- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
The on-Site Monitoring and Specimen-Making of Ectoparasites on Rodents and Other Small Mammals
Published: Vol 14, Iss 21, Nov 5, 2024 DOI: 10.21769/BioProtoc.5104 Views: 1569
Reviewed by: Zeeshan BandaySatya Ranjan SahuAnonymous reviewer(s)
Abstract
The ectoparasites of rodents and other small mammals usually involve five categories of arthropods—fleas, sucking lice, gamasid mites, chigger mites, and occasionally, ticks. These ectoparasites are medically important, serving as vectors for diseases such as plague, murine typhus, scrub typhus, forest encephalitis, Lyme disease, and other zoonoses. Field surveys, collection, and specimen preparation of ectoparasites are crucial for studying taxonomy, faunistics, ecology, and epidemiology. They are also essential for vector surveillance. The present protocol summarizes the on-site monitoring and specimen-making of ectoparasites of rodents and other sympatric small mammals. Besides the collection and specimen preparation of small mammal hosts, the protocol describes in detail the collection, fixation, specimen-making, and taxonomic identification of ectoparasites and provides some monitoring indices. The on-site monitoring indices include the host density index and the infestation indices of ectoparasites (prevalence, mean abundance, mean intensity). The methodologies outlined in this protocol provide technical guidance and references for vector monitoring (surveillance) and control.
Key features
• Collection and specimen preparation of small mammal hosts, including rodents (rats, mice, and voles) and other sympatric small mammals (shrews, tree shrews, and pikas)
• Collection, fixation, specimen-making, and taxonomic identification of ectoparasites, including fleas, sucking lice, gamasid mites, chigger mites, and ticks
• On-site monitoring indices—host density index and infestation indices of ectoparasites: prevalence, mean abundance, and mean intensity
Keywords: RodentGraphical overview
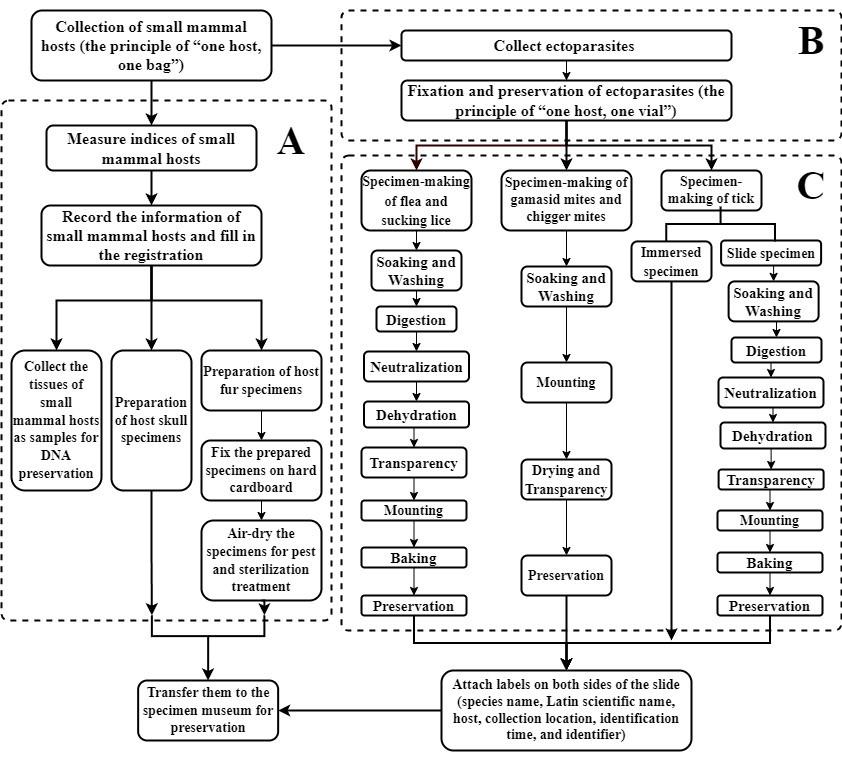
Flowchart for collection and specimen-making of ectoparasites and their hosts. A. Process for the collection and specimen preparation of small mammal hosts. B. Collection and fixation of ectoparasites. C. Process for specimen-making for different categories of ectoparasites.
Background
Rodents (rats, mice, and voles) and other sympatric small mammals (shrews, tree shrews, and pikas) often share habitats. They serve as significant infectious sources and reservoir hosts for numerous zoonotic diseases, including plague, murine typhus, scrub typhus, and hemorrhagic fever with renal syndrome [1,2]. These small mammals frequently harbor a multitude of ectoparasites such as fleas, sucking lice, gamasid mites, chigger mites, and occasionally ticks [3,4]. Rodents are of paramount importance among small mammal hosts [5]. Ectoparasites found on rodents and other small mammals act as vectors for a variety of zoonotic diseases, with fleas transmitting plague and murine typhus, ticks transmitting encephalitis and Lyme disease, chigger mites transmitting scrub typhus, and gamasid mites transmitting rickettsialpox. Gamasid mites may also serve as potential vectors for hemorrhagic fever with renal syndrome (HFRS) [6–12]. Although ectoparasites are of medical importance, to date, few sources of literature have provided a systematic and comprehensive protocol for on-site monitoring and specimen preparation of ectoparasites. Based on relevant literature and our research experience on ectoparasites, this protocol systematically describes methods for on-site monitoring and specimen-making of ectoparasites.
Small mammals in this protocol refer to rodents (Order Rodentia) and other sympatric small mammals found in the same habitats. These include insectivores (Order Eulipotyphla or Insectivora), tree shrews (Order Scandentia), and pikas (Order Lagomorpha), excluding flying bats (Order Chiroptera). Small mammals serve as hosts of ectoparasites.
Fleas and sucking lice, two groups of ectoparasites on small mammals, belong to the class Insecta from the phylum Arthropoda [13,14], and their collection and specimen preparation methodologies are similar. Gamasid mites and chigger mites, two tiny categories of arthropods, belong to the subclass Acari, class Arachnida of Arthropoda [15,16], and their collection and specimen preparation methodologies are also similar. Chigger mites are unique among Acari, with only the larval stage acting as ectoparasites; other stages are free-living [17–20]. Ticks predominantly parasitize large and medium-sized mammals, but occasionally, a few tick larvae and nymphs can be found parasitizing rodents and other small mammals [21-23]. Although ticks belong to the subclass Acari of Arachnida [24], their collection and specimen preparation methods differ from those of gamasid mites and chigger mites due to their relatively larger size.
Materials and reagents
Reagents
Cresol solution (Shanghai Hengyuan Biochemical Reagent, catalog number: 7784-24-9)
Potassium aluminum sulfate (Shanghai Hengyuan Biochemical Reagent, catalog number: 7784-24-9)
Arsenic trioxide (Yunnan Tin Co, catalog number: 1327-53-3)
Camphor powder (Shanghai Anpu Experimental Technology, catalog number: 464-49-3)
Xylene (Shanghai Aladdin Biochemical Technology, catalog number: 1330-20-7)
Distilled water
Canadian balsam (Shanghai Macklin Biochemical Technology, catalog number: 8007-47-4), neutral balsam (Shanghai Macklin Biochemical Technology, catalog number: 96949-21-2), or fir balsam (Aibixin Biotechnology, catalog number: 8016-42-0)
Pomegranate oil (Xi’an Rongbai Biological Technology, catalog number: 84961-57-9) or clove oil (Jiangxi Baicao Pharmaceutical, catalog number: 8000-34-8)
Arabic gum (Shanghai Yiji Industrial, catalog number: 9000-01-5)
Formaldehyde (Beijing Qinling Pharmaceutical Technology, catalog number: 79083-29-7)
Glycerin (Shanghai Aladdin Biochemical Technology, catalog number 56-81-5)
10% sodium hydroxide (Shanghai Macklin Biochemical Technology, catalog number 1310-73-2) or potassium hydroxide (Shanghai Aladdin Biochemical Technology, catalog number 1310-58-3) solution
5%–10% cresol soap solution (Lysol) (see Recipes)
Alum arsenic preservative (see Recipes)
Hoyer’s solution or Berlese’s solution (see Recipes)
Different concentrations of ethanol (30%, 50%, 70%, 80%, 90%, 95% and 100% anhydrous ethanol)
The different concentrations of ethanol can be prepared by diluting anhydrous ethanol with distilled water. Anhydrous ethanol is a kind of very commonly used reagent with a wide choice of catalog numbers and manufacturers. We use anhydrous ethanol from Wuhan Jisi Instruments & Equipment (catalog number: 64-17-5).
Recipes
5%–10% cresol soap solution (Lysol)
5 mL of cresol solution + 95 mL of distilled water for making 5% cresol soap solution.
10 mL of cresol solution + 90 mL of distilled water for making 10% cresol soap solution.
In a beaker, mix the cresol and water and shake or stir until the cresol is completely dissolved in the water.
Alum arsenic preservative
Potassium aluminum sulfate (powder, accounting for 40% or 70%) + arsenic trioxide (powder, 20%) + camphor powder (40% or 10%). Mix them together.
Hoyer’s solution or Berlese’s solution
Hoyer’s solution (or Hoyer’s medium)
50 mL of distilled water, 30 g of Arabic gum, 200 g of formaldehyde, 20 mL of glycerin.
Berlese’s solution (or Berlese medium)
20 mL of distilled water, 15 g of Arabic gum, 160 g of formaldehyde, 20 mL of glycerin.
Laboratory supplies
Collection and specimen preparation of small mammal hosts
Fieldwork equipment (field tent, backpack, raincoat, non-slip shoes, flashlight)
Disinfection and protection materials: 70% or 75% ethanol (Wuhan Jisi Instruments & Equipment, catalog number: 64-17-5), protective clothing and hat, medical latex gloves, mask
Trapping materials
Rat cage (18 cm × 12 cm × 9 cm, Jiangxi Guixi Li’s Rat Trap Equipment Co., Ltd.)
Food bait (peanuts, corn, cooked meat, fried dough sticks, steamed buns)
Disposable white cloth bag
Recording materials (pen, marker, pencil, collection information record book)
Specimen preparation materials for small mammals [lancet, surgical scissors, tweezers, steel ruler or tape measure, electronic scale, degreased cotton, bamboo sticks, wooden sticks, suture needles, specimen labels, beakers, alcohol lamps (for boiling small animal skulls), ether, alum arsenic preservative]
Host tissue sample collection and preservation materials: Cryopreservation tubes and 95%–100% alcohol
Collection and fixation of ectoparasites
White square plate (large size: 565 mm × 400 mm, small size: 385 mm × 285 mm)
Lidded centrifuge tubes
Ophthalmic forceps
70% or 75% ethanol
Marking pens
Specimen-making of flea and sucking lice
Glass slides
Coverslips
Baking plate or baking rack
Specimen box
Marker pen
Label paper
Specimen-making of gamasid mites and chigger mites
Petri dish
Brush
Slide
Coverslip
Marker pen
Label paper
Specimen-making of ticks
Wide-mouth bottle
Cover glass
Sealing wax
Equipment
Stereomicroscope (Leica, model: DM3000)
Baking oven (Shanghai Yiheng Scientific Instrument, model: DHG-9420A)
Procedure
Collection and specimen preparation of small mammal hosts
Collection of small mammal hosts
Select the survey site and set rat cages: Prior to placing the rat cage, you should observe signs such as food residues, feces, rat trails, and rat holes. Identify areas frequently visited by rodents and strategically place the rat cage along their activity route to enhance the capture rate (Figure 1). Concurrently, conceal the rat cage with branches or weeds and mark its location for easy retrieval.
Choose food baits: This can be flexible according to the specific situation. For trapping house rats and mice in residential areas, choose baits such as cooked meat, fried dough sticks, and steamed buns. For trapping wild rodents (rats, mice, and voles) in outdoor environments, choose baits such as peanuts and corn.
At the chosen field survey site, place rat cages (trap cages) with baits in the afternoon or evening of the survey day to trap rodents and other sympatric small mammals (hosts) in various geographical and ecological environments, including residential areas (mainly indoor habitats) and different types of outdoor habitats such as farmlands, grasslands, shrubs, woodlands and forests [25,26]. Based on our experience, it is advisable to set rat cages between 4:00 PM and 6:30 PM in the afternoon.
In monitoring the density of small mammal hosts, quantitative placement of rat cages is required. In indoor habitats (human houses, poultry sheds, pig pens, cattle stalls, stables, barns, and warehouses), place one cage every 15 square meters (per 15 m2) along the foot of the wall. In outdoor habitats, place all 25 cages in a group in a straight line, with a spacing of 5 m and a row spacing of 20 m. In paddy fields, place cages along the ridges.
The number of cages placed at each survey point should be as uniform as possible to ensure the homogeneity and comparability of the samples from each survey point.
After being placed for three consecutive days, relocate to a new location and repeat the process [27].
Collect the captured small mammal hosts: The following morning, collect the captured rodents and other sympatric small mammal hosts and place them, along with the rat cage, into a white cloth rat bag, ensuring it is securely tied to prevent ectoparasites from escaping (Figure 1). Adhere to the principle of “one host (one small mammal), one bag” when placing host mammals into the rat bag.
Transport the bag to a temporary on-site laboratory for ectoparasite collection.
Prior to reuse, the rat bag must be thoroughly cleaned and disinfected to prevent cross-contamination of ectoparasites between different host mammals [28].
Collect ectoparasites: Rodent hosts considered pests can be euthanized by cervical dislocation or anesthesia (using ether). Afterward, collect their ectoparasites. Small mammal hosts that do not require euthanasia can be anesthetized into a deep coma for ectoparasite collection.
Once collection is complete, release the small mammals back into their natural habitats after the effects of anesthesia have subsided.
After ectoparasite collection, collect tissue specimens (muscle, bone, or internal organ specimens) from the host mammals, if needed. Store these collected tissue specimens in cryopreservation tubes with 95%–100% alcohol or other specialized fixatives for future use (e.g., DNA extraction).
Record the relevant information of the collected host mammals according to Tables 1 and 2.
Critical: Rodents and other small mammals are frequently carriers of numerous pathogens. During the collection and preparation of specimens from these hosts, it is advised to wear protective clothing, hats, medical gloves, and masks to ensure personal safety. The carcasses and internal organs of euthanized rodents should be immersed in a 5%–10% cresol soap solution (Lysol) for disinfection for a period of 24–48 h. Subsequently, they should be buried deep to prevent the spread and transmission of pathogens present in the rodent body [29].
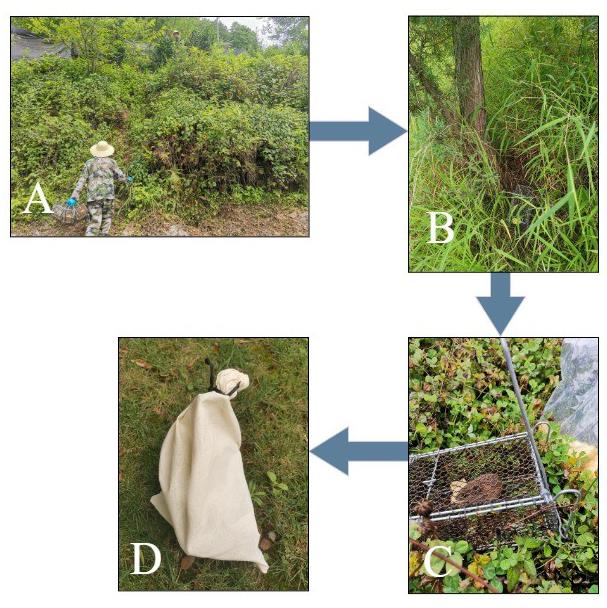
Figure 1. Collection of small mammal hosts. A. Place rat cages (trap cages) at the chosen site. B. Strategically place the rat cage along the activity route of rodents and other sympatric small mammals (hosts) and mark the location for easy retrieval. C. A captured host inside a rat cage. D. Place the animal and the rat cage into a white cloth rat bag, ensuring it is securely tied to prevent ectoparasites from escaping.Table 1. Basic information registration form for survey and collection sites of small mammal hosts
Date (year/month/day) Collection site name Geographic landscape, habitat, and altitude (m) of the collection site Climate factor records of the collection site Landscape Habitat Altitude Weather conditions Temperature ℃ Relative humidity % Other 2024/6/11 Dali Flatland Grasslands 2,005 m Sunny 27 ℃ 83% - 2024/6/12 Heqing Mountainous Forests 2,196 m Rainy 26 ℃ 76% -
Note: Geographic landscapes include mountainous and flatland landscapes. Habitats include residential areas (mainly indoor habitats) and different types of outdoor habitats such as farmlands, grasslands, shrubs, woodlands, and forests. Weather conditions include sunny, cloudy, and rainy (drizzle, moderate rain, heavy rain) conditions.Table 2. Basic information registration form for small mammal hosts
Collector: Host animal identifier: Information recorder:
Host number Collection time and location Host mammal name Host sex and age Host body index measurement Other information Date Collection site Sex Age Weight (g) Body length (cm) Tail length (cm) Ear height (cm) Hind foot length (cm) 1 2021/6/21 Bingchuan Mus caroli Male Adult 20 95 85 14 18 - 2 2021/6/21 Bingchuan Apodemus chevrieri Female Adult 34 110 90 15 21 - Note: Collection time and collection site are the same as in Table 1. Host age refers to adult or juvenile.
Preparation of host fur specimens:
Skinning: Euthanize small mammals via cervical dislocation or anesthesia. Under stringent personal protection measures, use surgical lancets and scissors to dissect and fully excise all muscles, bones, and internal organs from the euthanized rodent (dead rodent), ensuring a complete rodent skin is obtained.
Preservation: Uniformly paint an alum arsenic preservative to the inner surface of the rodent skin. This preservative serves to prevent the decay and pest infestation of the animal fur.
Filling or prosthesis support: If a posture specimen is required, first construct a dummy frame using wires, comparable in size and shape to the rodent body. Fill this frame with cotton or other suitable materials. For a general taxidermy specimen, provide support to the feet and tail using bamboo sticks or wooden sticks, filling the remaining parts directly.
Suturing and shaping: Sew the rodent skin using a needle and thread and adjust its shape and posture through a process termed “shaping.”
Fixation: Upon successful creation of the taxidermy specimen, affix the posture specimen to an appropriate platform for ease of display. Accompany each specimen with a label (either label paper or a label card) with information such as the scientific name of the rodent (ordinary name and complete Latin name), collection location and habitat, altitude, collection time, collector’s name, identifier’s name, and identification time (Figure 2, Figure 3A).

Figure 2. Posture specimen (Rattus tanezumi) (modified by Pan et al. [30])
Figure 3. Representative images of small animal host specimens (Rattus tanezumi). A-1. Dorsal view of taxidermy specimen. A-2. Ventral view of taxidermy specimen. A-3. Lateral view of taxidermy specimen. B-1. Overall dorsal view of skull specimen. B-2. Overall ventral view of skull specimen. B-3. Overall lateral view of skull specimen. B-4. View of mandibular tooth surface. B-5. Mandibular side view.
Preparation of host skull specimens
The skull and dental characteristics are important in the precise taxonomic identification of rodent hosts, thereby requiring the preparation of host skull specimens. Typically, rodent skull specimens are prepared using the boiling method:
Place the entire skull of the rodent (rat, mouse, or vole), detached from the stuffed specimen, in a container filled with water and boil it.
Once nearly cooked, remove the skull and eliminate all soft tissues such as muscles and fascia. If any soft tissue remains, return the skull to water (a small amount of sodium carbonate or sodium hydroxide can be added if necessary) and boil until all remaining soft tissue is removed.
Once all soft tissues are removed and the skull is naturally dried, it becomes the host skull specimen (Figure 3-B).
Collection and fixation of ectoparasites
Collection of ectoparasites
The collection procedures and methods for the aforementioned ectoparasites are similar and are thus described collectively here (Figure 4).
To prevent ectoparasites from attacking and biting the operator, anesthetize both the host mammals and the ectoparasites with ether prior to the collection of ectoparasites: place the host mammals, which have been captured on-site and enclosed in a sealed rat bag, into a sealed container (a large plastic bucket with a lid suffices).
Subsequently, add several cotton balls soaked in ether for anesthesia until the host mammals are either deceased or deeply comatose.
Then, place host mammals in a pre-prepared simple device, known as the “double-plate device,” to facilitate the collection of all ectoparasites from each host mammal individually. This device consists of a small white square plate of suitable size placed within a larger white square plate. Add an adequate volume of water to the large square plate and apply insect repellent around the edges of both plates. This special device is designed to prevent the escape of ectoparasites that have not been fully anesthetized by ether or those that have revived after anesthesia during the collection process. Here, the water in the large plate and the insect repellent form two types of barriers.
Use a toothbrush or comb to brush the host mammals from head to tail 2–3 times, aiming to brush the majority of the ectoparasites into the small plate. Combing 2–3 times allow most fleas, sucking lice, and gamasid mites to be obtained.
Use ophthalmic forceps to clamp the parasites that have been brushed into the small plate or a paintbrush dipped in fixative to dip them.
Simultaneously, check the parasites attached to the white rat bag and those that have fallen outside the small plate and into the water of the large plate.
After the combing collection is completed, carefully check the parasites remaining in the host’s fur from head to tail and collect them using the hair-turning method, including ticks that may be tightly attached to the host’s skin.
Only larvae of chigger mites (chiggers) parasitize the body surface of rodents and other small mammals. Chiggers are extremely small and typically attach to the thin skin of the host mammals’ bilateral auricles, the base of the external ear, and the opening of the external ear canal. Prioritize these parts for collection. A simple and practical method for collecting chiggers involves using a magnifying glass and a lancet or a scraper such as an ear spoon to scrape off the chiggers and chigger-like debris (suspected chiggers) attached to the bilateral auricles, ear canals, perianal area, perineum, and groin.
Place collected ectoparasites into a lidded centrifuge tube containing 70% or 75% ethanol for fixation and preservation (see step B2).
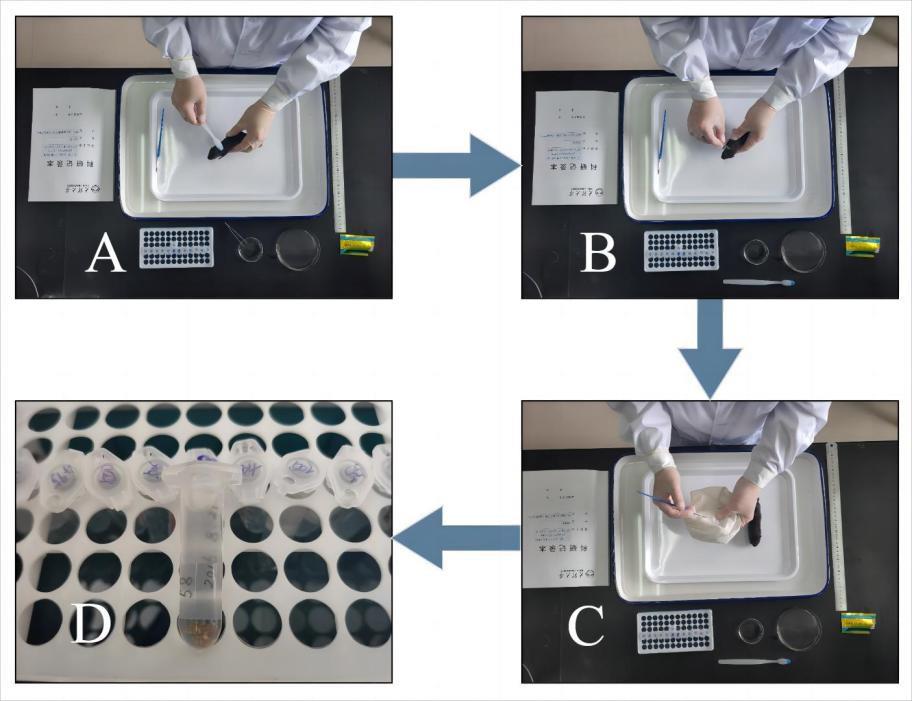
Figure 4. Collection and fixation of ectoparasites. A toothbrush or comb is used to brush the small mammal host from head to tail 2–3 times, causing ectoparasites to fall into the double-plate device for collection. B. An ophthalmic forceps is used to collect (lightly “clamp”) parasites from the body surface of the host. C. The parasites attached to the white rat bag are checked. D. The collected ectoparasites are placed into a lidded centrifuge tube containing 70% or 75% ethanol for fixation.Fixation and preservation of ectoparasites
Adhering to the principle of “one host, one vial”, place all collected ectoparasites into a container filled with 70% or 75% ethanol for fixation and preservation. Depending on the specific situation, containers such as standard glass vials, lidded centrifuge tubes, or Eppendorf tubes can be chosen.
Use an oil-based marker to label the container used for fixation and preservation.
Mark a label paper with a number using a pencil and place it inside the container to prevent confusion among ectoparasites from different hosts.
Note: Labels attached to the outside of the container can occasionally fade, and the pencil label here ensures that the number of samples collected can be accurately identified in the case of the fading of the external label, thus achieving double insurance.
Precautions: Gamasid mites and chiggers are small, with chiggers being particularly tiny. It is usually challenging to recognize chiggers with the naked eye, necessitating the use of a magnifying glass during the collection process. When collecting chiggers, also scrape off and collect the chigger-like debris (suspected chiggers) to prevent loss due to their minuscule size. If molecular biological research on the collected ectoparasites is required, or if there is a need to detect and isolate pathogens from the ectoparasites, fix and preserve collected ectoparasites with 95% ethanol or a special fixative in accordance with specific requirements. If long-term preservation is required, place ectoparasites in liquid nitrogen, which can be stored indefinitely.
Specimen-making of flea and sucking lice
Soaking and washing
Transfer the fleas and sucking lice, which are preserved in 70% or 75% ethanol, into a culture dish filled with clean or distilled water.
Allow them to soak and wash for a duration of 30–60 min, then proceed to remove any debris.
Digestion
Move the thoroughly washed flea and sucking lice into a solution of 5% or 10% potassium hydroxide or sodium hydroxide. This will corrode and digest their soft tissues and body contents.
The digestion process typically takes 1–3 days, depending on the size of specimens and ambient temperature. Larger specimens and lower room temperatures will extend the digestion time.
Neutralization
Transfer the digested flea and sucking louse specimens into clean or distilled water and wash them 2–3 times for 30 min each.
Then, move them into a 5% acetic acid solution and let them soak for 30 min. This step neutralizes the strong alkali (potassium hydroxide or sodium hydroxide) present in the fleas and sucking lice, halting the digestion process.
Finally, transfer them into clean or distilled water and soak and wash them 2–3 times for 30 min each.
Dehydration
Transfer the digested and neutralized specimens into a sequence of ethanol gradients with concentrations of 30%, 50%, 70%, 80%, 90%, 95%, and 100% for gradual dehydration.
Let soak with each ethanol gradient for 30–60 min.
Transparency
Move the dehydrated specimens into a mixture of anhydrous ethanol and xylene (mixed in a 1:1 ratio) and pure xylene for transparency.
Soak for 5–10 min each soaking until the specimens become transparent. Avoid excessive soaking time to prevent over-transparency.
Alternatively, directly transfer the dehydrated specimens into clove oil for a 30–60 min soak to achieve transparency.
Mounting
Place 2–3 drops of lipid-soluble adhesives such as Canada balsam, neutral balsam, or fir balsam on the center of a clean glass slide.
Use a dissecting needle to position the transparent fleas and sucking lice in the adhesive.
Under a stereomicroscope (dissecting microscope), adjust the position of the specimens so that the head end faces backward, and the limbs are extended. Then, cover it with a coverslip.
Ideally, only one specimen should be mounted on each glass slide. If two specimens of the same species are available, they can be mounted on one slide.
Baking
Place the mounted specimens flat on a special baking plate or rack and dry them in an oven at 45–60 °C.
Drying and curing generally takes 24–72 h.
Preservation
Observe the dried slide specimens under a microscope.
After completing taxonomic identification, attach a label to each side of the slide. The label should indicate the host animal collected and its Latin scientific name, collection location, collection time, collector, specimen’s species name and Latin scientific name, identification time, and identifier (Figure 5).
Store the labeled specimens individually in a specimen box in a special specimen cabinet.
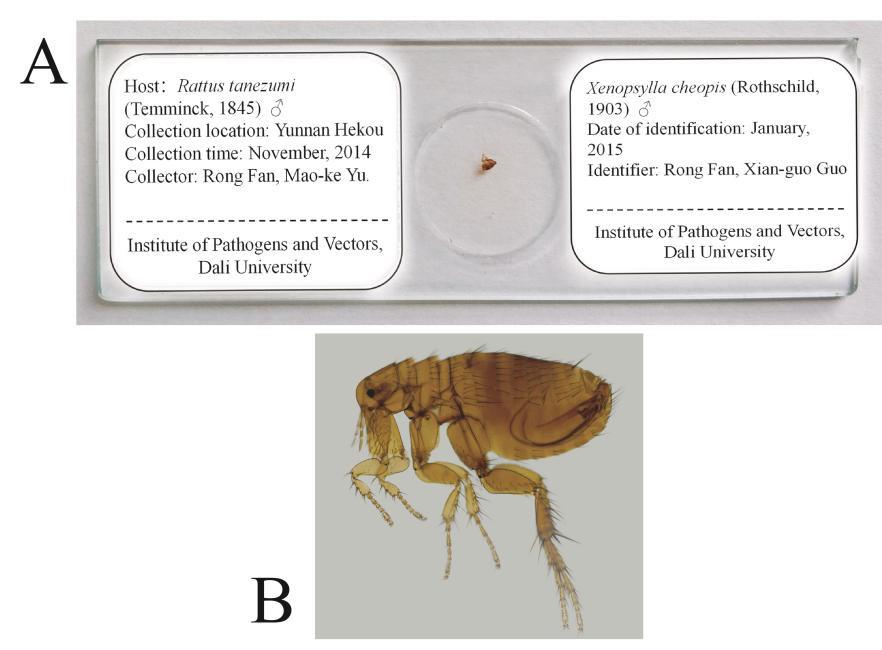
Figure 5. Specimen-making of fleas and sucking lice for the taxonomic identification under a microscope. A. The glass slide specimen of a flea with the Latin scientific name, collection location, collection time, and collector. B. A microscopic image of the flea Xenopsylla cheopis (Rothschild, 1903) [31], ♂.
Specimen-making of gamasid mites and chigger mites
Gamasid mites and chigger mites, both belonging to the class Arachnida, Arthropoda, share similar specimen-making steps and methods. Given their small size and soft chitinous exoskeletons, particularly in the case of chiggers, specimen-making typically involves the use of Hoyer’s or Berlese’s solution for direct mounting, with Hoyer’s solution being the more common choice. This method bypasses the need for digestion with potassium hydroxide (or sodium hydroxide) solution and gradual dehydration with an ethanol gradient of varying concentrations, followed by the application of water-soluble Arabic gum for mounting. However, for the few mites that are larger and possess thicker chitinous exoskeletons, the preparation method used for fleas and sucking lice is recommended, which involves digestion and gradual dehydration prior to mounting with lipid-soluble adhesives. The following outlines the steps and methods for the specimen-making of gamasid mites and chiggers:
Soaking and washing
Transfer the gamasid mites and chiggers, which have been fixed and preserved in 70% or 75% ethanol, into a Petri dish containing clean or distilled water.
Soak and wash for 30–60 min, depending on the size of the mites, and remove any debris.
Mounting
Place 2–3 drops of mounting fluid (Hoyer’s solution or Berlese’s solution) on the center of a clean slide.
Use a dissecting needle or brush to transfer the washed gamasid mites or chiggers into the mounting fluid.
Adjust the position under the stereomicroscope so that the head end faces backward, the ventral side faces upward, and the limbs are extended.
Cover with a coverslip.
Mount one specimen per slide; however, two specimens of the same species can also be mounted, one with the dorsal side facing up and the other with the ventral side facing up.
Drying and transparency
Lay the mounted slide specimens flat on a specialized baking plate or rack and bake in an oven at 45–60 °C for 7–15 days or longer until the mounting fluid has dried.
Monitor the process closely. If the mounting fluid shrinks, add more mounting fluid from the side of the coverslip using a dissecting needle.
Once dried and transparent, the specimens can be clearly observed under a microscope for morphological structure examination and taxonomic identification.
Preservation
After the dried slide specimens have been identified under a microscope, attach double labels to each side of the slide following the method described in Section C. Write relevant information on the labels.
Specimen-making of ticks
As mentioned above, ticks are also members of the class Arachnida, Arthropoda, which predominantly parasitize the body surface of large and medium-sized mammals. However, a minority of tick larvae and nymphs can be found in small mammals, such as rodents. Given their size, larger ticks do not typically require slide specimen-making and can be directly preserved in a fixed solution. Smaller ticks, including larvae and nymphs of certain species, can be prepared as slide specimens using the method outlined in Section C.
Immersed specimens
For larger ticks, field-collected specimens can be transferred to a wide-mouth bottle containing 70% or 75% ethanol for long-term preservation.
Seal the bottle cap with sealing wax.
Attach a label detailing the host’s name and Latin scientific name, collection location and time, collector, specimen’s name and Latin scientific name, identification time, and identifier.
Slide specimens
Refer to the method outlined in Section C.
Taxonomic identification of ectoparasites
Specimen preparation
Once the collection, fixation, preservation, and specimen-making of ectoparasites are completed, identify each type of ectoparasites as soon as possible.
Ticks, typically larger in size, can be directly placed under a stereomicroscope for taxonomic identification using fixed and preserved specimens. Fleas, sucking lice, gamasid mites, chiggers, and smaller ticks require slide specimen-making before being placed under the stereomicroscope for taxonomic identification.
Literature preparation for taxonomic identification: Prior to formal identification, it is essential to prepare the necessary identification tools, books, and literature related to taxonomic identification, including taxonomic keys.
Morphological observation
Using the taxonomic tools, books, literature, and taxonomic keys, carefully observe, measure, and compare the relevant morphological structures and characteristics.
Each ectoparasite specimen should be gradually identified according to the taxonomic levels of order, family, genus, and species, with an aim to identify it to the “species” level as much as possible.
Some specimens may be unclear due to the arthropod body being wrapped or contaminated by debris, broken limbs, hair loss, or excessive intestinal contents, and cannot be accurately identified. A small number of specimens may be new species that have not yet been recognized and cannot be identified for the time being. In such cases, they can be recorded as “tentative species” or “unidentified species” in the original data.
Data analysis
On-site monitoring indices for ectoparasites and their hosts
Host density indices.
For small mammal hosts of ectoparasites, it is usually necessary to calculate their density indices. The following indices (area density and cage-day density) can be used to reflect their density:
Where Da represents the area density, A represents the unit area (such as per square meter, etc.), H represents the number of small mammal hosts, Dm represents the cage-day density, MC represents the unit cage number (such as per 100 cages, etc.), H represents the number of hosts, and D represents the number of days. The area density (Da) is the number of hosts captured per unit area, and the cage-day density (Dm) is the number of hosts captured per unit cage number per day.
Ectoparasite composition ratio and infestation indices.
The constituent ratio (Cr) of ectoparasites is the percentage of the number of individuals of a certain parasite species (or a certain parasite group) to the total number of all parasite species (or all parasite groups) examined. The infestation indices include three indices: prevalence (P), mean abundance (MA), and mean intensity (MI). Prevalence is also known as the infestation rate, and mean abundance is also known as the infestation index. The calculation formula is as follows:
Where Ni is the number of individuals of a certain parasite species (or a certain parasite group), N is the total number of all parasite species (or all parasite groups), Hi is the number of host individuals infested with a certain parasite species (or a certain parasite group), and H is the total number of all host individuals examined.
Seasonal fluctuation curve
To dynamically monitor the seasonal fluctuation of a specific type of ectoparasite, a fixed survey point in the area under observation should be selected. A field survey lasting 5–7 days (one week) should be conducted every month for a year, during which small mammal host trapping and ectoparasite collection should be carried out. Following the taxonomic identification of ectoparasites, the number of ectoparasites, constituent ratio, and infestation indices should be counted monthly. Using a rectangular coordinate system, plot the month as the horizontal coordinate and the number of ectoparasites, constituent ratio, and infestation indices as the vertical coordinates to create the seasonal fluctuation curve of the ectoparasites (see Figure 6 and Figure 7) [32].
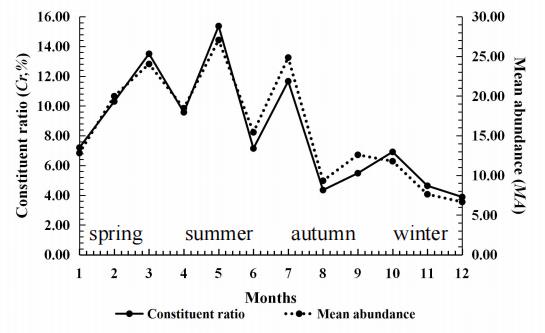
Figure 6. Seasonal fluctuation of chiggers (Walchia micropelta) on the rat Rattus brunneusculus at Jingha, southern Yunnan of China. April 2016–March 2017. Cited from Lv et al. [32].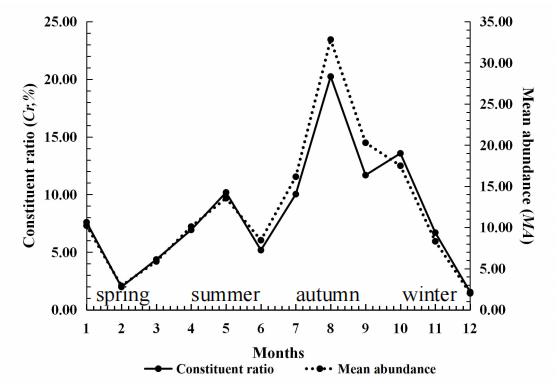
Figure 7. Seasonal fluctuation of chiggers (Ascoschoengastia indica) on the rat Rattus brunneusculus at Jingha, southern Yunnan of China. April 2016–March 2017. Cited from Lv et al. [32].
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
Song et al. [48]. Potential distribution of Leptotrombidium scutellare in Yunnan and Sichuan Provinces, China, and its association with mite-borne disease transmission. Parasites & Vectors.
General notes and troubleshooting
For taxonomic identification of small mammal hosts, refer to the following literature: Handbook of the Mammals of the World. Volume 6: Lagomorphs and Rodents I [33], Handbook of the Mammals of the World. Volume 7: Rodents II [34], Catalogue of mammals in China [35], and Taxonomy and distribution of mammals in China [36]. Host tissues (e.g., muscle, liver, or spleen) can be collected from deceased hosts for other research purposes, including DNA extraction of hosts and DNA detection of relevant pathogens (viruses, bacteria, etc.).
For taxonomic identification of ectoparasites, together with the relevant reports on new species, refer to the following literature: The Sucking Lice of North America: An Illustrated Manual for Identification [37], Economic Insect Fauna of China Fasc. 40 Acari: Dermanyssoidese [38], Trombiculid mites of China: studies on vector and pathogen of tsutsugamushi disease [39], Classification and retrieval of sucking lice in China [40], Fleas [41], Ticks [42], A Manual of Acarology [43], Biology of Ticks, Volume 2 [44], Hard Ticks (Acari: Ixodida: Ixodidae) Parasitizing Humans [45], Fleas and Ticks in Small Animals [46], and The Flea [47].
With the exception of ticks, most ectoparasites (fleas, sucking lice, chiggers, and gamasid mites) are too small to be directly observed and identified, and slide specimens must be made before the taxonomic identification under a microscope. Fleas and sucking lice fall within the class Insecta of Arthropoda, and their specimen-making steps and methods are similar. For optimal preservation, it is generally recommended to use lipid-soluble adhesives such as Canada balsam, neutral gum, or fir gum to mount the flea and sucking louse onto glass slides, which ensures long-term or permanent preservation. However, when faced with a large number of fleas and sucking lice collected on-site, achieving permanent mounting becomes challenging. As an alternative, Hoyer’s or Berlese’s solution (containing Arabic gum) can be used for mounting, in which each glass slide can accommodate multiple individuals of fleas or sucking lice.
Lysol is an irritating liquid, and arsenic is a highly toxic substance. Technicians and relevant operators must wear protective clothing, gloves, and masks to prevent skin contact, oral ingestion, or inhalation of the reagents. They must strictly comply with the regulations on the use and management of highly toxic substances in the process of purchasing, preparing, using, and storing arsenic.
Acknowledgments
We’d like to express our sincere thanks to the following people for their kind help and suggestions: Rong Fan, Cheng-Fu Zhao, Zhi-Wei Zhang, and Ya-Fei Zhao. The study was supported by the Major Science and Technique Programs from Yunnan Province (Grant No. 202102AA310055-X) to Xian-Guo Guo, and the expert workstation for Dao-Chao Jin in Dali Prefecture.
Competing interests
The authors declare they have no conflict of interest.
References
- Bowman, D. D. (1999). Georgis’ Parasitology for Veterinarians. 9th edition. Philadelphia, Saunders.
- Donham, K. J. (2016). Zoonotic diseases: overview of occupational hazards in agriculture. Agricultural Medicine: Rural Occupational and Environmental Health, Safety, and Prevention, pp.39–42.
- Walker, A. R. (1994). Arthropods of humans and domestic animals: a guide to preliminary identification, London, Britain, Springer Science & Business Media.
- Mathison, B. A. and Pritt, B. S. (2014). Laboratory Identification of Arthropod Ectoparasites. Clin Microbiol Rev. 27(1): 48–67. https://doi.org/10.1128/cmr.00008-13
- Baker, D.G. (2006). Parasitic diseases. The laboratory rat. pp.453-478.
- Moro, C. V., Chauve, C. and Zenner, L. (2005.) Vectorial role of some dermanyssoid mites (Acari, Mesostigmata, Dermanyssoidea). Parasite 12(2): 99-109. https://doi.org/10.1051/parasite/2005122099
- Eisen, R. J. and Gage, K. L. (2012). Transmission of Flea-Borne Zoonotic Agents. Annu Rev Entomol. 57(1): 61–82. https://doi.org/10.1146/annurev-ento-120710-100717
- Takayama-Ito, M., Lim, C. K., Yamaguchi, Y., Posadas-Herrera, G., Kato, H., Iizuka, I., Islam, M. T., Morimoto, K. and Saijo, M. (2018). Replication-incompetent rabies virus vector harboring glycoprotein gene of lymphocytic choriomeningitis virus (LCMV) protects mice from LCMV challenge. PLoS NeglTrop Dis. 12(4): e0006398. https://doi.org/10.1371/journal.pntd.0006398
- Boulanger, N., Boyer, P., Talagrand-Reboul, E. and Hansmann, Y. (2019). Ticks and tick-borne diseases. Medecine et maladies infectieuses. 49(2): 87–97.
- Chaisiri, K., Gill, A. C., Stekolnikov, A. A., Hinjoy, S., McGarry, J. W., Darby, A. C., Morand, S. and Makepeace, B. L. (2019). Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in Thailand. Animal Microbiome : 1–17. https://doi.org/10.1101/523845
- Elliott, I., Pearson, I., Dahal, P., Thomas, N. V., Roberts, T. and Newton, P. N. (2019). Scrub typhus ecology: a systematic review of Orientia in vectors and hosts. Parasites & Vectors 12(1): 1–36. https://doi.org/10.1186/s13071-019-3751-x
- Kim, S. Y., Gill, B., Song, B. G., Chu, H., Park, W. I., Lee, H. I., Shin, E. h., Cho, S. H. and Roh, J. Y. (2019). Annual Fluctuation in Chigger Mite Populations and Orientia Tsutsugamushi Infections in Scrub Typhus Endemic Regions of South Korea. Osong Public Health Res Perspect. 10(6): 351–358. https://doi.org/10.24171/j.phrp.2019.10.6.05
- Laroche, M., Raoult, D. and Parola, P. (2019). Insects and the Transmission of Bacterial Agents. Microbial Transmission : 195–202. https://doi.org/10.1128/9781555819743.ch10
- Di Giovanni, F., Wilke, A. B. B., Beier, J. C., Pombi, M., Mendoza-Roldan, J. A., Desneux, N., Canale, A., Lucchi, A., Dantas-Torres, F., Otranto, D., et al. (2021). Parasitic strategies of arthropods of medical and veterinary importance. Entomol Gen. 41(5): 511–522. https://doi.org/10.1127/entomologia/2021/1155
- Varma, M. R. G. (1993). Ticks and mites (Acari). Medical Insects and Arachnids: 597–658. https://doi.org/10.1007/978-94-011-1554-4_18
- OConnor, B. M. (2009). Mites. In Encyclopedia of Insects. Academic Press.
- Spieksma, F. T. M. (1990). Mite biology. Clinical Reviews in Allergy. 8: 31–49 https://doi.org/10.1007/BF02914435
- Elston, D. M. (2006). What's eating you? Chiggers. Cutis. 77(6): 350-352. https://www.mdedge.com/dermatology/article/67371/whats-eating-you-chiggers
- Literak, I., Stekolnikov, A. A., Sychra, O., Dubska, L. and Taragelova, V. (2008). Larvae of chigger mites Neotrombicula spp. (Acari: Trombiculidae) exhibited Borrelia but no Anaplasma infections: a field study including birds from the Czech Carpathians as hosts of chiggers. Exp Appl Acarol. 44(4): 307–314. https://doi.org/10.1007/s10493-008-9150-1
- Dhooria, M. S. (2016). Medical and Veterinary Acarology. Fundamentals of Applied Acarology : 425–439. https://doi.org/10.1007/978-981-10-1594-6_23
- Anderson, J. F. (2002). The natural history of ticks. Medical Clinics of North America 86(2): 205–218. https://doi.org/10.1016/s0025-7125(03)00083-x
- Sonenshine, D. E. (2009). Ticks. In Encyclopedia of Insects. Academic Press. https://doi.org/10.1016/B978-0-12-374144-8.00264-2
- Madder, M., Horak, I. and Stoltsz, H. (2014). Tick identification. Pretoria: Faculty of veterinary Science University of Pretoria. 58.
- Keirans, J.E. and Durden, L.A. (2005). Tick Systematics and Identification. In Tick-Borne Diseases of Humans (eds J.L. Goodman, D.T. Dennis and D.E. Sonenshine). https://doi.org/10.1128/9781555816490.ch7
- Mohd-Taib, F. S. and Ishak, S. N. (2021). Bait preferences by different small mammal assemblages for effective cage-trapping. Malays J Sci. 40(2): 1–15. https://doi.org/10.22452/mjs.vol40no2.1
- McCleery, R., Monadjem, A., Conner, L. M., Austin, J. D. and Taylor, P. J. (2022). Methods for ecological research on terrestrial small mammals. JHU Press.
- Herbreteau, V., Jittapalapong, S., Rerkamnuaychoke, W., Chaval, Y., Cosson, J. F. and Morand, S. (2011). Protocols for field and laboratory rodent studies. Kasetsart University.
- Ge, D. Y., Cheng, J. L., Wen, Z. X., Feijó, A., Xia, L. and Yang, Q. S. (2021). A protocol for field collection and specimen preparation of glires. Bio-101, e1010667.
- Hall, W. J. and Wehr, E. E. (1953). Diseases and parasites of poultry (No. 1652). US Department of Agriculture.
- Pan, Q. H., Wang, Y. X., Yan, K. (2007). A Field Guide to the Mammals of China, Beijing, China, China Forestry Publishing House (in Chinese).
- Wu, H. Y. (2007). Fauna Sinica: Insecta Siphonaptera. Second Edition. Beijing, China, Science Press (in Chinese).
- Lv, Y., Guo, X., Jin, D., Song, W., Peng, P., Lin, H., Fan, R., Zhao, C., Zhang, Z., Mao, K., et al. (2021). Infestation and seasonal fluctuation of chigger mites on the Southeast Asian house rat (Rattus brunneusculus) in southern Yunnan Province, China. International Journal for Parasitology: Parasites and Wildlife 14: 141–149. https://doi.org/10.1016/j.ijppaw.2021.02.005
- Freitas, T. (2016). Handbook of the mammals of the world–volume 6 Lagomorphs and Rodents I. Barcelona, Spain: Lynx Edicions.
- Wilson, D. E., Lacher, T. E. and Mittermeier, R. A. (2017). Handbook of the mammals of the world, volume 7: rodents Ⅱ. Barcelona, Spain: Lynx Edicions.
- Wei, F. W., Yang, Q. S., Wu, Y., Jiang, X. L., Liu, S. Y., Li, B. G., Yang, G., Li, M., Zhou, J., Li, S. et al. (2021). Catalogue of mammals in China. Acta Theriologica Sinica. 41: 487 (in Chinese).
- Wei, F. W., Yang, Q. S., Wu, Y., Jiang, X. L., Liu, S. Y. (2022). Taxonomy and distribution of mammals in China. Beijing, Science Press (in Chinese).
- Kim, K. C., Pratt, H. D., Stojanovich, C. J. (1986). The Sucking Lice of North America: An Illustrated Manual for Identification. United States, Pennsylvania State University Press.
- Deng, G. F. and Teng, K. F. (1993). Economic Insect Fauna of China Fasc. 40 Acari: Dermanyssoidese. Beijing, China, Science Press (in Chinese).
- Li, J. C., Wang, D. Q. and Chen, X. B. (1997). Trombiculid mites of China: studies on vector and pathogen of Tsutsugamushi disease. Guangzhou, Guangdong Science and Technology Publishing (in Chinese).
- Jin, D. X. (1999). Classification and retrieval of sucking lice in China. Beijing, Science press (in Chinese).
- Petrie, K. (2008). Fleas. United States, ABDO Publishing Company.
- Petrie, K. (2008). Ticks. United States, ABDO Publishing Company.
- Krantz, G. W. and Walter, D. E. (2009). A Manual of Acarology. Third Edition. Lubbock, Texas: Texas Tech University Press.
- Sonenshine, D. E., Roe, R. M. (2014). Biology of Ticks Volume 2. USA, Oxford University Press.
- Guglielmone, A. A., Robbins, R. G. (2018). Hard Ticks (Acari: Ixodida: Ixodidae) Parasitizing Humans: A Global Overview. Germany, Springer International Publishing.
- Gram, W. D., Short, J. (2020). Fleas and Ticks in Small Animals. Spain, Grupo Asis.
- Russell, H. (2021). The Flea. India, Alpha Editions.
- Song, W. Y., Lv, Y., Yin, P. W., Yang, Y. Y. and Guo, X. G. (2023). Potential distribution of Leptotrombidium scutellare in Yunnan and Sichuan Provinces, China, and its association with mite-borne disease transmission. Parasites & Vectors16(1): e1186/s13071–023–05789–y. https://doi.org/10.1186/s13071-023-05789-y
Article Information
Publication history
Received: Mar 17, 2024
Accepted: Sep 2, 2024
Available online: Oct 22, 2024
Published: Nov 5, 2024
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Yin, P., Guo, X., Song, W., Dong, W., Lv, Y. and Jin, D. (2024). The on-Site Monitoring and Specimen-Making of Ectoparasites on Rodents and Other Small Mammals. Bio-protocol 14(21): e5104. DOI: 10.21769/BioProtoc.5104.
Category
Environmental science > Parasites
Biological Sciences > Biological techniques
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










