- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Purification of Photorhabdus Virulence Cassette (PVC) Protein Complexes from Escherichia coli for Artificial Translocation of Heterologous Cargo Proteins
(*contributed equally to this work) Published: Vol 14, Iss 7, Apr 5, 2024 DOI: 10.21769/BioProtoc.4966 Views: 2221
Reviewed by: David A. CisnerosBhanu JagilinkiJoyce ChiuGuillaume Lenoir

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Prokaryotic Expression and Purification of the hSox2-HMG Domain
Lijie Yang [...] Jingjun Hong
Aug 20, 2025 2367 Views

Cluster FLISA—A Method to Compare Protein Expression Efficiency Between Cell Lines and Subunit Clustering of Proteins
Sabrina Brockmöller and Lara Maria Molitor
Nov 5, 2025 1207 Views

On-Column Dual-Gradient Refolding for Efficient Recovery of Insoluble Affinity-Tagged Recombinant Proteins
Anna Vlaskina [...] Maxim Patrushev
Feb 5, 2026 37 Views
Abstract
Contractile injection systems (CISs), one of the most important bacterial secretion systems that transport substrates across the membrane, are a collection of diverse but evolutionarily related macromolecular devices. Numerous effector proteins can be loaded and injected by this secretion complex to their specific destinations. One group of CISs called extracellular CIS (eCIS) has been proposed as secretory molecules that can be released from the bacterial cytoplasm and attack neighboring target cells from the extracellular environment. This makes them a potential delivery vector for the transportation of various cargos without the inclusion of bacterial cells, which might elicit certain immunological responses from hosts. We have demonstrated that the Photorhabdus virulence cassette (PVC), which is a typical eCIS, could be applied as an ideal vector for the translocation of proteinaceous cargos with different physical or chemical properties. Here, we describe the in-depth purification protocol of this mega complex from Escherichia coli. The protocol provided is a simpler, faster, and more productive way of generating the eCIS complexes than available methodologies reported previously, which can facilitate the subsequent applications of these nanodevices and other eCIS in different backgrounds.
Keywords: Contractile injection systemBackground
Typical contractile injection systems (CISs) have been studied for decades for their structure, assembly, and mechanism. They can take part in multiple interactions between microorganisms and hosts by delivering nucleic acids and protein substrates using contractile sheath–tube assemblies, such as the contractile tails of bacteriophage T4, P2, and Mu [1]. The type VI secretion system, which spans both the inner and outer membranes of Gram-negative bacteria and transports substrates from the cytoplasm, is a well-studied example of a contractile apparatus [2]. Extracellular CIS (eCISs), found in both prokaryotes and archaea, such as Photorhabdus virulence cassette (PVC), the antifeeding prophage [3], and the metamorphosis-associated contractile structure [4], have also been studied extensively in recent years.
Although the species from the Photorhabdus genus are generally considered pathogens infecting insects, P. asymbiotica has been reported to infect humans in the United States and Australia [5]. Five PVC clusters have been identified in the genome of P. asymbiotica, all phylogenetically related to the tail of T4 bacteriophage. PVC could translocate toxins into eukaryotic cells [6], which means PVC is different from T4 phage or R-type pyocins, which can only target prokaryotic cells. Moreover, a group of N-terminal signal peptides are identified within the PVC effectors to facilitate the cargo loading process [7]. Taking into consideration that PVC complexes could be released outside the bacterial cells for function, this apparatus would be an ideal model for manipulation as a potent delivery vector in precision medicine [8].
The purification workflow of PVC can be divided into four steps: bacterial collection, cell rupture, cell debris removal, and protein extraction. Centrifugation and ultracentrifugation are the primary methods for bacterial collection, cell debris removal, and protein extraction. As for the cell rupture, chemical and biological methods are applied to break the cell membrane and release the PVC protein complexes. This protocol has been used several times to purify PVC complexes for structural and functional characterization experiments, demonstrating the effectiveness of this method.
Materials and reagents
15 mL centrifuge tubes (Corning, catalog number: 430791)
50 mL centrifuge tubes (Corning, catalog number: 430829)
500 mL triangular culture flask (Corning, catalog number: 431145)
1.5 mL microtubes (Axygen, catalog number: MCT-150-C-S)
Sterile syringe filter (Millipore, catalog number: SLGVR33RB)
500 mL centrifuge bottle (Nalgene, catalog number: 3120-0500)
50 mL round bottom centrifuge tubes (Biosharp, catalog number: BS-500-MC)
13.2 mL centrifuge tubes (Beckman Coulter, catalog number: 344059)
Escherichia coli strain EPI300 (Lucigen, catalog number: EC300110)
Plasmids pCNM3, pBR60, and pBBRN Pnf N50-TkTcsT, which were used previously [4,7] (Figure 1)
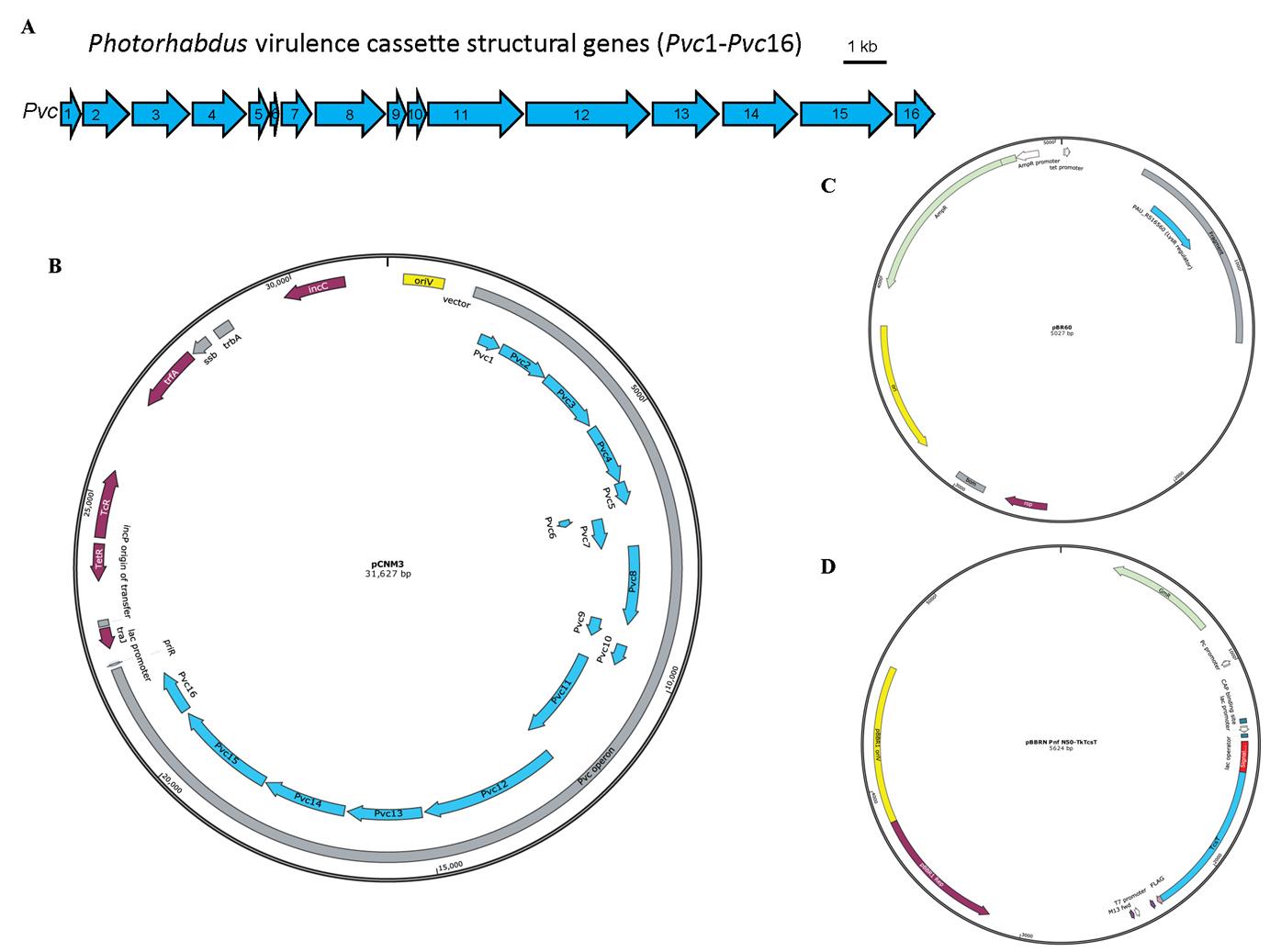
Figure 1. Schematic illustration of the Photorhabdus virulence cassette (PVC) locus and the map of plasmids used for PVC complex and cargo protein production. A. The 16 PVC structural genes (annotated Pvc1–Pvc16, shown in blue) involved in protein complex production are encoded consecutively within the P. asymbiotica genome. B–D. The plasmids pCNM3 (B), pBR60 (C), and pBBRN Pnf N50-TkTcsT (D) were constructed by our lab and the maps were generated using SnapGene. pCNM3 was constructed by ligating the complete PVC operon into pRK404 vector for constitutive expression under their own promoters [9]. pBR60 was created by ligating the DNA fragment containing the PAU_RS16560 (LysR regulator gene, shown in blue) into pBR322 plasmid (BamHI-SalI insertion) for constitutive expression of the regulator gene under its own promoter. pBBRN Pnf N50-TkTcsT was a plasmid used for cargo production. This plasmid was created by inserting the open reading frame of the toxic TkTcsT protein (shown in blue) from Trichosanthes kirilowii into a broad host range vector pBBR1MCS5, with a PVC signal peptide sequence (shown in red) fused at the N-terminus of the TkTcsT protein. This allows the production of signal peptide–fused proteins in E. coli.LB broth, powder (Solarbio, catalog number: L1010)
Phosphate-buffered saline (PBS) (Gibco, catalog number: C10010500BT)
Tetracycline HCl (Solarbio, catalog number: T8180)
Ampicillin, sodium salt (Solarbio, catalog number: A8180)
Gentamycin sulfate (Solarbio, catalog number: G8170)
Anhydrous ethanol (Beijing Chemical Works, catalog number: C320080)
TBS tablets (VWR, catalog number: K859-100TABS)
Triton X-100 solution (Sigma, catalog number: X-100)
Protease inhibitor cocktail (MCE, catalog number: HY-K0010)
Lysozyme (Sigma, catalog number: L6876)
DNase I (MCE, catalog number: HY-108882)
Sodium chloride (Solarbio, catalog number: S8210)
Magnesium chloride hexahydrate (Solarbio, catalog number: M8161)
Solution endotoxin erasol (TianDZ, catalog number: 60607-25)
Solutions
LB broth medium (see Recipes)
10 mg/mL tetracycline in solution (see Recipes)
100 mg/mL ampicillin in solution (see Recipes)
50 mg/mL gentamycin in solution (see Recipes)
5 mg/mL NaCl (see Recipes)
2 M MgCl2 (see Recipes)
20 mg/mL lysozyme (see Recipes)
5 mg/mL DNase I (see Recipes)
P buffer (see Recipes)
Recipes
LB broth medium
Add 25 g of LB broth powder to 1,000 mL of distilled water. Sterilize using a high-pressure steam sterilization pot at 121 °C for 15 min.
10 mg/mL tetracycline in solution
Add 0.5 g of tetracycline HCl to 50 mL of anhydrous ethanol. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
100 mg/mL ampicillin in solution
Add 1 g of ampicillin to 10 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
50 mg/mL gentamycin in solution
Add 1 g of gentamycin sulfate to 20 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
5 mg/mL NaCl
Dissolve 1 g of sodium chloride in 200 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
2 M MgCl2·6H2O
Dissolve 406 g of magnesium chloride hexahydrate in 800 mL of distilled water and adjust volume to 1 L. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
20 mg/mL lysozyme
Dissolve 1 g of lysozyme in 50 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
5 mg/mL DNase I
Dissolve a bottle of DNase I (100 mg) in 20 mL of NaCl solution (5 mg/mL). Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
P buffer
Take 50 mL of distilled water into a 50 mL centrifuge tube and add a piece of TBS tablet. Divide the solution into two 50 mL centrifuge tubes equally after the TBS tablet is completely dissolved.
Add 2.5 mL of 10% Triton X-100 solution, 500 μL of 20 mg/mL lysozyme, 500 μL of 5 mg/mL DNase I, 125 μL of 2 M MgCl2, and 500 μL of protease inhibitor cocktail to each tube. Add distilled water to fill 50 mL.
Notes:
P buffer should be freshly prepared.
TBS tablets could cause skin and eye irritation; avoid breathing dust, fumes, gas, mist, vapors, or spray.
Triton X-100 solution could cause serious eye damage; wear protection when using it.
The final concentration of lysozyme and DNase I are 200 µg/mL and 50 µg/mL in P buffer, respectively. Triton X-100 and MgCl2 are 50 mg/mL, 0.5%, and 5 mM, respectively.
Equipment
Innova44 Incubator Shaker Series (NBS, model: SHA1104)
Biological safety cabinet (Shanghai Lishen Scientific Equipment Co, model: Hfsafe 1200LC)
Milli-Q (Millipore, catalog number: IX 70XX)
High-pressure steam sterilization pot (YAMATO, model: SM830)
High-speed refrigerated centrifuge (Eppendorf, model: 5424 R)
Ultracentrifuge (Beckman, model: Optima L-100 XP)
-80 °C ultra-low temperature freezer (Haier, model: DW-86L728J)
Thermostatic water bath (GFL, model: GFL1002)
High-speed refrigerated centrifuge (Hitachi, model: CR22G)
Ice machine (Panasonic, model: SIM-F140BDL)
NanoDrop 1000 spectrophotometer
Procedure
Production of empty and cargo-loaded PVC complexes
The heterologous expression of the empty PVC complexes in E. coli requires two plasmids, pCNM3 and pBR60. Plasmid pCNM3 carries 16 structural genes required for PVC complexes assembly based on a tetracycline-resistant broad host range vector pRK404 (Figure 1A). Plasmid pBR60 harbors the LysR regulator gene (PAU_RS16560) fragment, which is indispensable for the maturation of PVC complexes (Figure 1C). The backbone vector for pBR60 is ampicillin-resistant pBR322 (the original tetracycline-resistance gene of pBR322 is disrupted by gene insertion). Both genes are expressed constitutively under their own promoters. The E. coli strain EPI300 co-expressing these two plasmids can be used to produce the empty PVC complexes accordingly (two plasmids in E. coli for empty PVC).
For the cargo-loaded PVC complex to be produced, a third plasmid based on pBBRN (a derivative of broad host range vector pBBR1MCS5, which introduces a NdeI site to generate a start codon at the beginning of LacZα gene) with gentamycin resistance can be used. In this protocol, the DNA fragment producing fusion protein of toxic TkTcsT and PVC signal peptide at the N-terminus is inserted into multiple cloning sites of pBBRN, whose expression is controlled by the lac promoter (Figure 1D). Similar operations can be applied to generate cargo-loaded PVC complexes using the empty PVC-producing E. coli strain mentioned above, co-transformed with the third cargo-expressing plasmid (three plasmids in E. coli for cargo-loaded PVC).
Bacterial collection
Prepare LB broth medium according to Recipe 1. Add antibiotics to the LB broth medium if needed. Prepare 10 mg/mL tetracycline solution (Recipe 2) and dilute 100 times to bring the final concentration to 10 μg/mL at the time of use. Prepare 100 mg/mL ampicillin solution (Recipe 3) and dilute 100 times to bring the final concentration to 100 μg/mL at the time of use. Prepare 50 mg/mL gentamycin solution (Recipe 4) and dilute 500 times to bring the final concentration to 10 μg/mL at the time of use.
Load 3–4 mL of LB broth medium supplemented with 100 μg/mL ampicillin and 10 μg/mL tetracycline (additional 10 μg/mL gentamycin for cargo-loaded PVC production) into 15 mL centrifuge tubes. The same type and concentration of antibiotics are used throughout step B.
Pick one single colony of E. coli EPI300 stain harboring corresponding plasmids on LB solid medium or frozen bacteria (in a -80 °C ultra-low temperature refrigerator) in LB broth medium with antibiotics and incubate overnight on a shaker at 220 rpm and 37 °C.
Inoculate 2 mL of bacteria incubated overnight (from steps B2 and B3) in 200 mL of LB broth medium with antibiotics and incubate for 24 h at 220 rpm and 30 °C in a shaker.
Load the 200 mL bacterial cultures into 500 mL centrifuge bottles and centrifuge using a high-speed refrigerated centrifuge at 12,000× g for 10 min at 4 °C.
Discard the supernatant and resuspend in 30 mL of sterile PBS.
Transfer the suspension to 50 mL centrifuge tubes and centrifuge at 3,500× g for 30 min at 4 °C.
Discard the supernatant and store the bacterial pellet in a -80 °C ultra-low temperature refrigerator overnight until use.
Cell rupture
Prepare P buffer (see Recipes).
Take out the frozen bacteria from the -80 °C ultra-low temperature refrigerator, add 10 mL of P buffer to each tube, resuspend, and incubate in a water bath at 37 °C for 30 min.
Cell debris removal
Transfer the lysate to round bottom centrifuge tubes and spin at 27,000× g for 10 min at 4 °C using a high-speed refrigerated centrifuge.
Protein extraction
Transfer the supernatant to ultracentrifuge tubes, balance with P buffer precooled at 4 °C, and centrifuge at 150,000–250,000× g for 1 h at 4 °C using an ultracentrifuge.
Discard the supernatant and resuspend sediment with 1 mL of sterile PBS precooled at 4 °C. Transfer the suspension into 1.5 mL microtubes and centrifuge at 18,400× g for 10 min at 4 °C using a high-speed refrigerated centrifuge.
Transfer the supernatant into ultracentrifuge tubes, balance with PBS precooled at 4 °C, and centrifuge at 150,000–250,000× g for 1 h at 4 °C using an ultracentrifuge.
Discard the supernatant and resuspend sediment with 200 μL of sterile PBS precooled at 4 °C. Transfer the suspension into 1.5 mL microtubes and centrifuge at 18,400× g for 10 min at 4 °C using a high-speed refrigerated centrifuge.
Collect supernatant and measure the concentration of PVC at 280 nm using a NanoDrop 1000 spectrophotometer. Store the supernatant containing the PVC particles at 4 °C (Figure 2).
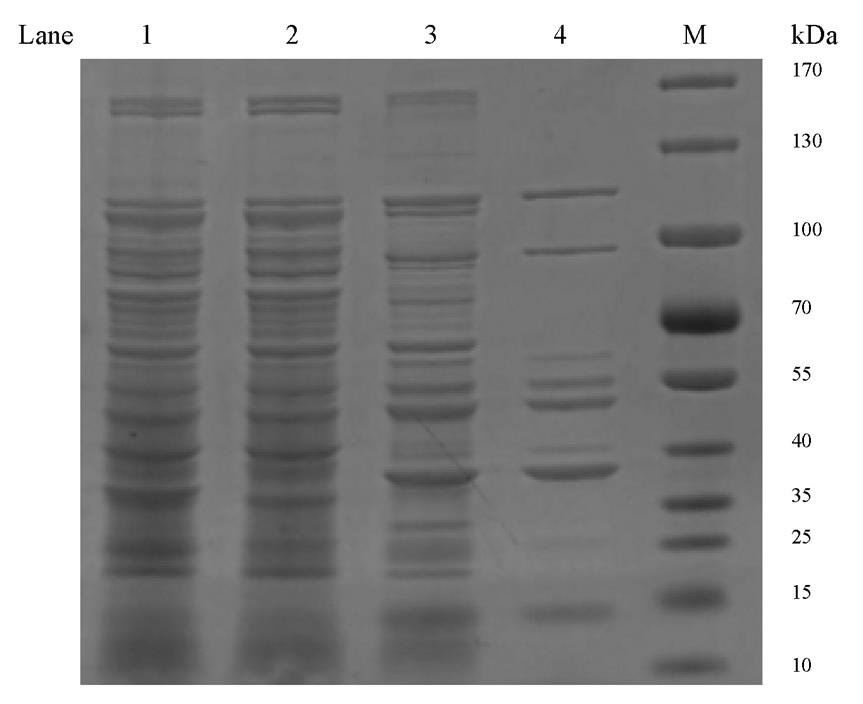
Figure 2. SDS-PAGE analysis of protein samples from major steps during Photorhabdus virulence cassette (PVC) purification. Lane 1: Whole-cell lysate from step D1. Lane 2: The supernatant from step E1. Lane 3: The supernatant from step E3. Lane 4: Final purified PVC from step E4, as used for negative staining analysis. M: Marker.
Endotoxin removal (optional)
Precool the solution endotoxin erasol reagent and the protein sample.
Add 50 μL of solution endotoxin erasol reagent to 500 μL of protein sample, mix, leave it on an ice bath for 10 min, and then incubate in a water bath at 65 °C for 20 min.
Centrifuge at 18,400× g for 10 min using a high-speed refrigerated centrifuge. Collect the supernatant.
Validation of protocol
This protocol has been used and validated in the following research articles:
Jiang et al. [7]. N-terminal signal peptides facilitate the engineering of PVC complex as a potent protein delivery system. Science Advances (Figure 1, panel D; Figure 2, panel B; Figure 3).
Wang et al. [6]. Characterization of Photorhabdus Virulence Cassette as a causative agent in the emerging pathogen Photorhabdus asymbiotica. Science China Life Sciences (Figure 1, panel B; Figure 2, panel F).
Jiang et al. [9]. Cryo-EM Structure and Assembly of an Extracellular Contractile Injection System. Cell (Figure 1; Figure 6, panel D; Figure 7, panel A; Figure S1, panel C–D).
General notes and troubleshooting
In steps B4 and B5, the OD600 of bacteria incubated overnight should be higher than 0.6. If the OD600 of bacteria is lower than 0.6, it generally indicates some problems in the culture process or that the cargo protein may be toxic to bacteria.
In step B4, incubate at 30 °C, because the protein expression and assembly at 30 °C is better than at 37 °C.
In step B8, the collected bacterial pellet can be stored at -80 °C overnight to facilitate complete rupture of the bacterial cells, which is helpful for a greater production of the PVC complexes.
In steps E1 and E3, any centrifugal force between the ranges of 150,000× g and 250,000× g can efficiently sediment the particles. For example, spinning at a higher speed produces a greater number of PVC particles but with many undesirable immature components.
In step E5, a NanoDrop 1000 spectrophotometer is used to measure the concentration of PVC protein samples. NanoDrop 1000 spectrophotometer is a convenient equipment for protein and nucleic acid concentration determination; samples as low as 2 μL can be accurately quantified. Here, the concentration of protein samples is estimated by measuring UV absorption at 280 nm. Although this method is not strictly quantitative, it is quick, convenient, and suitable for protein concentration determination in this protocol.
In step E5, the concentration of protein extracted under this protocol should generally be in the range of 1–5 mg/mL. If the concentration is higher than 10 mg/mL, it is recommended to repeat steps E4–E5 to exclude any remaining bacterial debris or other protein components.
Step F is optional; however, it is an essential step if the protein sample is to be tested on animals.
Data analysis
Negative staining is the best method to assess the quality of PVC samples in the solution [9]. Aliquots of 1–5 μL of PVC samples (depending on the protein concentration) are applied onto glow-discharged holey-carbon copper grids (300 mesh, Zhongjingkeyi, Beijing), washed, and stained with 2% uranyl acetate. The negative stain grids are dried at room temperature and checked using an electron microscope. PVC complexes, if present, are seen as bullet-shaped (Figure 3).
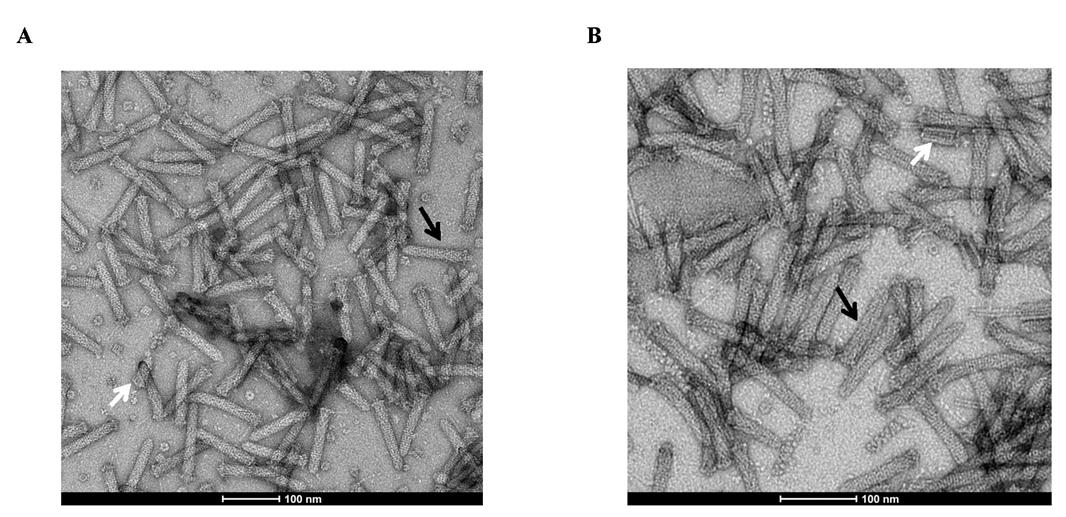
Figure 3. Representative negative-staining electron microscopy figure of Photorhabdus virulence cassette (PVC) particles purified from E. coli. Empty PVC (A) and PVC complexes loaded with TkTcsT cargos (B) share similar T4-tail-like morphology by negative staining electron microscopy observation. Mature (black arrows) and immature (white arrow) components of PVC are shown.
Acknowledgments
We thank Prof. Ning Gao and the Electron Microscopy Laboratory of Peking University for help with negative staining. We also thank all the lab members of Qi Jin and Feng Jiang for beneficial advice. The work was funded by the National Key Research and Development Program of China (2022YFC2303200), National Natural Science Foundation of China (NSFC) (32070081), the CAMS Innovation Fund for Medical Sciences (2021-I2M-1-037) and the Special Research Fund for Central Universities, Peking Union Medical College (3332023055). The original protocol was developed and used in previous works [6,7,9].
Competing interests
No competing interest is declared.
References
- Taylor, N. M. I., van Raaij, M. J. and Leiman, P. G. (2018). Contractile injection systems of bacteriophages and related systems. Mol. Microbiol. 108(1): 6–15.
- Wang, J., Brodmann, M. and Basler, M. (2019). Assembly and Subcellular Localization of Bacterial Type VI Secretion Systems. Annu. Rev. Microbiol. 73: 621–638.
- Desfosses, A., Venugopal, H., Joshi, T., Felix, J., Jessop, M., Jeong, H., Hyun, J., Heymann, J. B., Hurst, M. R. H., Gutsche, I., et al. (2019). Atomic structures of an entire contractile injection system in both the extended and contracted states. Nat. Microbiol. 4(11): 1885–1894.
- Rocchi, I., Ericson, C. F., Malter, K. E., Zargar, S., Eisenstein, F., Pilhofer, M., Beyhan, S. and Shikuma, N. J. (2019). A Bacterial Phage Tail-like Structure Kills Eukaryotic Cells by Injecting a Nuclease Effector. Cell Rep. 28(2): 295–301 e294.
- Costa, S. C., Girard, P. A., Brehelin, M. and Zumbihl, R. (2009). The emerging human pathogen Photorhabdus asymbiotica is a facultative intracellular bacterium and induces apoptosis of macrophage-like cells. Infect. Immun. 77(3): 1022–1030.
- Wang, X., Cheng, J., Shen, J., Liu, L., Li, N., Gao, N., Jiang, F. and Jin, Q. (2022). Characterization of Photorhabdus Virulence Cassette as a causative agent in the emerging pathogen Photorhabdus asymbiotica. Sci. China Life Sci. 65(3): 618–630.
- Jiang, F., Shen, J., Cheng, J., Wang, X., Yang, J., Li, N., Gao, N. and Jin, Q. (2022). N-terminal signal peptides facilitate the engineering of PVC complex as a potent protein delivery system. Sci. Adv. 8(17): eabm2343.
- Kreitz, J., Friedrich, M. J., Guru, A., Lash, B., Saito, M., Macrae, R. K. and Zhang, F. (2023). Programmable protein delivery with a bacterial contractile injection system. Nature 616(7956): 357–364.
- Jiang, F., Li, N., Wang, X., Cheng, J., Huang, Y., Yang, Y., Yang, J., Cai, B., Wang, Y. P., Jin, Q. and Gao, N. (2019). Cryo-EM Structure and Assembly of an Extracellular Contractile Injection System. Cell 177(2): 370–383 e315.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Wang, Y., Zhang, X., Feng, X., Wang, X., Jin, Q. and Jiang, F. (2024). Purification of Photorhabdus Virulence Cassette (PVC) Protein Complexes from Escherichia coli for Artificial Translocation of Heterologous Cargo Proteins. Bio-protocol 14(7): e4966. DOI: 10.21769/BioProtoc.4966.
- Jiang, F., Shen, J., Cheng, J., Wang, X., Yang, J., Li, N., Gao, N. and Jin, Q. (2022). N-terminal signal peptides facilitate the engineering of PVC complex as a potent protein delivery system. Sci. Adv. 8(17): eabm2343.
Category
Microbiology > Heterologous expression system > Escherichia coli
Biochemistry > Protein > Expression
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









