- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Protocol for Custom Biomineralization of Enzymes in Metal–Organic Frameworks (MOFs)
(*contributed equally to this work) Published: Vol 14, Iss 3, Feb 5, 2024 DOI: 10.21769/BioProtoc.4930 Views: 1885
Reviewed by: Salim GasmiNeha NandwaniAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Fluorescence-Based Flippase Assay to Monitor Lipid Transport by Drs2-Cdc50
Inja M. Van Der Linden [...] Huriye D. Uzun
Jul 20, 2025 3236 Views

An Optimized Enzyme-Coupled Spectrophotometric Method for Measuring Pyruvate Kinase Kinetics
Saurabh Upadhyay
Aug 20, 2025 2441 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 483 Views
Abstract
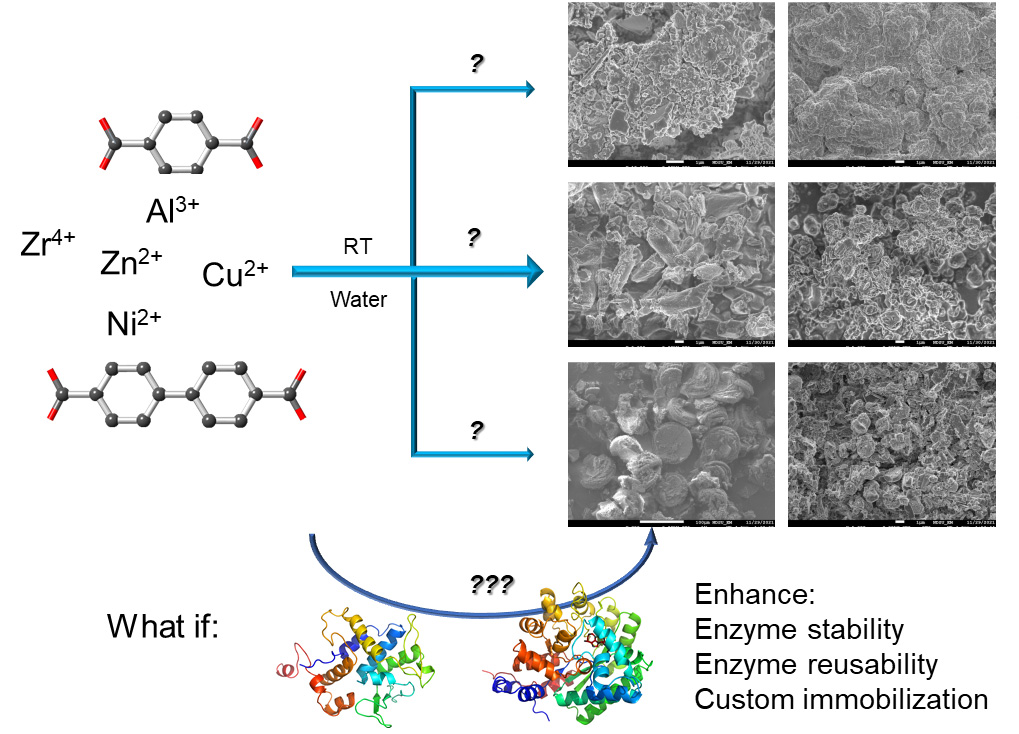
Enzyme immobilization offers a number of advantages that improve biocatalysis; however, finding a proper way to immobilize enzymes is often a challenging task. Implanting enzymes in metal–organic frameworks (MOFs) via co-crystallization, also known as biomineralization, provides enhanced reusability and stability with minimal perturbation and substrate selectivity to the enzyme. Currently, there are limited metal–ligand combinations with a proper protocol guiding the experimental procedures. We have recently explored 10 combinations that allow custom immobilization of enzymes according to enzyme stability and activity in different metals/ligands. Here, as a follow-up of that work, we present a protocol for how to carry out custom immobilization of enzymes using the available combinations of metal ions and ligands. Detailed procedures to prepare metal ions, ligands, and enzymes for their co-crystallization, together with characterization and assessment, are discussed. Precautions for each experimental step and result analysis are highlighted as well. This protocol is important for enzyme immobilization in various research and industrial fields.
Key features
• A wide selection of metal ions and ligands allows for the immobilization of enzymes in metal–organic frameworks (MOFs) via co-crystallization.
• Step-by-step enzyme immobilization procedure via co-crystallization of metal ions, organic linkers, and enzymes.
• Practical considerations and experimental conditions to synthesize the enzyme@MOF biocomposites are discussed.
• The demonstrated method can be generalized to immobilize other enzymes and find other metal ion/ligand combinations to form MOFs in water and host enzymes.
Graphical overview
Background
Enzyme immobilization is receiving increased interest in both research and industry due to the (potential) promise of enhanced cost efficiency and catalytic performance control [1–4]. The biggest challenge is still maintaining enzymatic function without disturbance to the enzyme itself [5,6]. Metal–organic frameworks (MOFs) are extended 3-dimensional crystalline networks formed by the coordination bonds between certain metal ions and ligands. MOFs typically contain supreme porosity and highly tunable properties, given the high diversity in metal ions and ligands that can form such a 3D network. MOFs are advanced enzyme immobilization platforms but are mostly limited to smaller enzymes and substrates [6–13]. Co-crystallization of enzymes and certain metal ions/ligands is a unique way to host enzymes in MOF crystal scaffolds, being adaptable to large enzymes and enzyme clusters [14,15]. This strategy also allows for a small portion of enzymes to be implanted at the surface of MOF crystals, thus being partially exposed to the reaction medium, allowing contact with substrates larger than MOF apertures [16]. This strategy can therefore be applied to biocatalytic reactions—involving large substrates such as proteins/polypeptides, polysaccharides, and cells—to be carried out while reusing/recycling immobilized enzymes [17–25].
Commonly, the co-crystallization process is performed in an organic solvent such as methanol, which is a challenging condition for most enzymes [15]. There is a natural co-crystallization process called biomineralization, which is essentially co-crystallization but takes place in water phase under ambient conditions [26–28]. Although the reaction can be slow in nature, the formed biominerals are sufficiently stable with immobilized and possibly functional proteins/enzymes. Inspired by nature, biomineralization has also been carried out on lab benches [29,30]. Although nature might find its own sophisticated way to biomineralize proteins or other biomacromolecules, on-bench biomineralization in a reasonable timeframe for enzymes needs additional planning. Furthermore, although natural biomineralization could generate enzyme@MOF composites with distinct morphology and crystallinity, certain considerations are needed to optimize the on-bench strategy in order to better preserve enzyme activity and reusability and the stability of co-crystals. Lastly, aqueous phase co-crystallization may generate imperfect crystals but retains enzyme activity and reusability, which would still improve biocatalysis and thus be worth pursuing. Focusing on aqueous phase co-crystallization, we have acquired extensive experience in immobilizing various enzymes on MOFs [16,20,21]. Our recent work has revealed a combination of 10 metal ions/ligands to carry out biomineralization for effective enzyme immobilization; broadening the spectrum of available metal/ligand pairs allows for custom immobilization of enzymes according to their characteristics and stability (in certain metal ions and ligands) [19]. However, there is a lack of detailed experimental protocols to carry out such a sophisticated mission.
In this protocol, we detail the biomineralization procedures, highlight the precautions, potential pitfalls, and suggested solutions, and summarize the assessment of successful enzyme biomineralization based on our recent experience. We will cover MOF preparation in aqueous phase (metal/ligand selection and preparation and criteria of a useful MOF) to obtain a background without enzymes, enzyme@MOF composite formation (enzyme preparation and activity assessment on MOFs), and additional experimental conditions that may help the formation of co-crystals without damaging the enzymes. The strategy and methods can be applicable to immobilizing other enzymes and searching for other metal ion/ligand combinations for custom immobilization of other enzymes.
Materials and reagents
Reagents
Terephthalic acid (BDC, 98%) (Millipore Sigma, catalog number: 185361-100G)
4,4’-Biphenyldicarboxylic acid (BPDC) (CHEM-IMPEX INT’L Inc., catalog number: 26841)
Bicinchoninic acid (BCA) kit for protein determination (Millipore Sigma, catalog number: BCA1)
MES (Millipore Sigma, catalog number: 475893-100GM)
Sodium acetate (Millipore Sigma, catalog number: S8750)
HEPES (Millipore Sigma, catalog number: PHG0001-100G)
Glycine (Millipore Sigma, catalog number: 410225)
Ethanol (EtOH) (standard resources—could be obtained from any commercial resources)
Acetic acid (standard resources)
Sodium hydroxide (NaOH) (standard resources)
Concentrated hydrochloric acid (HCl) (standard resources)
Double-distilled water (standard resources)
Thin test tubes (Wiretrol® II, catalog number: 5-000-2020)
Solutions
MES buffer 0.5 M, pH 6 (10 mL) (see Recipes)
Acetate buffer 0.05 M, pH 4.6 (40 mL) (see Recipes)
HEPES buffer 0.2 M, pH 6.8 (40 mL) (see Recipes)
Glycine buffer 0.05 M, pH 9 (40 mL) (see Recipes)
Recipes
MES buffer 0.5 M, pH 6 (10 mL)
8 mL of double-deionized water (ddH2O)
0.976 g of MES
pH to 6 using HCl
Dilute to 10 mL
Acetate buffer 0.05 M, pH 4.6 (40 mL)
30 mL of ddH2O
82.272 mg of sodium acetate
0.057 mL of glacial acetic acid
pH to 4.6 using HCl
Dilute to 40 mL
HEPES buffer 0.2 M, pH 6.8 (40 mL)
30 mL of ddH2O
1.91 g of HEPES
pH to 6.8 using HCl
Dilute to 40 mL
Glycine buffer 0.05 M, pH 9 (40 mL)
30 mL of ddH2O
150 mg of glycine
10 mg of sodium hydroxide
pH to 9 with NaOH
Dilute to 40 mL
Equipment
Centrifuge (Thermo Fisher, model: Sorvall Legend Micro 21R)
Thermogravimetric analyzer (TA instruments Water, model: TGA-Q500)
XRD–Brucker’s Single Crystal Diffractometer, Apex 2 Duo with Cu IμS X-ray Source (Bruker Corporation, model: Apex 2 Duo)
Field-emission scanning electron microscope (STEM, model: JEOL JSM-7600F)
Millipore concentrators (Merck KGaA, catalog number: UFC500396)
Mortar and pestle setup (Millipore Sigma, catalog number: Z136077)
Spectrometer (Thermo Scientific, model: NanoDrop 2000)
Procedure
To minimize mineral impact on our MOF preparation, all water in this procedure is double-deionized water (ddH2O) and all solutions are prepared using ddH2O. The pH in all buffers is adjusted with 1 M HCl or NaOH. Enzyme activity often requires specific pH; however, certain MOFs are unstable in certain pHs. In this case, alternative buffers in the same pH range are provided in the Recipes section. All enzymes and enzyme@MOF composites should be stored at 4 and delivered on ice prior to activity tests. Enzyme@MOF composites do not need to be freshly prepared unless left in the fridge for over a month. All involved equipment operation and data analysis should follow the corresponding users’ manuals/guidance and will only be briefly covered here. A general overview of this protocol is shown in Figure 1.

Figure 1. An overview of the procedures involved in this protocol
Metal ion/ligand selection and aqueous phase MOF preparation
It is necessary to prepare MOFs without enzyme because these can serve as the background or control signals for characterization. The best pool to select metal ions and ligands are the published MOFs prepared via solvothermal or hydrothermal methods [6–8], bearing in mind that the same pair may not form the same crystal structures in the aqueous phase under ambient conditions or may not form crystal at all in water. A few summative lists of commonly seen combinations of metal ions and ligands can be found in the literature [8,14,31]. Enzyme stability and functionality in the presence of excess metal ions and ligands, solubility of ligands, and potential toxicity should be considered during the selection and scanning of metal ion–ligand combinations [32–35]. It is common to scan a number of metal ions and ligands but only find a few combinations that can form crystals [19]. Normally, +2 oxidation state of metal ions is preferred according to our experience, although +3 and +4 are possible in certain circumstances. Once a combination is found as judged by direct visualization, proceed with the following.
Optimize MOF forming conditions. This step is necessary because the optimal metal:ligand ratio often deviates from the content ratio in a MOF. We have found that the quality of co-crystals formed this way is dependent on the reaction volume even under the same metal and ligand concentrations. In rare cases, the anions of the metal ion stock can affect the formation and stability of co-crystals [36]. We found the following steps useful for a new metal/ligand combination:
Improve ligand solubility in water. Organic linkers/ligands can have a low solubility in water. To be able to reach the concentration needed for co-crystallization, a typical operation is to react the ligand with NaOH to prepare a salt-based ligand as detailed in our recent work. In brief, 50 mM NaOH reacting with 25 mM ligand in water under vigorous stirring at room temperature for 1–2 h should be sufficient, although the concentration and reaction time may vary depending on ligands. Taking terephthalic acid (BDC) and 4,4’-Biphenyldicarboxylic acid (BPDC) as example ligands, the following procedures were proved effective.
To prepare the more soluble disodium terephthalate (BDC-Na2), terephthalic acid (4.16 g, 25 mM) and NaOH (2.0 g, 50 mM) were mixed by stirring in 20 mL of ddH2O at room temperature until transparent (~1 h). Then, the mixture was precipitated in 400 mL of cold isopropanol by mixing. Precipitate was washed via centrifugation with isopropanol until filtrate reached pH 7 and dried overnight at 75 °C in an oven.
Disodium BPDC (BPDC-Na2) was synthesized by adding 6 g (22.2 mM) of BPDC along with 2.65 g of NaOH in 100 mL of ddH2O. The mixture was stirred at 95 °C for 3 h until transparent and then precipitated, washed, and dried in a similar fashion to the BDC-Na2 synthesis above.
Metal ion selection. Typical salts containing the needed metal ions with a low charge are preferred, as high negative charges may interference with the formation of MOFs [i.e., Zn(NO3)2, Ca(NO3)2, Al(NO3)2].
Caution: In water co-crystallization, the anion of the metal ion stock solution may affect the formation of the co-crystals. A typical example is ZIF: Zn(AoC)2 forms more stable, smaller ZIF, yet Zn(NO3)2 forms larger ones. When choosing commercial resources for metal ions, the anions should be carefully selected.
Forming MOF. We typically start with 1 mL of water containing 25 mM metal ions and 50 mM of ligands under gentle nutation at room temperature. MOFs can be formed from minutes to hours depending on metal and ligand selection.
Caution: We found it more effective to add the metal first followed by the ligand.
Washing off unreacted species. We suggest centrifuging the composites (13,000× g for 5 min) and removing the water from the supernatant as much as possible. Then, 1 mL of EtOH or MeOH should be used to wash additional reaction residuals as water may crush the formed crystals, which can be weak given the formation condition. Usually, three washes are sufficient. The obtained co-crystals should be stored at 4 for further use.
Caution: We suggest avoiding extensive washes with water as it may disrupt the crystal lattice. In addition, organic solvents are easier to dry out for crystal characterization.
MOF characterization. All characterization techniques are well-established with standard operation procedures. Here, we only highlight the differences/precautions when dealing with MOFs formed in the aqueous phase.
Powder X-ray diffraction (PXRD). Upon removal of EtOH via drying, a PXRD sample should be prepared by loading the powder to the bottom of a thin test tube (Figure 2). The sample height should be ~1.6 mm with a ~2.5 mm seal. If a single crystal is formed (which may happen in aqueous phase too), then the crystal should be directly loaded into the X-ray diffractometer for data acquisition. For powder samples, it is very important to finely grind the particles in order to obtain a high-quality diffraction pattern. Usually, we use a regular mortar and pestle setup. Once data are acquired, we typically compare the pattern from 4–70° of 2θ to those reported in the literature on the same metal/ligand. If no match can be found, there is a chance that we are forming multi-phase co-crystals or a completely new crystal, either of which can be resolved with a powder X-ray diffractometer and proper analysis.

Figure 2. Preparing a powder sample for powder X-ray diffraction (PXRD) data acquisition by loading the powder to the bottom of a thin test tubeScanning electron microscope (SEM). SEM is needed to confirm the morphology and size of the formed co-crystals. Regular operation on SEM data acquisition is applicable here without special cautions (~2 mg of dried sample is needed).
Thermalgravimetric analysis (TGA). TGA needs special caution because the sample holders can be sensitive and thus be damaged during data acquisition for MOFs made of Al, Zn, Ni, Fe, etc. Our typical suggestion is to select ceramic holds for these MOFs and regular holds for the rest (to save the cost). In most cases, 10 mg of sample is needed.
Caution: It is very important to completely dry all samples.
N2 isotherm. This is necessary to confirm the porosity, which is needed for substrate diffusion and enzyme activity. N2 isotherm measurements require a high amount of sample and high crystallinity. A collapsed N2 isotherm plot often indicates poor crystallinity and porosity [19]. Typical data analysis reported in the literature is applicable here. We found 10 mg of sample is needed, with typical porosity of 0.05–0.5 cm3/g being the normal range for an acceptable MOF.
Caution: Avoiding washing with water can reduce the potential crystal damage during wash. MeOH or EtOH are good options.
pH stability. Due to the potential interaction between metal ions and anions in buffers, certain buffers can disassemble certain MOFs. PBS buffer is a typical example, wherein the highly negatively charged phosphate group could coordinate with cations and disassemble the MOF scaffolds. We found citrate buffer also capable of disassembling MOFs. For low pH ranges, we typically use acetate and MES buffer; for near-neutral pH, we found HEPES buffer the best; at high pH range, glycine buffer is optimal. These buffers (usually at 50 mM concentration) also do not significantly affect the activities of most enzymes and thus should be tested on the formed MOFs in order to guide the pH stability for the next steps. Once soaked in a buffer, we monitor the turbidity at 450 nm over time using a spectrometer. If no turbidity changes over a certain time (one day for example), the pellets will then be subjected to PXRD and SEM studies to confirm the presence of co-crystals.
Thermal stability. For thermal stability testing, we usually place the co-crystals in an oven and set the temperature to the target temperature. For most biological applications, 37–45 is the typical temperature range. The turbidity is measured approximately every hour to confirm the presence of crystals. After 1–2 days, if a crystal is still present, then PXRD and SEM should be applied to confirm, as broken crystals can also show turbidity. The same test should be carried out for crystals stored at 4 over a long period of time to document the long-term stability of the formed crystals.
Enzyme@MOF composite preparation
Enzymes may participate in the co-crystallization of metal ions and ligands by serving as the nuclei, which can affect the kinetics of co-crystal formation and even the structure. Thus, all data on enzyme@MOF composites should be compared to those on MOFs alone. Because enzymes’ properties differ significantly, the following procedures were only based on our experience on a few enzymes. Specific enzymes’ biomineralization should be dealt with in a case-by-case manner.
Prepare enzymes and control experiments. This step is necessary to retain enzyme functionality and validate the measured enzyme activity in the next steps.
Enzyme preparation. If an enzyme is ordered in the powder form, then an appropriate buffer or ddH2O should be used to dissolve it under the manufacturer’s instructions if available. If no instructions are given, we typically start with HEPES buffer. The typical enzyme stock concentration should follow the ones that are suggested in the literature, as high enzyme concentration can cause enzyme precipitation. We suggest storing enzymes below 1 mM at 4 . If possible, it is also ideal to store enzymes in the buffers that are favored by MOF (see above). If an enzyme is ordered or expressed/purified in the solution form in a low concentration, then a buffer exchange via dialysis or centrifugation-concentrators is needed to maximize the effectiveness of MOF encapsulation.
Caution: Minimize enzyme loss by optimizing concentration and buffer selection during buffer exchange.
Positive control of enzyme activity. Typical activity assays should be carried out to confirm that the enzymes are active. Usually, results are compared to the activity data from a reliable resource with known activity. Often, the dependence of activity as a function of enzyme concentration is needed in order to confirm that enzymes are active.
Negative controls of activity. It is necessary to confirm that metal ions, ligands, and MOFs alone do not show activity using the typical activity assays. Should a metal/ligand combination influence the activity of the target enzyme, an alternative MOF should be used to biomineralize this specific enzyme. Enzymes physically mixed with MOFs after washing (on ice with sonication under 50% duty cycle with medium power) should also be subjected to activity tests to confirm no physical adsorption of enzymes. This is important because physical adsorption can result in enzyme leaching and poor reusability after multiple rounds of activity tests. Only enzymes implanted in MOFs are needed.
Caution: Although metal ions and ligands often do not affect enzyme activity measurements, depending on the activity kit and mechanism certain metal ions and ligands may affect the reading of the involved equipment. Thus, negative controls are necessary and have to be carefully designed.
Prepare enzyme@MOF composites.
Typical recipe: usually, mixing 1 mL of ddH2O, 25 μL of 0.5 M metal salt solution, 1 mM enzyme, and 0.5–0.25 M ligand (total volume is usually ~1.1 mL after mixing) depending on the ligand can form the needed composites. The concentrations are mostly applicable to the MOFs reported in our recent work. Should a new combination of metal and ligand be needed, the concentrations of both metal ion and ligand need to be optimized.
Caution: Avoiding high enzyme concentrations can reduce enzyme loss.
Reaction at room temperature overnight under nutation.
Wash with EtOH at 4 as described above (step A.1.d). The low temperature is needed for retaining enzyme activity.
The prepared enzyme@MOF composites can be stored at 4 .
Enzyme@MOF characterization. All characterization techniques are well-established with standard operation procedures. Here, we only highlight the differences/cautions when dealing with enzyme@MOF composites.
PXRD. Upon removal of EtOH via drying, a similar loading procedure and analysis should be applied as described above (step A.2.a). Example PXRD data is shown in Figure 3A.
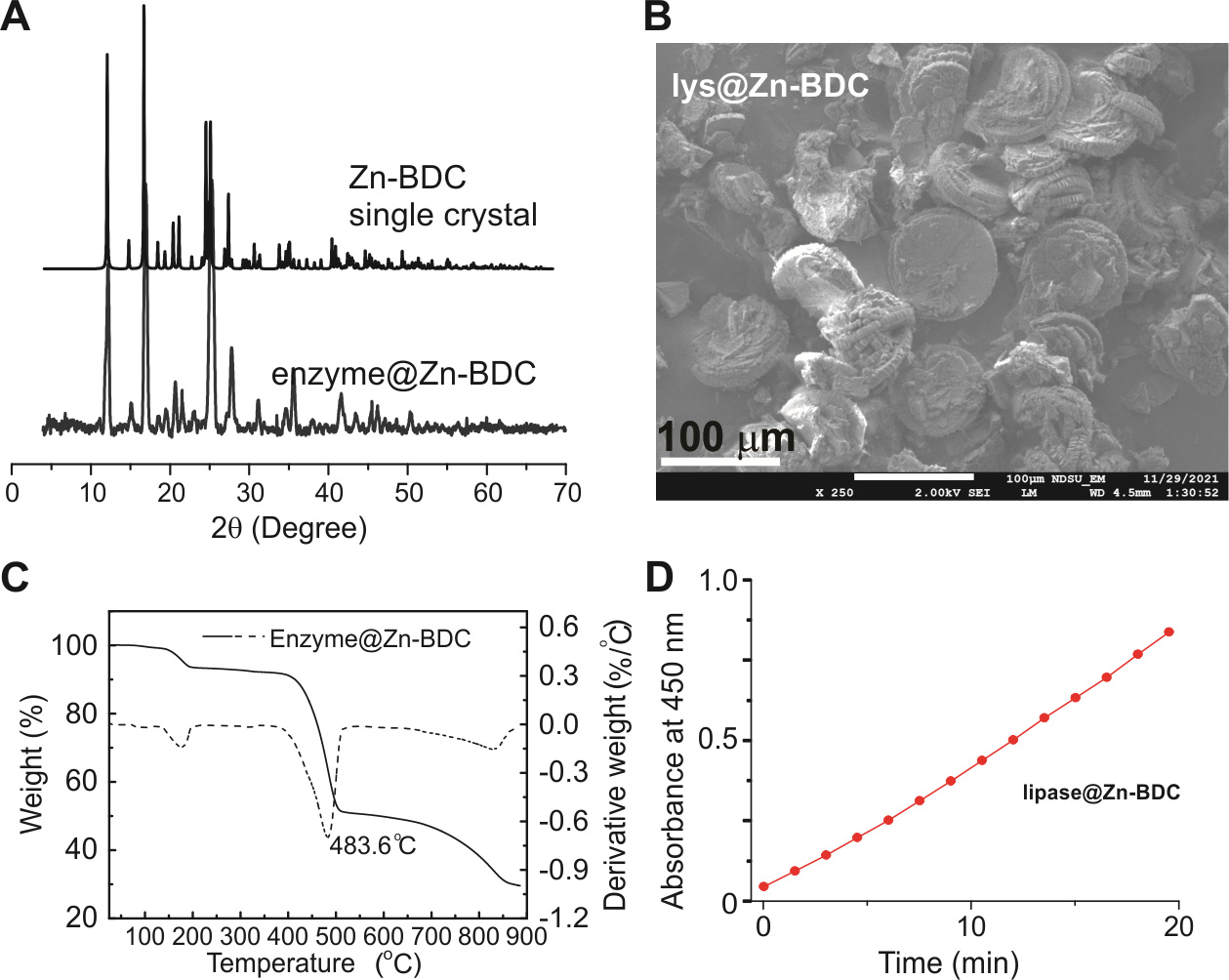
Figure 3. Example powder X-ray diffraction (PXRD) (A), scanning electron microscope (SEM) (B), thermalgravimetric analysis (TGA) (C), and lipase activity (D) data when lipase was immobilized in a model metal–organic framework (MOF), Zn-BDC [19]. The close PXRD patterns in the absence and presence of enzyme indicate that the enzyme did not cause significant alterations to the crystal structure (A). SEM images displayed the general shape of the enzyme@Zn-BDC biocomposites (A). TGA data suggested the loading capacity (~1%; C). The activity assay indicates that lipase is active in Zn-BDC (D).SEM. SEM is needed to confirm the morphology and size of the formed co-crystals. Regular operation on SEM data acquisition is applicable here without special cautions (2 mg of the composite sample is needed). Example SEM data is shown in Figure 3B.
TGA. TGA needs special precautions because the sample holders can be sensitive and thus damaged during data acquisition for MOFs made of Al, Zn, Ni, Fe, etc. Our typical suggestion is to select ceramic holds for these MOFs and regular holds for the rest (to save the cost). In most cases, 10 mg of sample is needed. The TGA of enzyme@MOF composites should be compared to that of MOF alone, which should highlight the weight loss due to enzyme encapsulation. Example TGA data is shown in Figure 3C.
N2 isotherm. Typical data analysis reported in the literature is applicable here. We found 10 mg of sample is needed. The N2 isotherm of enzyme@MOF composites should be compared to that of MOF alone, which should highlight the porosity loss due to enzyme encapsulation. The typical porosity loss range is 0.05–0.3 cm3/g. Most N2 isotherm instruments directly provide digital numbers of porosity.
pH and thermal stability. A similar procedure to test the pH and thermal stability should be carried out for the enzyme@MOF composites as well.
Enzyme activity on MOFs. Enzyme@MOF composites should be subjected to the activity assays mentioned above to confirm the functionality of the encapsulated enzymes. Also, if enzyme performance needs to be compared among different MOFs, then the same loading capacity should be used (or at least normalized) for comparison. This is typically done via BCA assay. In detail, the formation and reduction of Cu2+ to Cu+ with the help of specific amino acids (cystine, tryptophan, tyrosine, etc.) is proportional to the amount of protein present. The commercially available BCA working reagent is light green and turns purple-blue in the presence of protein. Approximately 0.1 mL of protein sample is mixed with 2 mL of working BCA reagent and incubated for 30 min at 37 °C. Absorbance of standard solutions and samples are measured at 562 nm. Under the same enzyme quantity, a fair comparison can be carried out to determine which MOF best reserves enzyme activity. Example activity data when lipase is immobilized in Zn-BDC is shown in Figure 3D.
If multiple combinations of metal ions and ligands are all able to immobilize enzymes with acceptable activity remaining, usually large crystalline MOFs are favorable in biocatalysis applications, although smaller particles may improve the catalytic efficiency against large-size substrates.
Data analysis
The typical data analysis procedure is to compare the characterization data with published ones. For example, the PXRD pattern of a MOF with and without enzyme immobilization should be compared to the literature on the same MOF without enzymes (for examples, see Figure 4).
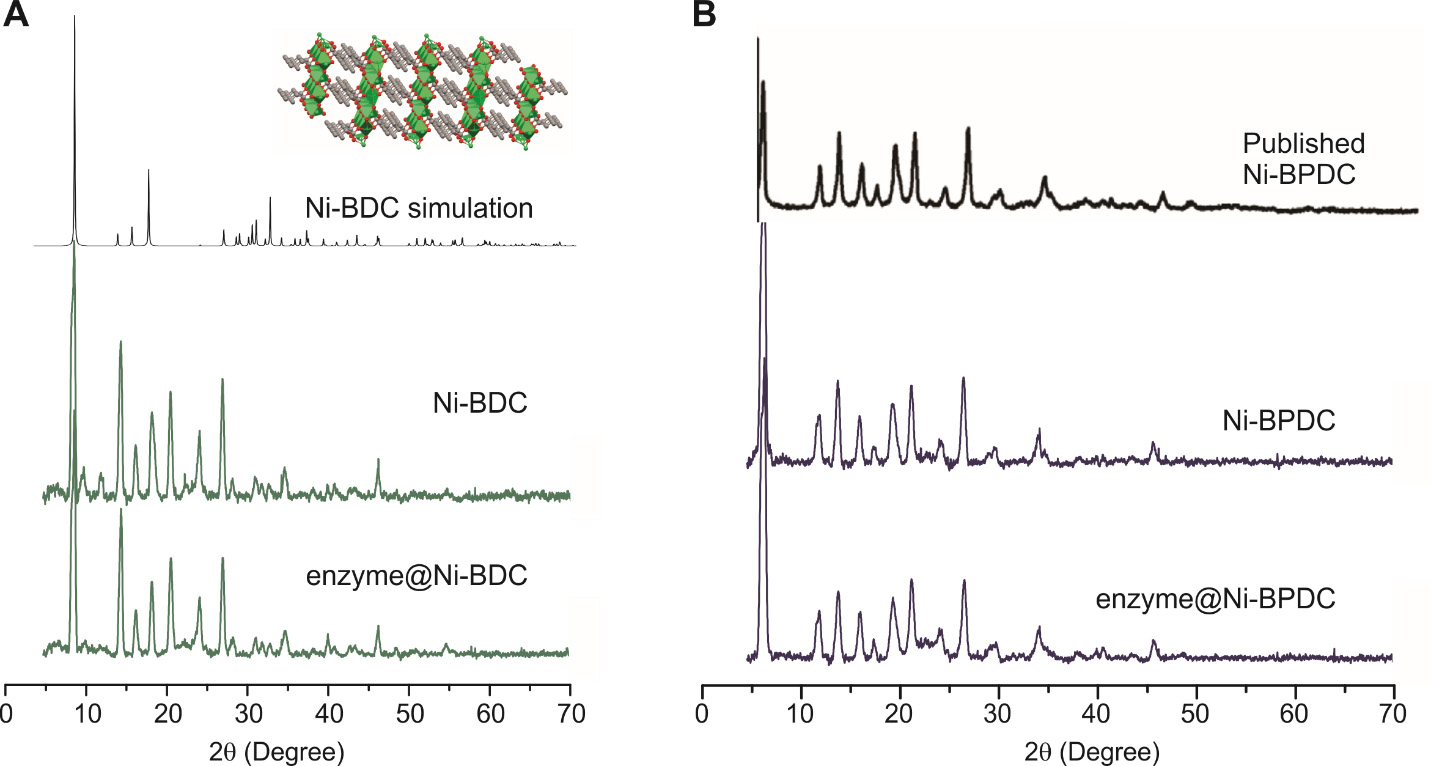
Figure 4. Simulation of the powder X-ray diffraction (PXRD) pattern of Ni-BDC (A) and Ni-BPDC (B) based on published structures upon overlapping with the experimental data [37,38]. Simulation was done using the freeware Mercury developed by the Cambridge Crystallographic Data Centre, which is accessible to most public academic users. Based on the simulation, we were able to propose the possible crystal structure of our biomineralization products.
It is possible to find multiple PXRDs for a certain MOF in the literature. The obtained MOFs could be a combination of several PXRD patterns, indicating the presence of multiple crystal phases as in the case shown in Figure 5, wherein Al-BDC MOF synthesized in the aqueous phase is most likely a multi-phase MOF with at least two possible structures. It is not uncommon to see multi-phase MOF when the synthesis is carried out in water under ambient conditions.
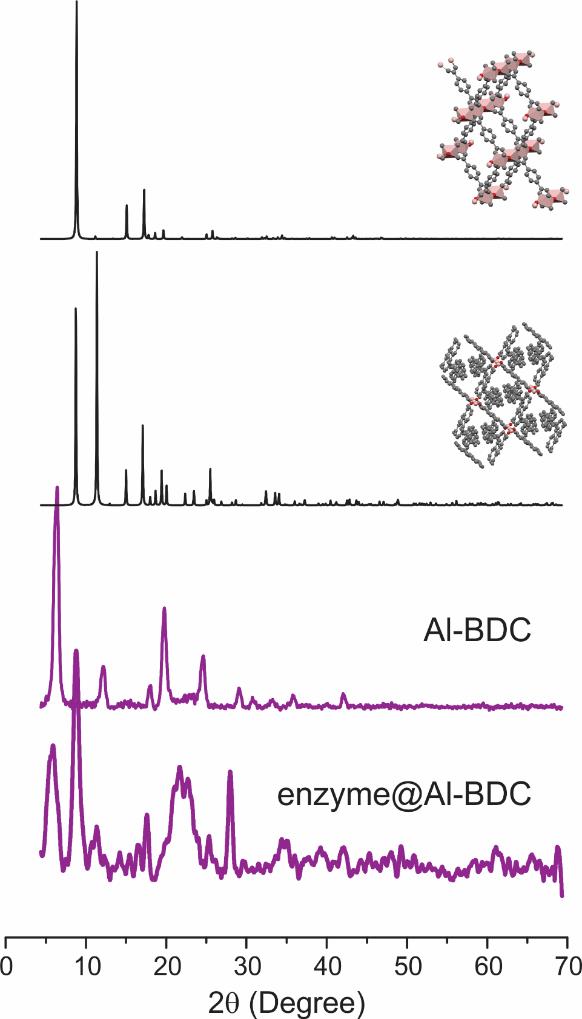
Figure 5. Simulation of the powder X-ray diffraction (PXRD) pattern of Al-BDC (black) based on two published structures (inset) upon overlapping with the experimental data (purple) [39]. Simulation was done using the freeware Mercury developed by the Cambridge Crystallographic Data Centre, which is accessible to most public academic users. Our PXRD patterns do not match either simulated spectrum, suggesting the possibility of multiple phases coexisting in our products.
The activity assay of the enzyme in solution and upon immobilization in MOFs should also be compared. Depending on the enzyme being studied, different data analysis and interpretation could be carried out. For example, for lysozyme enzyme, we typically compare the drop in OD450 and compare the slope to that of the free enzyme in the lysozyme activity assay. For lipase, we compare the slope of increase in optical density to assess the efficiency of catalysis by lipase. Details of the comparison are shown in our recent work [19].
Validation of protocol
The whole procedure is validated in our recent work and supplemental information [19].
General notes and troubleshooting
All procedures assumed normal conditions. Once an enzyme is immobilized on various MOFs, additional considerations could be worth mentioning to better utilize the formed composites.
Cause of activity difference. For biomaterials/biocatalyst development purposes, molecular-level details of the performance of enzymes are often needed in order to understand the functionality and guide the rational design of future MOF platforms. Depending on enzyme and metal/ligand selection, multiple reasons could cause the differences in enzyme activity on different MOFs even under the same loading quantity. For example, different MOFs may present distinct hydrophilicity and thus result in different enzyme intrinsic dynamics (most enzymes are hydrophilic and could be less active in a hydrophobic scaffold). Smaller ligands may present smaller gaps/pores and thus tight restrictions to enzymes, which would also reduce the activity. For large-substrate enzymes, the amount of active site being exposed to the solution is directly related to the activity. These structure/dynamic details of enzymes upon biomineralization in MOFs can be probed using our recently developed techniques [40,41].
Disassemble MOFs to release enzymes. This is a common practice to confirm the loading capacity and enzyme functionality after MOF encapsulation. Most MOFs are unstable under either acidic or basic pHs as well as specific buffers (e.g., PBS buffer). Thus, it is possible to disassemble MOFs to release the enzyme and double-check the integrity using circular dichroism and activity assay. This is also an effective approach to confirm the loading capacity.
Other synthetic conditions of enzyme@MOF composites. We found it to be practically useful if slightly higher temperatures (< 60 ) can help the formation of co-crystals without damaging some enzymes. It is also possible to use some modulators to adjust the rate of crystal formation.
Precautions on metal/ligand selection according to the target enzyme. Metalloenzymes should receive additional care when being biomineralized this way because the endogenous metal binding site may be occupied by the metal ions required for MOF formation. Our typical suggestion would be to use MOFs with charges different from the endogenous metal. For example, to immobilize human Cu/Zn superoxide dismutase (SOD1) [42,43], Al3+ should be used as the metal center of MOF.
Imperfect crystallinity and low porosity of MOFs formed in the aqueous phase. High crystallinity definitely enhances stability and substrate diffusivity. However, if an amorphous or multi-phase crystal is formed when the enzyme is co-crystallized with certain metal ions and ligands, which can be easily and quickly confirmed by PXRD, we still suggest testing the reusability of enzymes on these MOFs. It is likely that the imperfect crystals are still able to immobilize enzymes and retain enzyme activity and thus be useful for biocatalysis applications. It is especially useful when only specific metal ions can be applied to immobilize a metalloenzyme and imperfect crystals are the only option.
Stability and reusability. The enzyme@MOF composites are generally stable after interacting with substrates under reaction conditions. This has been confirmed with our reusability tests in the key reference [19].
Acknowledgments
This work is supported by the National Science Foundation (NSF: MCB 1942596 and DMR 2306137). We appreciate Dr. Peter G. Fajer for generously donating the Bruker ECS-106 to our institution (North Dakota State University) and Dr. Wayne Hubbell for generously providing the EPR data analysis software.
Competing interests
The authors declare no competing interests.
Ethical considerations
No human subjects are involved in this work.
References
- Hoarau, M., Badieyan, S. and Marsh, E. N. G. (2017). Immobilized enzymes: understanding enzyme – surface interactions at the molecular level. Org. Biomol. Chem. 15(45): 9539–9551.
- Sheldon, R. (2007). Enzyme Immobilization: The Quest for Optimum Performance. Adv. Syn. Catal. 349: 1289–1307.
- Hartmann, M. and Jung, D. (2010). Biocatalysis with enzymes immobilized on mesoporous hosts: the status quo and future trends. J. Mater. Chem. 20(5): 844–857.
- Datta, S., Christena, L. R. and Rajaram, Y. R. S. (2013). Enzyme immobilization: an overview on techniques and support materials. 3 Biotech. 3(1): 1–9.
- Hartmann, M. and Kostrov, X. (2013). Immobilization of enzymes on porous silicas – benefits and challenges. Chem. Soc. Rev. 42(15): 6277–6289.
- Li, P., Modica, J. A., Howarth, A. J., Vargas L., E., Moghadam, P. Z., Snurr, R. Q., Mrksich, M., Hupp, J. T. and Farha, O. K. (2016). Toward Design Rules for Enzyme Immobilization in Hierarchical Mesoporous Metal-Organic Frameworks. Chem 1(1): 154–169.
- Li, P., Chen, Q., Wang, T. C., Vermeulen, N. A., Mehdi, B. L., Dohnalkova, A., Browning, N. D., Shen, D., Anderson, R., Gómez-Gualdrón, D. A., et al. (2018). Hierarchically Engineered Mesoporous Metal-Organic Frameworks toward Cell-free Immobilized Enzyme Systems. Chem 4(5): 1022–1034.
- Du, Y., Jia, X., Zhong, L., Jiao, Y., Zhang, Z., Wang, Z., Feng, Y., Bilal, M., Cui, J., Jia, S., et al. (2022). Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 454: 214327.
- Wang, X., Lan, P. C. and Ma, S. (2020). Metal–Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 6(9): 1497–1506.
- Drout, R. J., Robison, L. and Farha, O. K. (2019). Catalytic applications of enzymes encapsulated in metal–organic frameworks. Coord. Chem. Rev. 381: 151–160.
- Gkaniatsou, E., Sicard, C., Ricoux, R., Benahmed, L., Bourdreux, F., Zhang, Q., Serre, C., Mahy, J. and Steunou, N. (2018). Enzyme Encapsulation in Mesoporous Metal–Organic Frameworks for Selective Biodegradation of Harmful Dye Molecules. Angew. Chem. Int. Ed. 57(49): 16141–16146.
- Lian, X., Fang, Y., Joseph, E., Wang, Q., Li, J., Banerjee, S., Lollar, C., Wang, X. and Zhou, H. C. (2017). Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 46(11): 3386–3401.
- Gkaniatsou, E., Sicard, C., Ricoux, R., Mahy, J. P., Steunou, N. and Serre, C. (2017). Metal–organic frameworks: a novel host platform for enzymatic catalysis and detection. Mater. Horiz. 4(1): 55–63.
- An, H., Song, J., Wang, T., Xiao, N., Zhang, Z., Cheng, P., Ma, S., Huang, H. and Chen, Y. (2020). Metal–Organic Framework Disintegrants: Enzyme Preparation Platforms with Boosted Activity. Angew. Chem. 132(38): 16907–16912.
- Lyu, F., Zhang, Y., Zare, R. N., Ge, J. and Liu, Z. (2014). One-Pot Synthesis of Protein-Embedded Metal–Organic Frameworks with Enhanced Biological Activities. Nano Lett. 14(10): 5761–5765.
- Pan, Y., Li, H., Farmakes, J., Xiao, F., Chen, B., Ma, S. and Yang, Z. (2018). How Do Enzymes Orient When Trapped on Metal–Organic Framework (MOF) Surfaces? J. Am. Chem. Soc. 140(47): 16032–16036.
- Pan, Y., Li, Q., Liu, W., Armstrong, Z., MacRae, A., Feng, L., McNeff, C., Zhao, P., Li, H., Yang, Z., et al. (2023). Unveiling the orientation and dynamics of enzymes in unstructured artificial compartments of metal–organic frameworks (MOFs). Nanoscale 15(6): 2573–2577.
- Li, Q., Armstrong, Z., MacRae, A., Ugrinov, A., Feng, L., Chen, B., Huang, Y., Li, H., Pan, Y., Yang, Z., et al. (2023). Metal–Organic Materials (MOMs) Enhance Proteolytic Selectivity, Efficiency, and Reusability of Trypsin: A Time-Resolved Study on Proteolysis. ACS Appl. Mater. Interfaces 15(7): 8927–8936.
- Jordahl, D., Armstrong, Z., Li, Q., Gao, R., Liu, W., Johnson, K., Brown, W., Scheiwiller, A., Feng, L., Ugrinov, A., et al. (2022). Expanding the “Library” of Metal–Organic Frameworks for Enzyme Biomineralization. ACS Appl. Mater. Interfaces 14(46): 51619–51629.
- Pan, Y., Li, Q., Li, H., Farmakes, J., Ugrinov, A., Zhu, X., Lai, Z., Chen, B. and Yang, Z. (2021). A general Ca-MOM platform with enhanced acid-base stability for enzyme biocatalysis. Chem Catal. 1(1): 146–161.
- Pan, Y., Li, H., Lenertz, M., Han, Y., Ugrinov, A., Kilin, D., Chen, B. and Yang, Z. (2021). One-pot synthesis of enzyme@metal–organic material (MOM) biocomposites for enzyme biocatalysis. Green Chem. 23(12): 4466–4476.
- Li, Q., Pan, Y., Li, H., Lenertz, M., Reed, K., Jordahl, D., Bjerke, T., Ugrinov, A., Chen, B., Yang, Z., et al. (2021). Cascade/Parallel Biocatalysis via Multi-enzyme Encapsulation on Metal–Organic Materials for Rapid and Sustainable Biomass Degradation. ACS Appl. Mater. Interfaces 13(36): 43085–43093.
- Li, Q., Pan, Y., Li, H., Alhalhooly, L., Li, Y., Chen, B., Choi, Y. and Yang, Z. (2020). Size-Tunable Metal–Organic Framework-Coated Magnetic Nanoparticles for Enzyme Encapsulation and Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 12(37): 41794–41801.
- Farmakes, J., Schuster, I., Overby, A., Alhalhooly, L., Lenertz, M., Li, Q., Ugrinov, A., Choi, Y., Pan, Y., Yang, Z., et al. (2020). Enzyme Immobilization on Graphite Oxide (GO) Surface via One-Pot Synthesis of GO/Metal–Organic Framework Composites for Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 12(20): 23119–23126.
- Neupane, S., Patnode, K., Li, H., Baryeh, K., Liu, G., Hu, J., Chen, B., Pan, Y. and Yang, Z. (2019). Enhancing Enzyme Immobilization on Carbon Nanotubes via Metal–Organic Frameworks for Large-Substrate Biocatalysis. ACS Appl. Mater. Interfaces 11(12): 12133–12141.
- Harding, J. H., Duffy, D. M., Sushko, M. L., Rodger, P. M., Quigley, D. and Elliott, J. A. (2008). Computational Techniques at the Organic−Inorganic Interface in Biomineralization. Chem. Rev. 108(11): 4823–4854.
- Nudelman, F. and Sommerdijk, N. A. J. M. (2012). Biomineralization as an Inspiration for Materials Chemistry. Angew. Chem. Int. Ed. 51(27): 6582–6596.
- Dunleavy, R., Lu, L., Kiely, C. J., McIntosh, S. and Berger, B. W. (2016). Single-enzyme biomineralization of cadmium sulfide nanocrystals with controlled optical properties. Proc. Natl. Acad. Sci. U.S.A. 113(19): 5275–5280.
- Liao, F. S., Lo, W. S., Hsu, Y. S., Wu, C. C., Wang, S. C., Shieh, F. K., Morabito, J. V., Chou, L. Y., Wu, K. W., Tsung, C. K., et al. (2017). Shielding against Unfolding by Embedding Enzymes in Metal–Organic Frameworks via a de Novo Approach. J. Am. Chem. Soc. 139(19): 6530–6533.
- Liang, K., Ricco, R., Doherty, C. M., Styles, M. J., Bell, S., Kirby, N., Mudie, S., Haylock, D., Hill, A. J., Doonan, C. J., et al. (2015). Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nat. Commun. 6(1): 7240.
- Zheng, Z., Alawadhi, A. H. and Yaghi, O. M. (2023). Green Synthesis and Scale-Up of MOFs for Water Harvesting from Air. Mol. Front. J. 7: 20–39.
- Zhao, M., Wang, H. B., Ji, L. N. and Mao, Z. W. (2013). Insights into metalloenzyme microenvironments: biomimetic metal complexes with a functional second coordination sphere. Chem. Soc. Rev. 42(21): 8360–8375.
- Cohen, S. M. (2017). A Bioinorganic Approach to Fragment-Based Drug Discovery Targeting Metalloenzymes. Acc. Chem. Res. 50(8): 2007–2016.
- Mesbah, N. M. and Wiegel, J. (2018). Biochemical characterization of halophilic, alkalithermophilic amylopullulanase PulD7 and truncated amylopullulanases PulD7ΔN and PulD7ΔC. Int. J. Biol. Macromol. 111: 632–638.
- Chen, G., Kou, X., Huang, S., Tong, L., Shen, Y., Zhu, W., Zhu, F. and Ouyang, G. (2020). Modulating the Biofunctionality of Metal–Organic‐Framework‐Encapsulated Enzymes through Controllable Embedding Patterns. Angew. Chem. Int. Ed. 59(7): 2867–2874.
- Jian, M., Liu, B., Liu, R., Qu, J., Wang, H. and Zhang, X. (2015). Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv. 5(60): 48433–48441.
- Gumilar, G., Kaneti, Y. V., Henzie, J., Chatterjee, S., Na, J., Yuliarto, B., Nugraha, N., Patah, A., Bhaumik, A., Yamauchi, Y., et al. (2020). General synthesis of hierarchical sheet/plate-like M-BDC (M = Cu, Mn, Ni, and Zr) metal–organic frameworks for electrochemical non-enzymatic glucose sensing. Chem. Sci. 11(14): 3644–3655.
- Rawool, C. R., Karna, S. P. and Srivastava, A. K. (2019). Enhancing the supercapacitive performance of Nickel based metal organic framework-carbon nanofibers composite by changing the ligands. Electrochim. Acta 294: 345–356.
- Alaerts, L., Maes, M., Giebeler, L., Jacobs, P. A., Martens, J. A., Denayer, J. F. M., Kirschhock, C. E. A. and De Vos, D. E. (2008). Selective Adsorption and Separation of ortho-Substituted Alkylaromatics with the Microporous Aluminum Terephthalate MIL-53. J. Am. Chem. Soc. 130(43): 14170–14178.
- Pan, Y., Li, H., Li, Q., Lenertz, M., Zhu, X., Chen, B. and Yang, Z. (2021). Site-directed spin labeling-electron paramagnetic resonance spectroscopy in biocatalysis: Enzyme orientation and dynamics in nanoscale confinement. Chem Catal. 1(1): 207–231.
- Pan, Y., Li, H., Li, Q., Lenertz, M., Schuster, I., Jordahl, D., Zhu, X., Chen, B. and Yang, Z. (2021). Protocol for resolving enzyme orientation and dynamics in advanced porous materials via SDSL-EPR. STAR Protoc. 2(3): 100676.
- McCord, J. M. and Fridovich, I. (1969). Superoxide Dismutase: An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244 (22): 6049–6055.
- Tainer, J. A., Getzoff, E. D., Richardson, J. S. and Richardson, D. C. (1983). Structure and mechanism of copper, zinc superoxide dismutase. Nature 306(5940): 284–287.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Armstrong, Z., Jordahl, D., MacRae, A., Li, Q., Lenertz, M., Shen, P., Botserovska, A., Feng, L., Ugrinov, A. and Yang, Z. (2024). A Protocol for Custom Biomineralization of Enzymes in Metal–Organic Frameworks (MOFs). Bio-protocol 14(3): e4930. DOI: 10.21769/BioProtoc.4930.
Category
Biological Engineering > Biomedical engineering
Biochemistry > Other compound > Ion
Biochemistry > Protein > Activity
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link