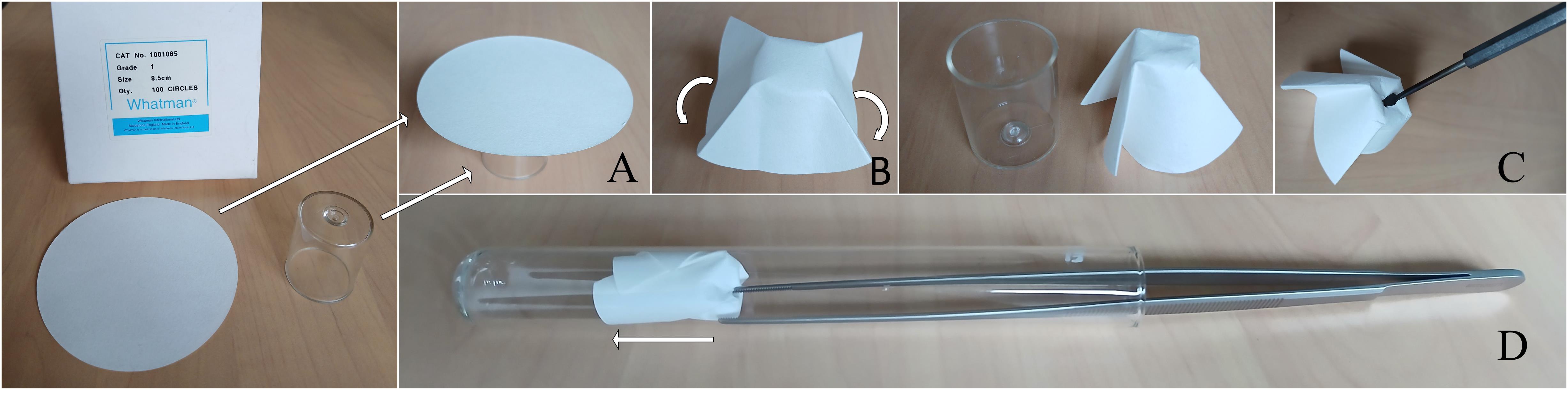
Figure 1. Folding the filter paper into the glass tube. A. Place the paper disc on the lid of a glass tube. B. Fold the paper disc over the lid. C. Pierce the center of the folded disk with a pointed object. This will allow the taproot to pass through. D. Insert the folded disc into the tube that will contain the grafted seedling.
B. Rootstock preparation (under a magnifying glass)
1. Carefully remove the in vitro plantlets out of the glass tube (Figure 2A).
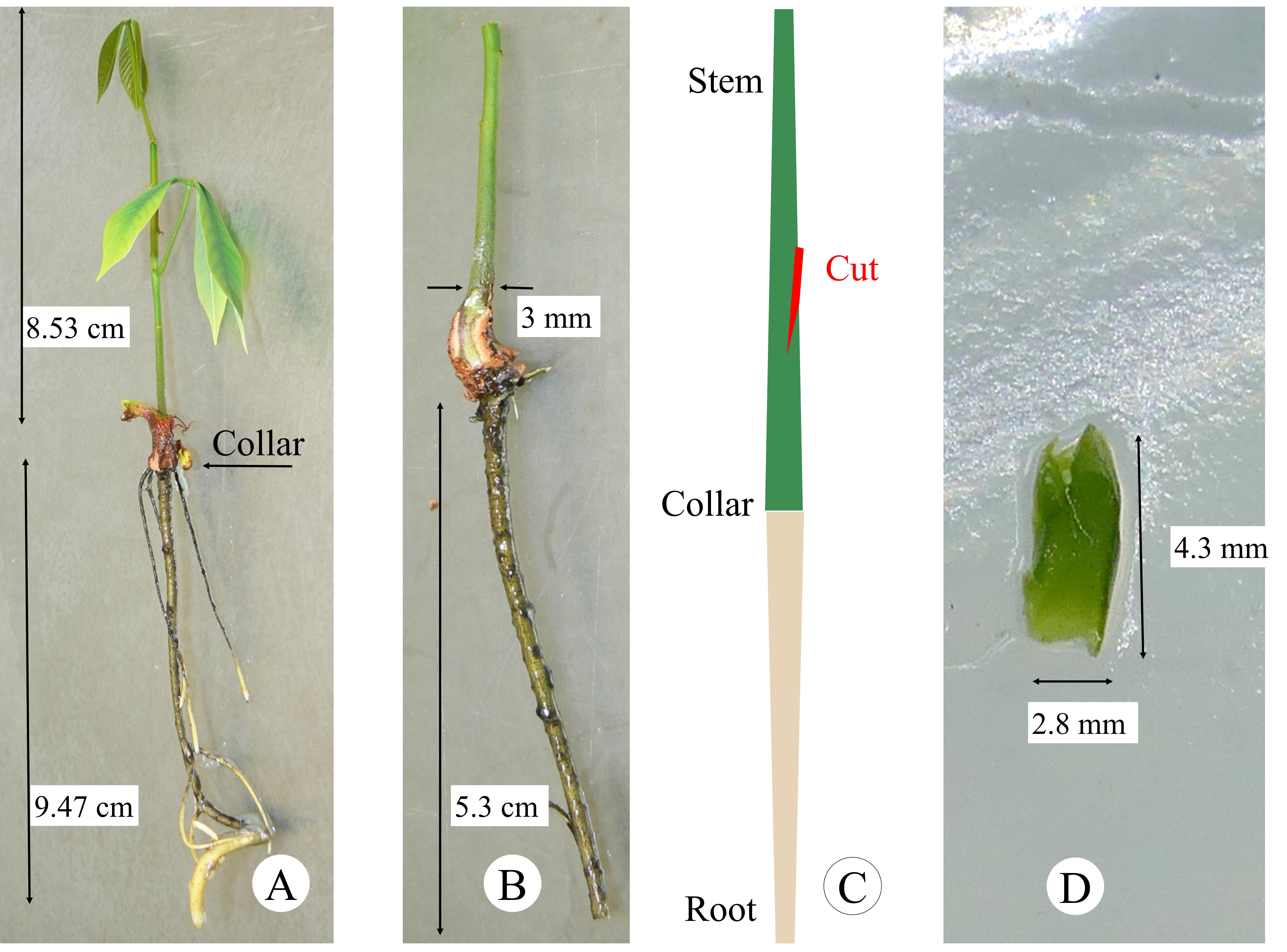
Figure 2. Plant material preparation. A. In vitro plantlet at the right stage of development. B. Rootstock after pruning of cotyledons and taproot. C. Grafting window preparation. D. Scion at the correct size equivalent to the diameter of the rootstock.
2. Prune cotyledons and stem from in vitro plantlets used as rootstock (Figure 2B).
3. Prune taproot at 6 cm below the collar (Figure 2B).
4. Prepare the grafting window by cutting a slightly oblique slit in the stem at least 2 cm above the collar (Figure 2C). Take care not to weaken or break the rootstock stem.
C. Scion preparation (under a magnifying glass)
1. Cut a beveled graft from the apex or another bud of the selected in vitro plant. Its size should match the diameter of the rootstock stem (Figure 2D).
2. While waiting to hold the rootstock under the magnifying glass, leave the scion on GER semi-solid medium for a few seconds to avoid drying and oxidation, without closing the Petri dish with its lid (Figure 2D).
D. Grafting (under a magnifying glass)
1. Place the scion in the oblique slit and surround it with a piece of semi-solid medium (grafting steps, Figure 3A).
2. Transfer the grafted material into a glass tube containing liquid GER medium; stabilize the grafted plant by placing it in the middle of the folded filter paper (Grafting steps, Figure 3B).
3. Hermetically seal the capped culture tubes with food-grade plastic foil. No aeration is required until bud break and graft development.
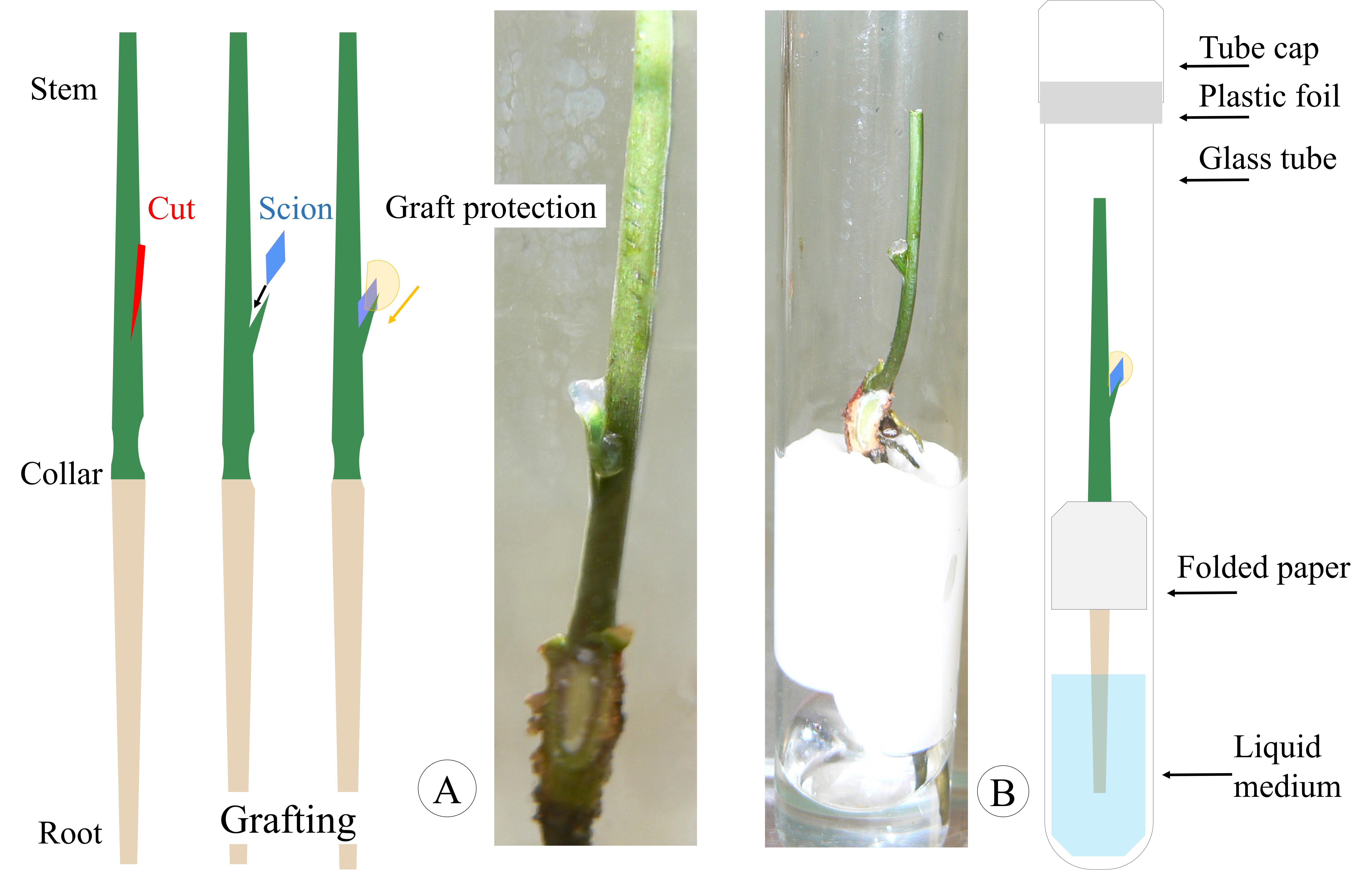
Figure 3. Grafting steps in optimal conditions. A. Placement of the graft in the slit and protection with a piece of solid medium. B. Correct positioning of the grafted plant into the filter paper base.
E. Graft re-growth
1. Grow plantlets for one month at 27 °C, under a light intensity of 60 μmol m-2·s-1 with a 12/12 h light/dark photoperiod.
2. Monitor and suppress the restart of cotyledon buds twice a week. Under sterile conditions, partially remove the plantlet from the disinfected tube and remove the bud with a sterile blade.
3. When the grafted bud breaks and regenerates a growth unit (Figure 4A), transfer the plant to the greenhouse for acclimatization when the leaves are fully expanded (Figure 4B). Use a 2 L pot for full development for two months. During this step, use a transparent plastic cup for 2 weeks to maintain humidity around the young grafted plant (Figure 4C). Then, transfer plantlets into 7 L pots for up to one year (Figure 4D). The soil used here was GO M2 (Jiffy), pH 5, with the following growing conditions: average temperature of 28–32 °C, humidity 50%–70%, manual watering 2–3 times a week, and natural lighting.
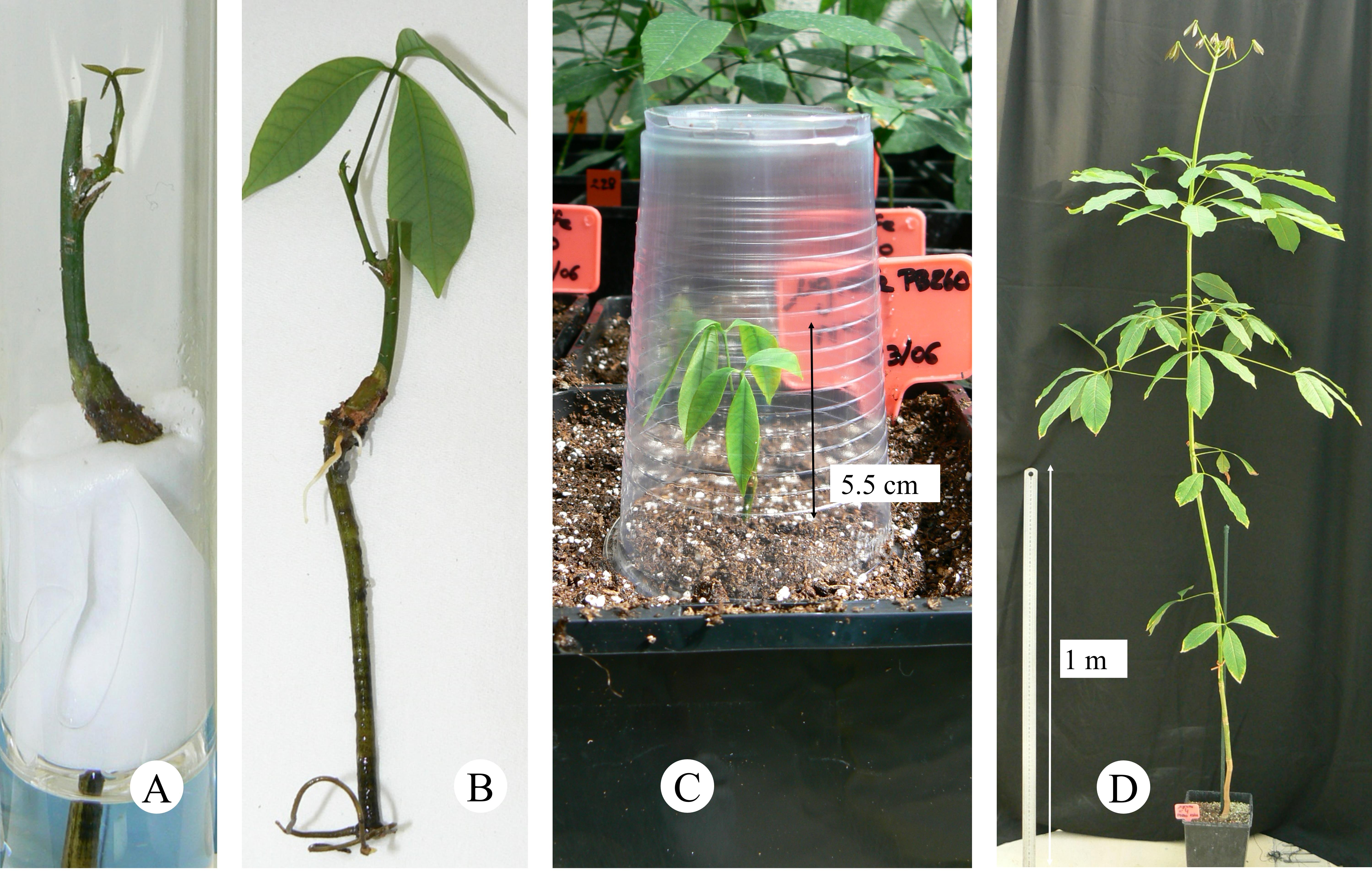
Figure 4. Graft re-growth, acclimatization, and plant development in the greenhouse. A. Graft re-growth. B. Grafted plantlet with fully developed leaves. C. Acclimatization step in the greenhouse. D. Development of the grafted plant after one year.
Data analysis
To establish a robust protocol in rubber trees, several parameters were tested on enough plants to allow statistical analysis using XLSTAT software. These parameters were tested on in vitro plants of clone PB 260 regenerated by somatic embryogenesis. In fact, two types of media (liquid or semi-solid), as well as a junction protection treatment (none, liquid, or semi-solid) and various times of rootstock bud pruning (day of grafting, 3 weeks after grafting) were tested. Statistical analysis was carried out by parametric k-proportions comparison including khi2, Montecarlo, and Marascuilo tests.
Validation of protocol
Throughout the process, adverse effects such as root damage and bud break on the rootstocks were monitored (Figure 5). Root damage that occurred in the semi-solid medium was avoided in the liquid medium. Bud break on the rootstock was avoided by pruning all the cotyledonary buds on the rootstock on the day of grafting. Grafted buds survived thanks to the use of a drop of semi-solid medium at the junction. The two best treatments, consisting of a treatment at the scion-rootstock junction with semi-solid medium and a pruning of the rootstock buds, resulted in a grafted bud break rate ranging from 63% to 73% (Figure 5).
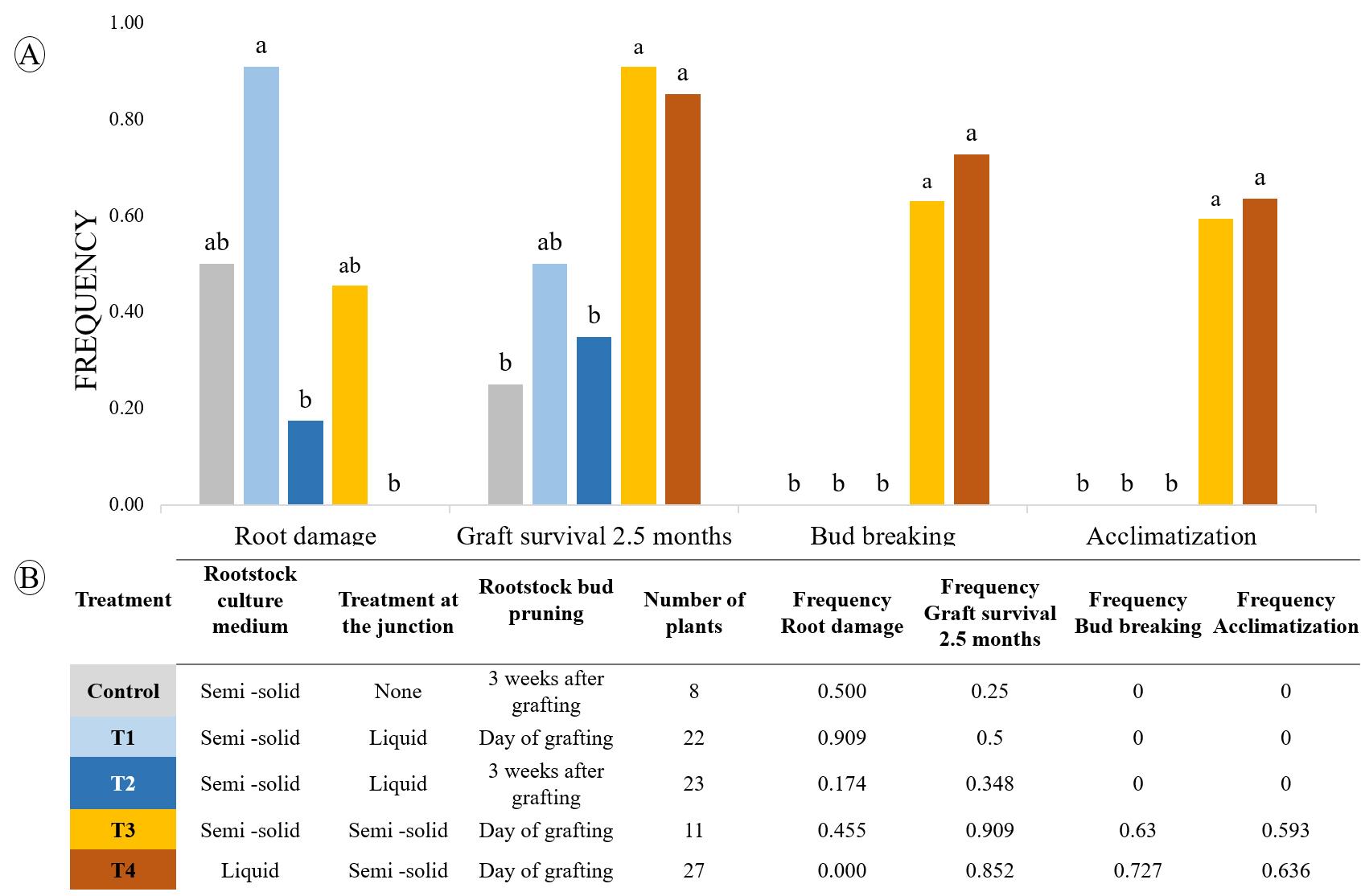
Figure 5. Effect of rootstock culture medium, the treatment at the junction, and the pruning of the rootstock buds on the development of the grafted buds. A. Histograms showing the effectiveness of treatments on root damage, graft survival, bud breaking, and acclimatization in the greenhouse. B. Details of treatments applied and number of grafted plants used. Statistical analyses were carried out using a parametric k-proportions comparison. Values with the same letter are not significantly different at the 0.05 probability level.
General notes and troubleshooting
Preliminary trials revealed several problems, including root damage due to the resistance of the semi-solid medium, bud break on the rootstock competing with the grafted bud, and, most importantly, drying of the grafted buds. According to the literature, stem and root pruning reduced early bud break on rootstock in citrus [14]. These authors also showed that root damage could be reduced using liquid medium. Interestingly, a drop of medium at the junction prevents the drying of grafted buds in pear and apple shoot tip grafting [15]. The first critical step is pruning the cotyledons, which consists of removing the cotyledons and underlying buds to avoid competition with the graft, as soon as the rootstock is prepared and without damaging the stem (see section B). Grafting is, therefore, done on a long internode without any developed buds. Monitoring the buds is the second critical step; they are removed carefully once or twice a week (see section E). This successful micrografting method developed on rubber clone PB 260 should then be tested on several other commercial clones. This technique will speed up the production of plant material for rootstock–scion interaction studies for commercial rubber clones as well as transgenic material for various agronomic traits. This technique may also facilitate rubber tree rootstock breeding.
Acknowledgments
The authors would like to thank Dr. Pablo Aleza (IVIA) and Dr. Mirea Bordas (Agromillora) for fruitful discussions and advice.
Competing interests
The authors declare that they have no financial and non-financial competing interests.
References
- Carron, M. P., Enjalric, F., Lardet, L. and Deschamps, A. (1989). Rubber (Hevea brasiliensis Müll. Arg.). In: Trees II (p. 222–245). Springer.
- Carron, M. P., Etienne, H., Lardet, L., Campagna, S., Perrin, Y., Leconte, A. and Chaine, C. (1995). Somatic embryogenesis in rubber (Hevea brasiliensis Müll. Arg.). In: Somatic Embryogenesis in Woody Plants: Volume 2—Angiosperms (p. 117–136). Springer.
- Montoro, P., Carron, M. P., Granet, F., Lardet, L., Leclercq, J., Dessailly, F., Martin, F., Gaurel, S., Uche, E., Rio, M., et al. (2012). Development of new varietal types based on rejuvenation by somatic embryogenesis and propagation by conventional budding or microcutting in hevea brasiliensis. Acta Hortic. 961: 553–576.
- Lê, C. L., Changins, A. and Abdelhamid, S. (s.d.) (2004). Microgreffage in vitro du châtaignier.
- Perrin, Y., Lardet, L., Enjalric, F. and Carron, M. P. (1994). Rajeunissement de clones matures d'Hevea brasiliensis (Müll. Arg.) par microgreffage in vitro. Can J Plant Sci. 74(3): 623–630.
- Lardet, L., Dessailly, F., Carron, M.-P., Rio, M., Ferrière, N. and Montoro, P. (2009). Secondary somatic embryogenesis in Hevea brasiliensis (Müll. Arg.): An alternative process for long-term somatic embryogenesis.
- Blanc, G., Baptiste, C., Oliver, G., Martin, F. and Montoro, P. (2006). Efficient Agrobacterium tumefaciens-mediated transformation of embryogenic calli and regeneration of Hevea brasiliensis Müll Arg. plants. Plant Cell Rep. 24(12): 724–733.
- Lardet, L., Leclercq, J., Bénistan, E., Dessailly, F., Oliver, G., Martin, F. and Montoro, P. (2011). Variation in GUS activity in vegetatively propagated Hevea brasiliensis transgenic plants. Plant Cell Rep. 30(10): 1847–1856.
- Leclercq, J., Lardet, L., Martin, F., Chapuset, T., Oliver, G. and Montoro, P. (2010). The green fluorescent protein as an efficient selection marker for Agrobacterium tumefaciens-mediated transformation in Hevea brasiliensis (Müll. Arg). Plant Cell Rep. 29(5): 513–522.
- Leclercq, J., Martin, F., Sanier, C., Clément-Vidal, A., Fabre, D., Oliver, G., Lardet, L., Ayar, A., Peyramard, M., Montoro, P., et al. (2012). Over-expression of a cytosolic isoform of the HbCuZnSOD gene in Hevea brasiliensis changes its response to a water deficit. Plant Mol Biol. 80(3): 255–272.
- Lestari, R., Rio, M., Martin, F., Leclercq, J., Woraathasin, N., Roques, S., Dessailly, F., Clément‐Vidal, A., Sanier, C., Fabre, D., et al. (2017). Overexpression of Hevea brasiliensis ethylene response factor HbERF‐IXc5 enhances growth and tolerance to abiotic stress and affects laticifer differentiation. Plant Biotechnol J. 16(1): 322–336.
- Martin, F., Abati, V., Burel, A., Clément-Vidal, A., Sanier, C., Fabre, D., Woraathasin, N., Rio, M., Besret, P., Farinas, B., et al. (2018). Overexpression of EcGSH1 induces glutathione production and alters somatic embryogenesis and plant development in Hevea brasiliensis. Ind Crops Prod. 112: 803–814.
- Lumivero (2024). XLSTAT statistical and data analysis solution. Paris, France. https://www.xlstat.com/fr
- Navarro, L. and Juárez, J. (2007). Shoot-tip grafting in vitro: impact in the citrus industry and research applications. In: Khan I. A. (Ed.). Citrus genetics, breeding and biotechnology (1st ed.) p. 353–364. Citrus genetics, breeding and biotechnology.
- Rafail, S. and Mosleh, M. (2010). Factors involved in micropropagation and shoot tip grafting of apple Malus domestica Borkh.) and pear (Pyrus sp. L.). Tropentag World Food System, 14–16.
Article Information
Publication history
Received: Sep 5, 2024
Accepted: Dec 23, 2024
Available online: Feb 9, 2025
Published: Feb 20, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Dessailly, F., Montoro, P., Melliti, S. and Leclercq, J. (2025). Micrografting Technique of Hevea brasiliensis In Vitro Plantlets. Bio-protocol 15(4): e5215. DOI: 10.21769/BioProtoc.5215.
Category
Plant Science > Plant breeding > Micropropagation
Plant Science > Plant developmental biology > General
Biological Sciences > Biological techniques > Microbiology techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








