- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples
Published: Vol 15, Iss 1, Jan 5, 2025 DOI: 10.21769/BioProtoc.5145 Views: 1917
Reviewed by: Vivien J. Coulson-ThomasThirupugal GovindarajanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

An Integrated Workflow for Three-Dimensional Visualization of Human Skeletal Muscle Stem Cell Nuclei
Jeremy R. Pearson [...] Eduardo Rosa-Molinar
Apr 20, 2025 2500 Views

Cryopreservation of Bulk-Produced Primary Rat Oligodendrocyte Progenitor Cells
Hanki Kim [...] Jun Young Choi
Jun 20, 2025 1417 Views

Generation of Agarose-Based FFPE Cancer Organoids for Morphology Preservation
Mi Rim Lee [...] Yun-Hee Kim
Oct 5, 2025 1703 Views
Abstract
Histological techniques to study muscle are crucial for assessing skeletal muscle health. To preserve tissue morphology, samples are usually fixed in formaldehyde or cryopreserved immediately after excision from the body. Freezing samples in liquid nitrogen, using isopentane as a mediator for efficient cooling, preserves the tissue in its natural state. However, this method is highly susceptible to freeze-fracture artifacts, which alter or destroy tissue architecture. Isopentane is most commonly used in a semi-frozen/liquid state that is visually assessed by the experimenter, which can pose a challenge when freezing multiple tissues at a time or maintaining a consistent temperature. Furthermore, tissue size is also a confounding factor; depending on the size, freezing times can vary. In this study, we compare two different options for using isopentane while cryopreserving tissue. We also present an easy and reproducible method of freezing the soleus tissue of mice using frozen isopentane. This method decreased the occurrence of freeze-fractures by an order of magnitude, to ~4%, whereas the traditional method of cryopreservation resulted in ~56% freeze-fracturing.
Key features
• A uniform and highly reproducible protocol for freezing any tissue that is prone to freeze-fracture.
• Removes the need to maintain a mixed state of isopentane.
• Optimized cryopreservation method for the soleus muscle of mice.
• Allows for prevention of peripheral freeze-fracture in tissue, which is the most susceptible region to freeze-fracture damage.
Keywords: Freeze-fractureGraphical overview
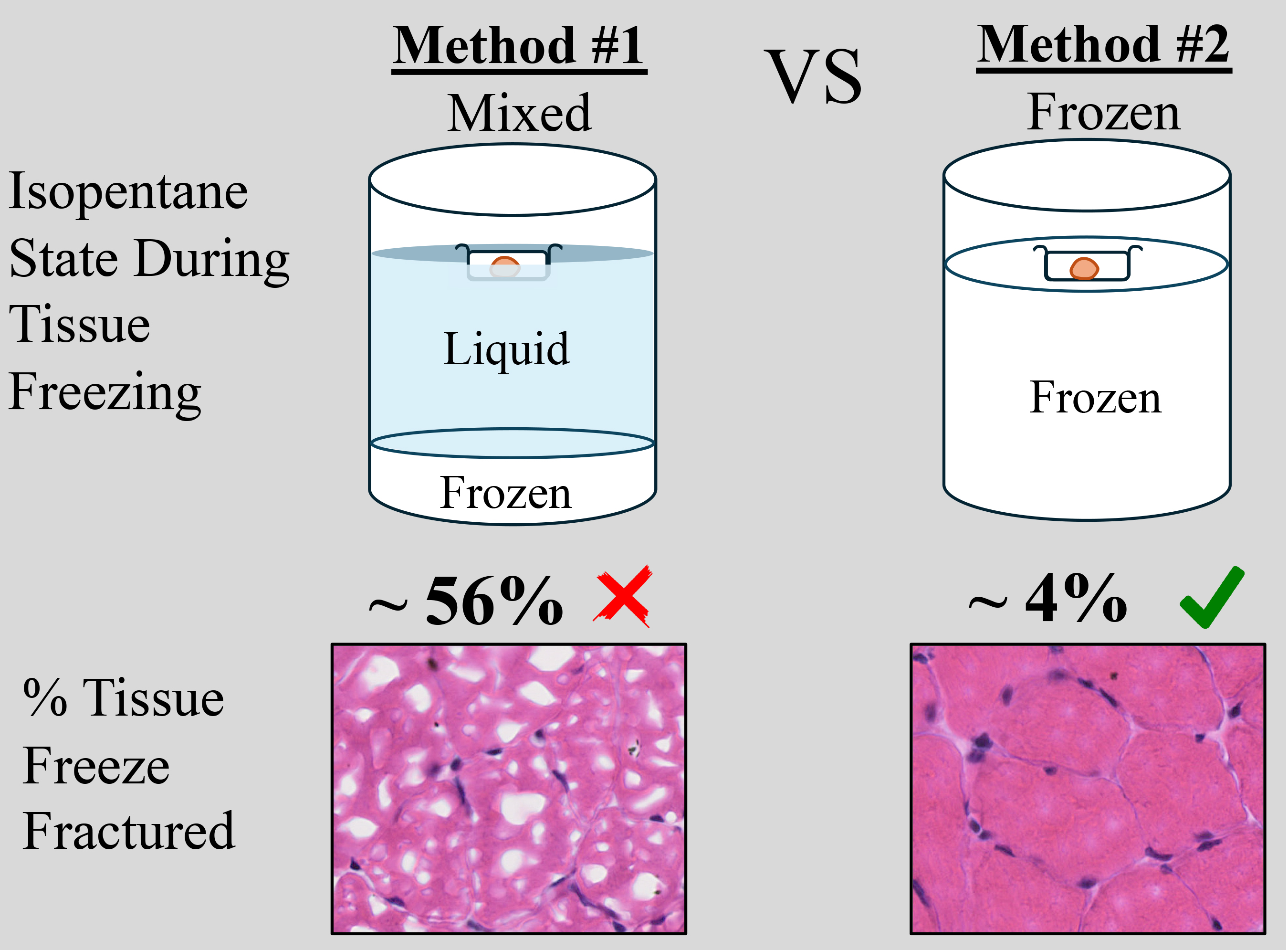
Background
Preserving tissue integrity is paramount for comprehensive morphological studies. Previous methods employed for tissue preservation, including formaldehyde fixation and cryopreservation, have demonstrated both advantages and challenges [1]. Formaldehyde fixation, while stabilizing tissues through protein crosslinking, may alter the natural conformation of cellular structures and impede antigen accessibility during immunohistochemical analysis. Cryopreservation, involving rapid freezing in liquid nitrogen without the need for fixation, is a widely used method for long-term tissue storage. However, the sudden temperature drop associated with liquid nitrogen often distorts the morphology of skeletal muscles. The liquid nitrogen evaporates immediately as it comes into contact with the warm tissue. This vapor becomes trapped between the mold surface and the liquid nitrogen, essentially creating an insulated barrier preventing freezing [2]. This process creates artifacts where intracellular water crystallizes immediately, resulting in the formation of huge crystals that rupture the muscle fibers. To address this, isopentane is utilized as a medium to facilitate controlled freezing [3].
A beaker filled with isopentane is placed in liquid nitrogen such that one-third of the isopentane is submerged. The isopentane is considered good for cryopreservation when a frozen layer forms at the bottom. At this stage, the bottom of the tissue mold is submerged in the chilled liquid. The duration of tissue submersion varies depending on the size of the tissue. Larger tissue requires longer freezing times than smaller tissues. The freezing times for small tissues like the diaphragm or extensor digitorum longus (EDL) is 6–12 s [3]. However, mice have even smaller tissues (e.g., soleus) that would require a freezing time of less than 6 s. Hence, small skeletal muscle tissues remain susceptible to freeze-fractures due to their higher surface-to-volume ratio. This method of freezing is highly inconsistent due to the many variables, i.e., liquid-to-frozen phase change, temperature fluctuations, visual assessment of phase state, tissue size, submerged depth of the mold, and batch-to-batch variability. In this study, rather than visual assessment, we monitor the temperature of isopentane when used for cryopreservation and present a reliable and reproducible method of preserving mouse skeletal muscles for both large and small tissues.
Materials and reagents
Biological materials
1. Tibialis anterior and soleus from 6-week-old C57BL/6 males (Jackson Laboratory, strain #000664)
Reagents
1. Bendable thermocouple probes for liquids and gases (McMaster Carr, catalog number: 39095K96)
2. 2-methylbutane/isopentane (Sigma-Aldrich, catalog number: 277258)
3. Dry ice
4. Liquid nitrogen
5. OCT (Optimal Cutting Temperature) (Sakura, catalog number: 4583)
6. CitriSolv (Fisher Scientific, catalog number: 04-355-121)
7. Bluing reagent (Fisher Scientific, catalog number: 22-050-114, Supplier No.: 7301)
8. Formalin (Fisher Scientific, catalog number: SF100-20)
9. Ethanol (Decon Laboratories, Inc, catalog number: 2701)
10. Hydrochloric acid (HCl) (Fisher Scientific, catalog number: A144-500)
11. Hematoxylin 560-Surgipath (Leica Biosystems, catalog number: 3801570)
12. Alcoholic eosin Y515-Suripath (Leica Biosystems, catalog number: 3801615)
Laboratory supplies
1. Modified big aluminum can (23Fl Oz), see section B: Cryopreservation method #1
2. Modified small aluminum can (11.5Fl Oz), see section C: Cryopreservation method #2
3. Handheld thermometer (McMaster Carr, catalog number: 9281T52)
4. Styrofoam ice box
5. Tissue molds (VWR, catalog number: 25608-916, Supplier No.: 4557)
6. Needles 23G (Exel International, catalog number: 26407)
7. Microscope slides (Epic Scientific, catalog number: 187200810RE)
8. Superfrost plus slides (Fisher Scientific, catalog number: 1255015)
Equipment
1. Blunt and sharp forceps (Fisher Scientific, catalog numbers: 12740916 and 11340705)
2. Scissors (Fisher Scientific, catalog number: 08-940)
3. Locking forceps (Fisher Scientific, catalog number: 13-812-16)
4. Cryostat blade (C.L. Sturkey, Inc. catalog number: 22-210-045)
5. Nanozoomer (Hamamatsu, model: C9600-12, catalog number: 000382)
Software and datasets
1. NDPI viewer software (Hamamatsu version 2.9.29)
Procedure
A. Dissection of muscle
1. Isolate tibialis anterior (TA) and soleus muscles from mice (6-week-old C57BL/6 males were used here) as previously described [4].
2. Cut each muscle in half at the mid-belly and place each half in molds filled with OCT. Use a needle to orient the muscle so that the cross-sectional side faces the bottom of the mold (Figure 1A).
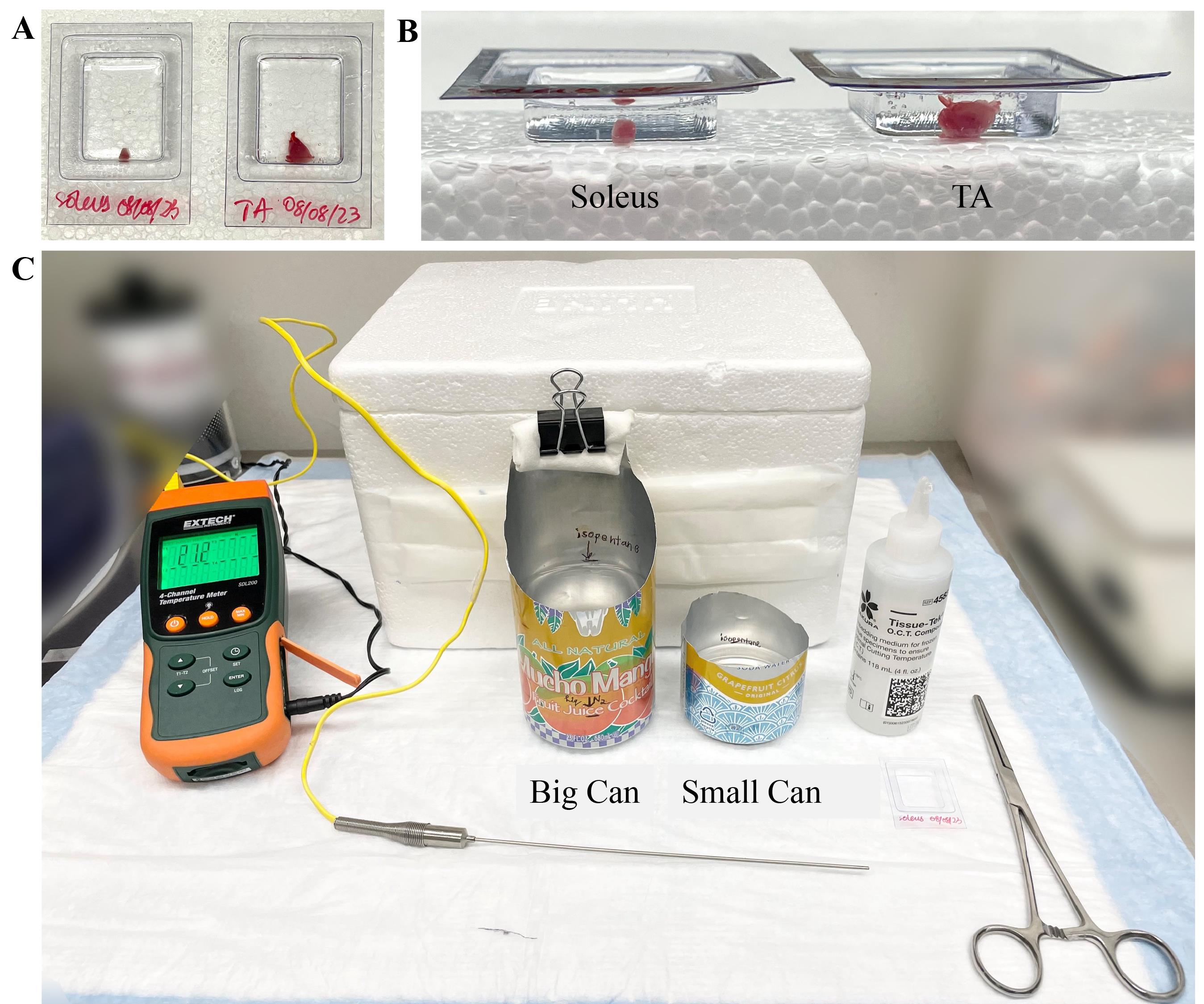
Figure 1. Muscle orientation and equipment used for cryopreservation. A, B) TA and soleus were cut at the mid-belly and placed in molds containing OCT. The tissue was placed at the bottom half of the mold; A) top view and B) side view. C) The instrument setup for cryopreservation methods was laid out on the bench. Temperature probe and temperature meter were used to monitor the temperatures in both methods. Isopentane was filled up to the marks in the aluminum can. OCT was used to embed the tissues. Forceps were used to insert the molds in the cans with isopentane. A styrofoam box with dry ice was kept ready to temporarily hold the molds until they were transferred to a -80 °C freezer. Not shown in the picture are the two differently sized styrofoam boxes (large for larger can/small for smaller can) containing liquid nitrogen.
3. Confirm orientation visually from the side (Figure 1B). By using this technique for embedding, the experimenter already has an understanding of the tissue location and orientation. This method also prevents the tissue from easily floating in the OCT while the mold is being handled by the experimenter.
B. Cryopreservation method #1
This is the conventional method for freezing muscle tissues for histology [1].
1. Cut the front part of the large aluminum can with a pair of scissors as shown in Figure 1C. This will allow the evaporation of isopentane fumes resulting from the contact of room-temperature mold with the colder isopentane. This way, the experimenter can easily visualize the mold when placed inside the can.
2. Fill the can with approximately 300 mL of isopentane and place it in a styrofoam box containing liquid nitrogen such that one-third of the can, containing 100 mL of isopentane, is submerged. Mark the level of isopentane inside the can with a sharpie (Figure 1C).
3. Use a larger can for cryopreserving in liquid isopentane as more volume is required.
4. To easily transfer an open-filled can, keep one side of the can taller to make it easier to hold. Attach a paper towel and binder clip to the can to protect the user from the cold temperature when handling the can.
5. Freeze the isopentane until the bottom part of the can has a frozen white layer and temperature reaches -120 °C (Figure 2). Place the temperature probe on the top of the isopentane layer where the mold will be placed.
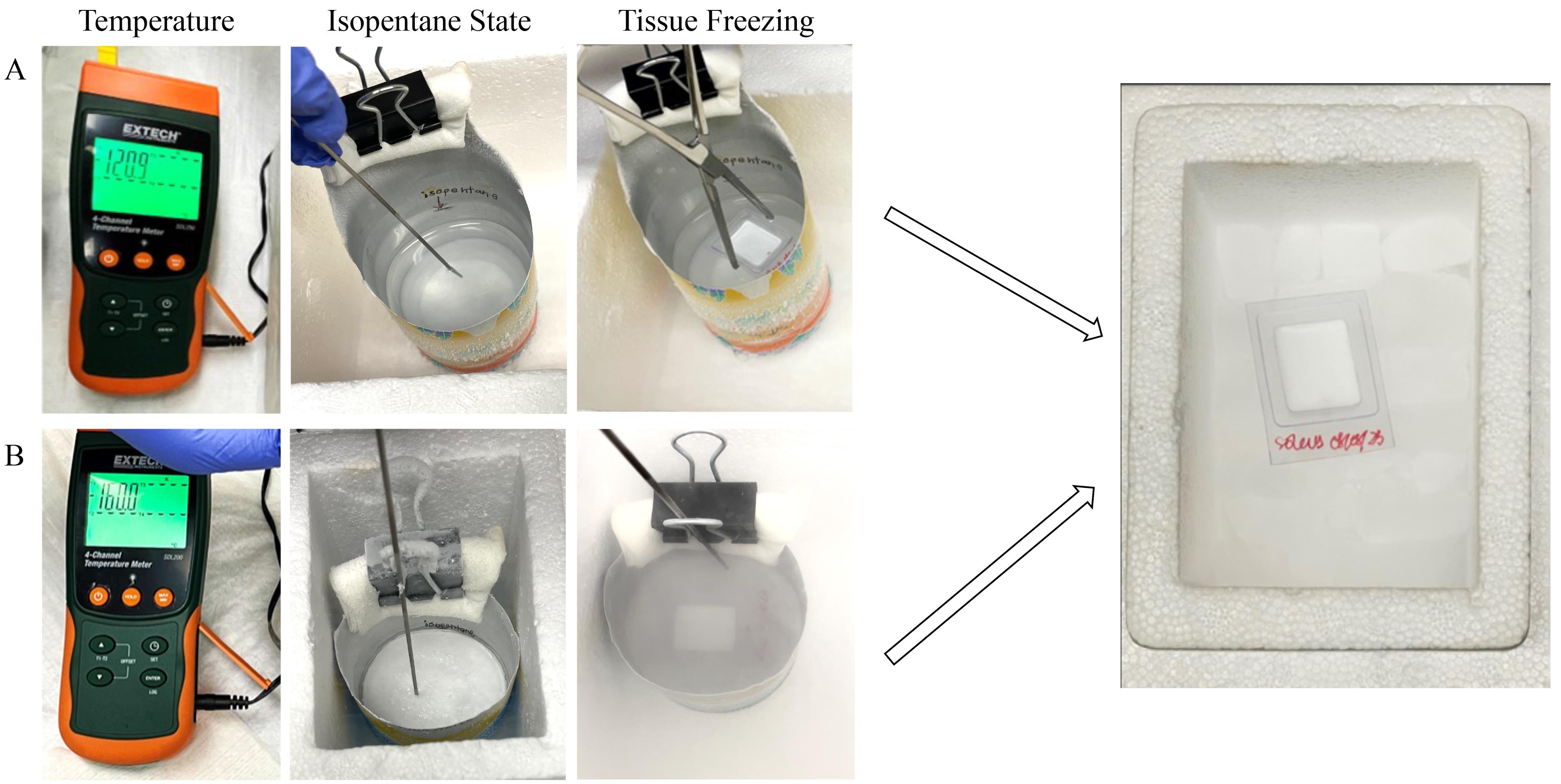
Figure 2. Detailed process of preserving the muscle tissue by two different methods. A) A bigger aluminum can was filled up to the mark with isopentane and inserted in a styrofoam box filled with liquid nitrogen. The temperature of the liquid isopentane was checked after the bottom layer was frozen (changes from translucent to white). Once the temperature reached -120 °C, the tissue mold was held in the liquid isopentane with forceps and freezing of the tissue was achieved. Precaution was taken not to submerge the tissue mold inside the isopentane. It was transferred with forceps to dry ice until storage at -80 °C. B) A small aluminum can was filled up to the mark with isopentane. It was placed inside a small styrofoam box filled up to one-third with liquid nitrogen to achieve quick and complete freezing of isopentane. Frozen isopentane was allowed to reach a temperature of -160 °C (as shown in the temperature meter); the tissue mold was placed on the frozen surface. Tissue mold was transferred using forceps on dry ice until storage in a -80 °C freezer.
6. Prepare the mold with tissue embedded in OCT. Hold the mold with locking forceps and place it in the chilled isopentane, ensuring the mold edges rest on the serrations to prevent tilting (Video 1).
7. Do not submerge the mold completely.
8. Freeze until OCT turns completely white. The outer border begins to freeze first and expands to the center of the mold. When the entire OCT turns from colorless to white, transfer the frozen mold to dry ice for 5–10 min to allow any residual isopentane to evaporate.
9. Store tissue samples at -80 °C until further analysis.
C. Cryopreservation method #2
1. Trim a small aluminum can and fill it with approximately 30 mL of isopentane to completely cover the bottom and maintain a flat, leveled surface on the top.
2. Freeze the isopentane completely by submerging the can in a styrofoam box filled to one-third with liquid nitrogen. As smaller volumes are needed when isopentane is used in the solid state, no added support is required. The same paper towel and binder clip can be used with the small can to allow the user to handle it safely.
3. Ensure the temperature of frozen isopentane reaches -160 °C (freezing point of isopentane) by placing the tip of the temperature probe on it (Figure 2).
4. Prepare the mold with tissue embedded in OCT. Place it on the frozen surface of isopentane using locking forceps (Video 1). Holding the mold with forceps is unnecessary, as the isopentane surface is solid.
5. Freeze until OCT turns completely white. Using forceps, transfer the frozen mold to dry ice for 15–20 min to allow residual isopentane to evaporate.
6. Wrap the mold in aluminum foil or place it in a sample bag.
7. Store tissue samples at -80 °C until further use.
D. Cryosectioning
1. Remove tissue samples from the -80 °C freezer and allow them to equilibrate to -20 °C in the cryostat.
2. Place tissue blocks on the cryostat sample holder with the cross-sectional side of the tissue facing up.
3. Cut the tissue blocks to produce 10 µm thick sections.
4. Place sections on Superfrost plus slides and store the slides in a -80 °C freezer.
E. H&E staining
1. Thaw slides to room temperature and air-dry them for 30 min.
2. Fix slides in formalin for 1 min, then wash them in distilled water for 2 min.
3. Dip slides in hematoxylin stain for 3 min. Hematoxylin stains the nuclei and gives it a reddish-purple color.
4. Wash slides in distilled water for 2 min.
5. Briefly dip slides in 0.25% acid alcohol (made from ethanol and HCl) to remove excess stain and then wash them in distilled water for 2 min.
6. Dip slides in bluing reagent for 1 min to change the previously stained nuclei to a blue color and then wash them in distilled water for 2 min.
7. Immerse slides in 95% ethanol for 30 s, followed by eosin stain for 1.5 min.
8. Wash slides in 95% ethanol for 30 s.
9. Dip the slides for 1 min in three separate containers with absolute ethanol.
10. Wash slides for 3 min in three separate containers with CitriSolv reagent.
11. After staining, image the slides using a Nanozoomer for analysis. H&E images for TA and soleus are presented in Figures 3 and 4, respectively.
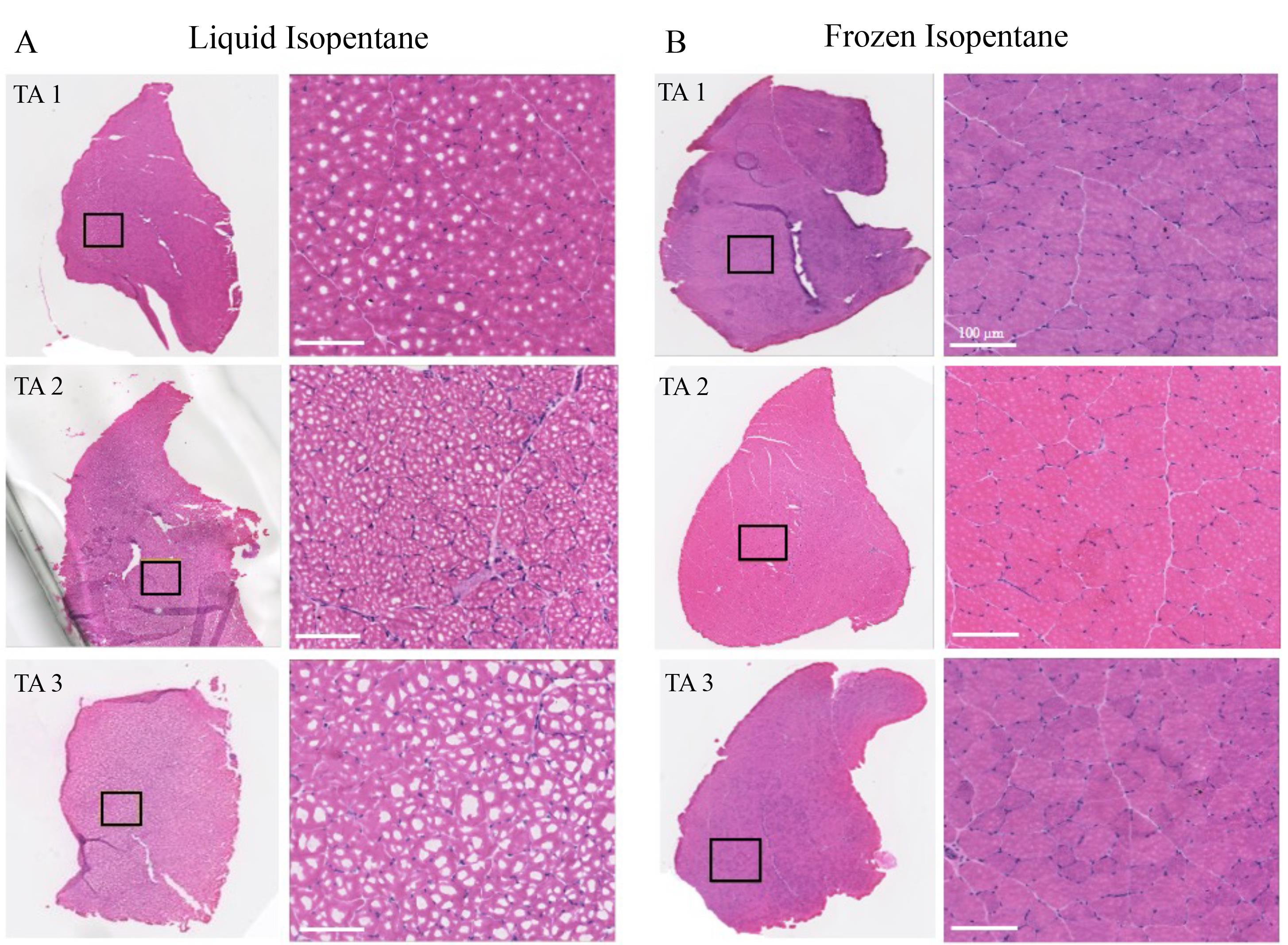
Figure 3. Comparison of H&E images of mouse tibialis anterior (TA) muscles embedded via liquid and frozen isopentane. A) Three TA muscle tissues were used for each of the preservation methods. Cell cytoplasms appear pink, and nuclei are stained blue. Images were zoomed in at randomly selected areas to look for freeze-fractures. Freeze-fracture was evident in tissues preserved by liquid isopentane. B) TA muscle preserved using frozen isopentane shows the absence of freeze-fractures and displays increased tissue integrity. Scale bar: 100 μm.
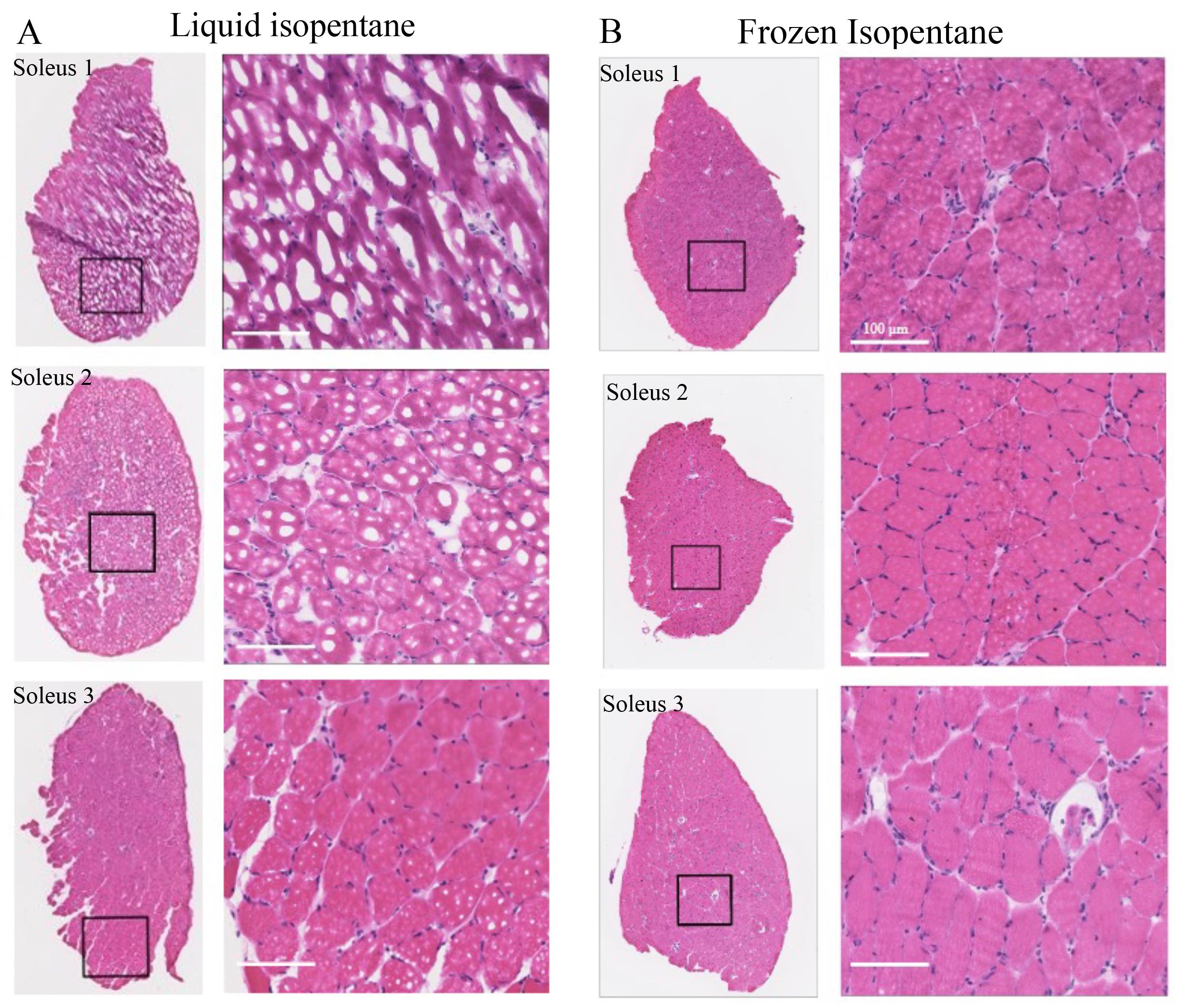 Figure 4. Comparison of H&E images of mouse soleus muscles embedded via liquid and frozen isopentane. A) Three soleus muscles were used for each of the preservation methods. As soleus is smaller than tibialis anterior (TA) and most of the other mouse muscles, it is more prone to freeze-fracture. Images were zoomed in to look for freeze-fractures. Freeze-fracture was evident in tissues preserved by liquid isopentane. B) Soleus muscles preserved using frozen isopentane show the absence of freeze-fractures. Scale bar: 100 μm.
Figure 4. Comparison of H&E images of mouse soleus muscles embedded via liquid and frozen isopentane. A) Three soleus muscles were used for each of the preservation methods. As soleus is smaller than tibialis anterior (TA) and most of the other mouse muscles, it is more prone to freeze-fracture. Images were zoomed in to look for freeze-fractures. Freeze-fracture was evident in tissues preserved by liquid isopentane. B) Soleus muscles preserved using frozen isopentane show the absence of freeze-fractures. Scale bar: 100 μm.
Data analysis
1. Five soleus muscle tissues were selected per method.
2. Total tissue area was determined by outlining the tissue in NDPI viewer software.
3. Multiple regions of damage were identified and outlined to determine their area. All the areas were summed to obtain a total freeze-fracture area.
4. Percentage of freeze-fracture was calculated. A mean of freeze-fracture percentage was obtained.
5. Statistical comparison was performed using the Student’s t-test. A p-value equal to or less than 0.05 was considered statistically significant.
Validation of protocol
Mouse muscle tissues are often frozen using ice-cold isopentane in liquid state to minimize tissue damage. This method is highly susceptible to variations caused by the phase state of isopentane and the duration of the technique, resulting in freeze-fracture in tissues. We optimized this method of cryopreservation using frozen isopentane. We validated this protocol by comparing the extent of freeze-fracture in both methods using soleus muscle and observed that when using frozen isopentane, tissue damage is minimal (~4%) compared with when freezing with liquid isopentane (~56%) (Table 1). We observed a significant difference between the two methods (N5/method). Additionally, when using frozen isopentane, the entire tissue morphology is protected. The periphery of the tissue is highly susceptible to freeze-fracture when cryopreserved in liquid isopentane; however, with the use of frozen isopentane, freeze-fracture is minimal at the periphery (Figure 5).
Table 1. Measurement of freeze-fractured area in H&E
| Total area (mm2) | Freeze-fracture area (mm2) | % Freeze-fracture | |||
| Liquid | Frozen | Liquid | Frozen | Liquid | Frozen |
| 1.3 | 1.8 | 0.5 | 0.14 | 40 | 8 |
| 1.4 | 1.4 | 1.15 | 0.04 | 81 | 3 |
| 0.8 | 1.7 | 0.29 | 0.05 | 36.1 | 2.8 |
| 1.5 | 1.5 | 1.29 | 0.06 | 88.9 | 4.1 |
| 1 | 1.2 | 0.33 | 0.02 | 34.2 | 1.5 |
| Mean | 56.04 | 3.88** | |||
| SD | 26.62073 | 2.48133 | |||
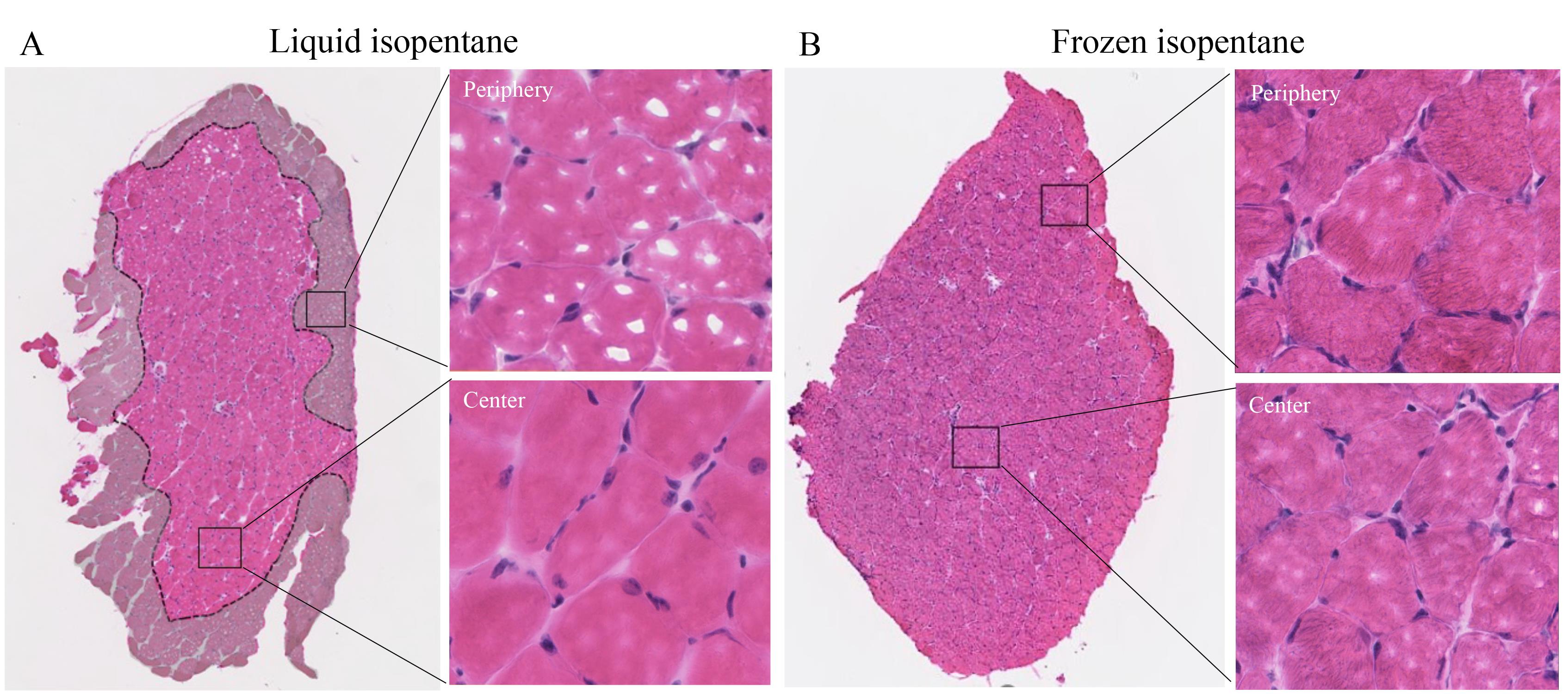
Figure 5. Identifying regions of freeze-fracture in soleus muscles. A) The tissue boundary is most susceptible to damage by freeze-fracture compared with the center. In the liquid isopentane method, tissue periphery displays significant freeze-fracture, as indicated by the greyed-out area. Zoomed views of the peripheral vs. central regions of the tissue. B) The frozen isopentane method is critical in eliminating this peripheral freeze-fracture to the tissue.
General notes and troubleshooting
1. A cold-resistant PTFE beaker or a cryogenic bowl/dish can be used instead of a modified aluminum can.
2. A large styrofoam ice box was used for the larger can. Liquid nitrogen was added to the box such that one-third of the can was submerged. The can was placed in the corner of the box for added support and to prevent the can from tipping over in the ice box. A smaller styrofoam ice box was used for the small can. The can occupied one corner of the box and was the appropriate size to be securely placed. Liquid nitrogen was added such that one-third of the can was submerged. As the box was smaller, very little liquid nitrogen was needed.
3. As the room-temperature mold containing the sample is placed on the frozen isopentane, fumes begin to form until the mold reaches the temperature of the isopentane. Meanwhile, the frozen surface beneath the mold will melt. If the mold is left too long and the can is still in liquid nitrogen, eventually the liquid isopentane will refreeze, and the mold may become stuck. In that case, remove the can and quickly extract the mold as soon as it becomes loose.
Acknowledgments
This work was funded in part by the Musculoskeletal Injury Rehabilitation Research for Operational Readiness (MIRROR), Department of Physical Medicine & Rehabilitation, Uniformed Services University, Bethesda, MD, and The Wellman Center, Boston, MA collaboration (HU00011920056). The views expressed herein are those of the authors and do not necessarily reflect the official policy or position of the Uniformed Services University, Defense Health Agency, Department of Defense, or the U.S. Government. R.R.A. was partially supported by the Lancer Endowed Chair in Dermatology.
Competing interests
The authors confirm that there are no known financial interests or personal relationships that could have influenced the work reported in this paper.
Ethical considerations
The authors state that there are no ethical conflicts associated with this research.
References
- Leiva-Cepas, F., Ruz-Caracuel, I., Peña-Toledo, M. A., Agüera-Vega, A., Jimena, I., Luque, E. and Peña-Amaro, J. (2018). Laboratory methodology for the histological study of skeletal muscle. Arch Med Deporte. 35: 254–262.
- Dubowitz, V., Sewry, C. and Oldfors, A. (2021). Preface to the Fifth Edition. In: Dubowitz, V., Sewry, C. A. and Oldfors, A. (Eds.). Muscle Biopsy (Fifth Edition). pp. vii. Elsevier.
- Kumar, A., Accorsi, A., Rhee, Y. and Girgenrath, M. (2015). Do's and Don'ts in the Preparation of Muscle Cryosections for Histological Analysis. J Visualized Exp. (99): e52793.
- Wang, C., Yue, F. and Kuang, S. (2017). Muscle Histology Characterization Using H&E Staining and Muscle Fiber Type Classification Using Immunofluorescence Staining. Bio Protoc. 7(10): e2279.
Article Information
Publication history
Received: Aug 1, 2024
Accepted: Oct 18, 2024
Available online: Nov 12, 2024
Published: Jan 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Ghag, N., Tam, J., Anderson, R. R. and Cheema, N. (2025). Cryopreservation Method for Preventing Freeze-Fracture of Small Muscle Samples. Bio-protocol 15(1): e5145. DOI: 10.21769/BioProtoc.5145.
Category
Cell Biology > Cell isolation and culture > Cryopreservation
Cell Biology > Tissue analysis > Histomorphology
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









