- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Quantitative Determination of Cholesterol Hydroxylase Specificities by GC–MS/MS in Living Mammalian Cells
Published: Vol 14, Iss 2, Jan 20, 2024 DOI: 10.21769/BioProtoc.4924 Views: 2406
Reviewed by: Faraz RashidSuprabhat MukherjeeAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Synthesis and Purification of Lipid-conjugated Fluorescent pH Sensors
Wiebke Wiesner [...] Thomas Guenther Pomorski
Jun 5, 2023 1965 Views

Lipidomics Workflow for Analyzing Lipid Profiles Using Multiple Reaction Monitoring (MRM) in Liver Homogenate of Mice with Non-alcoholic Steatohepatitis (NASH)
Hai Ning Wee [...] Jianhong Ching
Jul 5, 2023 2498 Views

Computational Analysis of Plasma Lipidomics from Mice Fed Standard Chow and Ketogenic Diet
Amy L. Seufert [...] Brooke A. Napier
Sep 20, 2023 2656 Views
Abstract
Cholesterol is oxygenated by a variety of cholesterol hydroxylases; oxysterols play diverse important roles in physiological and pathophysiological conditions by regulating several transcription factors and cell-surface receptors. Each oxysterol has distinct and overlapping functions. The expression of cholesterol hydroxylases is highly regulated, but their physiological and pathophysiological roles are not fully understood. Although the activity of cholesterol hydroxylases has been characterized biochemically using radiolabeled cholesterol as the substrate, their specificities remain to be comprehensively determined quantitatively. To better understand their roles, a highly sensitive method to measure the amount of various oxysterols synthesized by cholesterol hydroxylases in living mammalian cells is required. Our method described here, with gas chromatography coupled with tandem mass spectrometry (GC–MS/MS), can quantitatively determine a series of oxysterols endogenously synthesized by forced expression of one of the four major cholesterol hydroxylases—CH25H, CYP7A1, CYP27A1, and CYP46A1—or induction of CH25H expression by a physiological stimulus. This protocol can also simultaneously measure the amount of intermediate sterols, which serve as markers for cellular cholesterol synthesis activity.
Key features
• Allows measuring the amount of a variety of oxysterols synthesized endogenously by cholesterol hydroxylases using GC–MS/MS.
• Comprehensive and quantitative analysis of cholesterol hydroxylase specificities in living mammalian cells.
• Simultaneous quantification of intermediate sterols to assess cholesterol synthesis activity.
Graphical overview
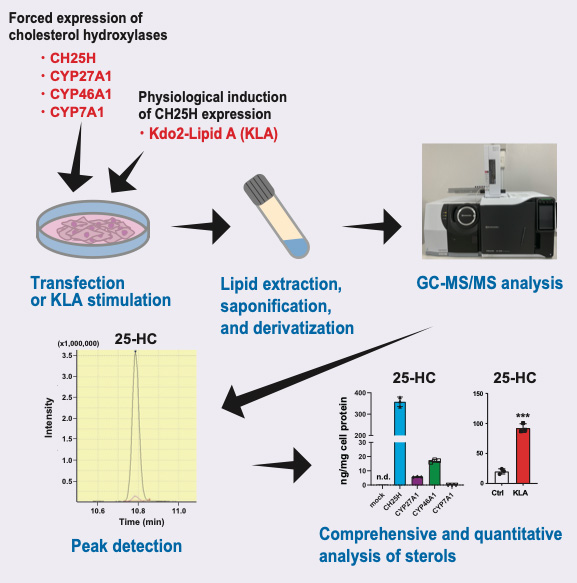
Background
Cholesterol plays diverse important biological roles, including function regulation of biological membranes and membrane proteins, and is the precursor for steroid hormones and bile acids; thus, cellular cholesterol homeostasis is tightly controlled by multiple mechanisms (Chang et al., 2006; Yamauchi and Rogers, 2018; Luo et al., 2020). Mammalian cells cannot break down the sterol backbone. Instead, cholesterol is converted to cholesteryl ester for storage (Chang et al., 2006) and to various oxysterols (Mutemberezi et al., 2016). Oxysterols exert different functions depending on the hydroxylation site(s). Multiple oxysterols whose side chain is hydroxylated, including 25-hydroxycholesterol (25-HC) and 27-HC, regulate cellular cholesterol homeostasis; they modulate two important transcription factors: sterol regulatory element-binding protein-2 (SREBP-2) and liver X receptor (LXR) (Gill et al., 2008; M.S. Brown et al., 2018). SREBP-2 transactivates most genes involved in cholesterol acquisition (biosynthesis and uptake) (Horton et al., 2002). On the other hand, LXR upregulates the expression of several ATP-binding cassette (ABC) transporters including ABCA1 and ABCG1 that mediate the export of excess cellular cholesterol (Tontonoz, 2011). Mechanistically, side-chain oxysterols bind to Insig-1 and Insig-2—retention factors for SREBP-2 in the endoplasmic reticulum (ER)—and protect them from proteasomal degradation, thereby inhibiting SREBP-2 activation. Side-chain oxysterols also serve as natural LXR ligands when added exogenously.
A series of hydroxylases catalyze the site-specific hydroxylation of cholesterol (Mutemberezi et al., 2016). Although a variety of oxysterols have been identified in the body, 7α-HC, 27-HC, 24S-HC, and 25-HC are the most abundant. These four oxysterols are synthesized largely by CYP7A1, CYP27A1, CYP46A1, and CH25H, respectively, in the ER or mitochondria (Figure 1), while some of these hydroxylases also produce other oxysterols as minor products (Mutemberezi et al., 2016; Saito et al., 2023). Recent studies show that the expression of cholesterol hydroxylases is highly regulated in physiological and pathophysiological conditions (Cyster et al., 2014; A.J. Brown et al., 2021). CH25H expression and 25-HC biosynthesis are markedly upregulated upon infection in immune cells such as macrophages, and 25-HC itself exhibits anti-bacterial and anti-viral effects, protecting cells from infection (Bauman et al., 2009; S.Y. Liu et al., 2013; Zhou et al., 2020). 25-HC can be further hydroxylated at the 7-position by the hydroxylase CYP7B1, generating 7α,25-dihydroxycholesterol (7α,25-diHC). This dihydroxycholesterol is a ligand for the G-protein coupled receptor GPR183 (also known as EBI2) involved in immune cell migration (C. Liu et al., 2011). Higher circulating 27-HC levels associate with the risk of estrogen receptor-positive breast cancer, since 27-HC serves as an endogenous selective estrogen receptor modulator (Nelson et al., 2013). CYP46A1 is a neuron-specific cholesterol hydroxylase that converts cholesterol into 24S-HC for eliminating excess cholesterol in the brain (Russell et al., 2009). CYP7A1 is a liver-specific hydroxylase that serves as the rate-limiting enzyme for bile acid synthesis (Russell, 2009). Although the activity of these cholesterol hydroxylases is biochemically studied using radiolabeled cholesterol as the substrate, comprehensive and quantitative characterization of their specificities remains poorly explored in living cells. Furthermore, precise measurement of the products of these cholesterol hydroxylases is crucial for better understanding their physiological and pathophysiological roles.
Here, we provide a highly sensitive method using gas chromatography coupled with tandem mass spectrometry (GC–MS/MS) to determine various oxysterols synthesized in living mammalian cells where a cholesterol hydroxylase is forcedly expressed or is upregulated by lipopolysaccharide, a component of Gram-negative bacteria known to upregulate CH25H expression in macrophages (Bauman et al., 2009). The current GC–MS/MS protocol enabled us to reveal cholesterol hydroxylase–specific production of oxysterols (Saito et al., 2023). Our protocol can also simultaneously determine the amount of intermediate sterols to monitor the activity of cholesterol biosynthesis. Accordingly, our GC–MS/MS-based sterol analysis, in combination with biochemical and gene expression studies, has suggested that side-chain oxysterols enzymatically synthesized within cells primarily regulate SREBP-2 but not LXR (Saito et al., 2023).
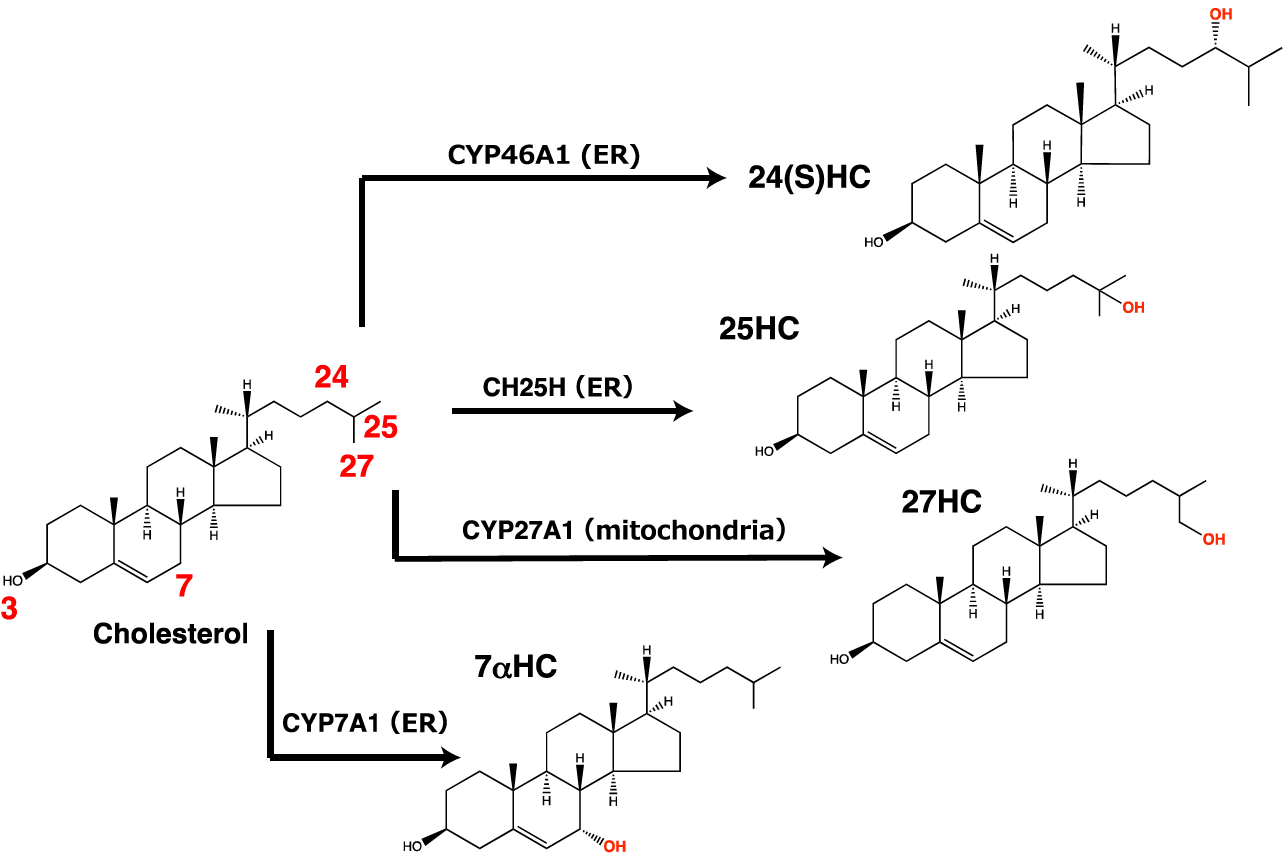
Figure 1. Hydroxylation of cholesterol by the four cholesterol hydroxylases handled in this protocol. CH25H, CYP27A1, CYP46A1, and CYP7A1 mainly produce 25-HC, 27-HC, 24(S)-HC, and 7α-HC, respectively.
Materials and reagents
1.5 mL sampling tubes (WATSON, catalog number: 131-8155C)
Screw-top test tube (Maruemu, catalog number: NR-10)
Mighty Vials (Maruemu, catalog number: 84-0561)
TORAST vial (Shimadzu GLC, catalog number: GLCTV-902)
TORAST vial insert (Shimadzu GLC, catalog number: GLCTV-I04)
TORAST vial cap (Shimadzu GLC, catalog number: GLCTV-903)
Hexane (Fuji Film Wako, catalog number: 085-00416)
2-propanol (Fuji Film Wako, catalog number: 166-04836)
Dibutyl hydroxytoluene (BHT) (Fuji Film Wako, catalog number: 029-07392)
Nitrogen gas
Helium gas
Argon gas
Chloroform (Fuji Film Wako, catalog number: 038-02606)
Ethanol (Fuji Film Wako, catalog number: 057-00456)
Milli-Q water
Pyridine (Fuji Film Wako, catalog number: 161-18453)
Note: Store pyridine in a desiccator with silica gel at room temperature and use within four weeks after opening.
N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA) (GL Science, catalog number: 1022-11061)
Cholesterol (purity ≥ 99%) (Sigma-Aldrich, catalog number: C8667)
Cholesterol-d7 (purity ≥ 99%) (Avanti Polar Lipids, catalog number: 700041P)
Desmosterol (purity ≥ 99%) (Nagara Science, catalog number: NS460402)
Lanosterol (purity ≥ 99.5%) (Nagara Science, catalog number: NS460102)
Lathosterol (purity ≥ 99%) (Nagara Science, catalog number: NS460502)
7-dehydrocholesterol (purity ≥ 95%) (Sigma-Aldrich, catalog number: 30800)
24,25-epoxycholesterol (purity ≥ 95%) (Abcam, catalog number: Ab141633)
24,25-dihydrolanosterol (purity ≥ 99.5%) (Nagara Science, catalog number: NS460201)
4β-hydroxycholesterol (purity ≥ 95%) (Cayman, catalog number: 19518)
7α-hydroxycholesterol (purity ≥ 99%) (Avanti Polar Lipids, catalog number: 700034P)
7β-hydroxycholesterol (purity ≥ 95%) (Sigma-Aldrich, catalog number: H6891)
7α,25-dihydroxycholesterol (purity ≥ 98%) (Sigma-Aldrich, catalog number: SML0541)
7α,27-dihydroxycholesterol (purity ≥ 99%) (Avanti Polar Lipids, catalog number: 700024P)
25-hydroxycholesterol (purity ≥ 98%) (Sigma-Aldrich, catalog number: H1015)
24(S)-hydroxycholesterol (purity ≥ 98%) (Sigma-Aldrich, catalog number: SML1648)
25-hydroxycholesterol-d6 (Avanti Polar Lipids, catalog number: LM4113-1EA)
27-hydroxycholesterol (purity ≥ 98%) (Sigma-Aldrich, catalog number: SML2042)
Sodium chloride (Fuji Film Wako, catalog number: 196-01665)
Potassium chloride (SIGMA, catalog number: P9541)
Disodium hydrogen phosphate dodecahydrate (Fuji Film Wako, catalog number: 196-02835)
Potassium dihydrogen phosphate (Fuji Film Wako, catalog number: 196-04245)
Potassium hydroxide (Fuji Film Wako, catalog number: 168-21815)
Sodium hydroxide (Fuji Film Wako, catalog number: 192-15985)
D-MEM/Ham’s F-12 with L-Glutamine and Phenol Red (Fuji Film Wako, catalog number: 048-29785)
RPMI-1640 with L-Glutamine and Phenol Red (Fuji Film Wako, catalog number: 189-02025)
Opti-MEMTM I reduced serum medium (Thermo Fisher Scientific, catalog number: 31985070)
Fetal bovine serum (FBS) (lot number: 27419002) (Corning, catalog number: 35-079-CF)
Penicillin G potassium (Meiji Seika Pharma, catalog number: 6111400D3051)
Streptomycin sulfate (Meiji Seika Pharma, catalog number: 6161400D1034)
Lipofectamine LTX reagent (Thermo Fisher Scientific, catalog number: 15338100)
Doxycycline hyclate (LKT Labs, catalog number: D5897)
Kdo2-Lipid A (Avanti Polar Lipids, catalog number: 69500P)
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, catalog number: 23227)
Biological materials
CHO-K1 cells (gift from Dr. Ta-Yuan Chang, Geisel School of Medicine at Dartmouth)
CHO-CH25Htet-on cells (Saito et al., 2023)
J774.1 cells (RIKEN Cell Bank, catalog number: RCB0434)
Expression plasmids encoding cholesterol hydroxylase (Saito et al., 2023): pFLAG-CH25H, pCYP27A1-FLAG, pCYP46A1-FLAG, and pCYP7A1-FLAG
Note: Detailed information on CHO-CH25Htet-on cells and the four hydroxylase expression plasmids are described in the original paper (Saito et al., 2023).
Solutions
70% Ethanol (see Recipes)
75% Ethanol (see Recipes)
10 N KOH (dissolved in 75% ethanol) (see Recipes)
Hexane/2-propanol (3:2) with 0.01% BHT (see Recipes)
0.1 N NaOH (see Recipes)
PBS (see Recipes)
Recipes
70% Ethanol
Reagent Volume Ethanol (absolute) 35 mL H2O 15 mL Total 50 mL 75% Ethanol
Reagent Volume Ethanol (absolute) 37.5 mL H2O 12.5 mL Total 50 mL 10 N KOH (dissolved in 75% ethanol)
Reagent Quantity Potassium hydroxide 28.1 g 75% Ethanol up to 50 mL Total 50 mL Hexane/2-propanol (3:2) with 0.01% BHT
Reagent Quantity Hexane 60 mL 2-propanol 40 mL Dibutyl hydroxytoluene 10 mg Total 100 mL 0.1 N NaOH
Reagent Quantity Sodium hydroxide 0.2 g H2O up to 50 mL Total 50 mL PBS
Reagent Quantity Sodium chloride 8 g Potassium chloride 0.2 g Disodium hydrogen phosphate dodecahydrate 3.6 g Potassium dihydrogen phosphate g H2O up to 1 L Sodium hydroxide 1 L
Equipment
GC–MS/MS (Shimadzu, model: GCMS-TQ8040NX) equipped with an auto-sampler (AOC-20i)
BPX5 GC capillary column (30 m × 0.25 mm, 0.25 μm) (TRAJAN, catalog number: SGE-054101)
Rxi Guard column (5 m × 0.25 mm) (Restek, catalog number: 10029)
Eppendorf ThermoMixer C (Eppendorf, catalog number: 5382000023)
Hamilton micro syringe 710RN (Hamilton, catalog number: 72-5004)
Pressured gas blowing concentrator (EYELA, model: MGS-2200)
Aluminum block MGB-1540 (EYELA, catalog number: 207580)
Aluminum block MGB-1624 (EYELA, catalog number: 207610)
Low-speed refrigerated centrifuge (TOMY, model: AX-511)
SpectraMax (Molecular Devices, model: M2e)
Software and datasets
GC-MSsolution v4 (Shimadzu)
RStudio software (Posit)
GraphPad Prism 9 (GraphPad)
Procedure
Cell culture
Preparation of CHO-K1 cells expressing cholesterol hydroxylaseSeed CHO-K1 cells in triplicate into 6-well plates at a density of 2 × 105 cells per well and incubate in DMEM/Ham’s F-12 medium supplemented with 7.5% FBS, penicillin (100 unit/ mL), and streptomycin (0.1 mg/mL) at 37 °C and 5% CO2 for 18–24 h.
Change the medium to Opti-MEM 2–3 h before transfection.
Transfect cells with 2 μg of plasmid encoding either CH25H, CYP7A1, CYP27A1, or CYP46A1 (pFLAG-CH25H, pCYP7A1-FLAG, pCYP27A1-FLAG, or pCYP46A1-FLAG, respectively) using Lipofectamine LTX reagent according to the manufacturer’s protocol.
Note: These four hydroxylase expression constructs are described in the original paper (Saito et al., 2023).
Change the medium to DMEM/Ham’s F-12 medium containing 0.1% FBS, penicillin (100 unit/ mL), and streptomycin (0.1 mg/mL) 5 h after transfection.
Incubate cells for 24 h at 37 and 5% CO2 before harvesting cells for lipid extraction.
Preparation of CHO-CH25Htet-on cellsPlate 2 × 105 CHO-CH25Htet-on cells in triplicate into a well of a 6-well plate and incubate for 18–24 h in DMEM/Ham’s F-12 medium with 7.5% FBS, penicillin (100 unit/ mL), and streptomycin (0.1 mg/mL) at 37 °C and 5% CO2.
Treat the cells for 24 h with or without 0.4 or 1 μg/mL of doxycycline hyclate in DMEM/Ham’s F-12 medium containing 0.1% FBS and penicillin/streptomycin to induce FLAG-CH25H expression and harvest the cells to extract cell lipids.
Preparation of J774.1 cellsSeed J774.1 cells in triplicate into 6-well plates at a density of 5 × 105 cells per well and incubate for two days in RPMI-1640 medium supplemented with 10% FBS and penicillin/streptomycin at 37 and 5% CO2.
Incubate the cells with or without 100 ng/mL of Kdo2-Lipid A, a toll-like receptor 4 ligand, in RPMI-1640 medium containing 10% FBS and penicillin/streptomycin for 20 h at 37 before extracting cell lipids.
Extraction of cellular lipids
Wash cells twice in 6-well plates with PBS (1.5 mL/well) and let cells dry at room temperature.
Add 1.5 mL/well of hexane/2-propanol (3:2) with 0.01% BHT (see Recipes) into 6-well plates and place at room temperature for 1 h to extract cellular lipids.
Collect hexane/2-propanol into a glass tube (screw-top test tube).
Add 1 mL/well of hexane/2-propanol (3:2) with 0.01% BHT into the same well and place at room temperature for 30 min.
Transfer hexane/2-propanol into the same glass tube to pool the cellular lipids extracted.
Add cholesterol-d7 (100 ng/tube from 5 μg/mL stock in chloroform) and 25-hydroxycholesterol-d6 (10 ng/tube from 1 μg/mL stock in methanol) to each sample as internal controls.
Evaporate the organic solvent under the nitrogen gas stream at room temperature using a pressured gas blowing concentrator MGS-2200.
Add 1 mL of 100% ethanol and 300 μL of 10 N KOH (dissolved in 75% ethanol) (see Recipes) to each tube, close with a screw cup, and mix gently.
Incubate the tubes at 80 °C for 1 h to saponify lipids.
Cool the tubes on ice.
Add 2 mL of chloroform into each tube and vortex.
Add 2.5 mL of distilled water into each tube and vortex.
Centrifuge the tubes at 2,380× g for 10 min at room temperature using the low-speed refrigerated centrifuge AX-511.
Remove the aqueous phase (upper layer) using an aspirator.
Add 2 mL of distilled water into each tube again and vortex for 1 min.
Centrifuge the tubes at 2,380× g for 10 min at room temperature.
Remove the aqueous layer again using an aspirator.
Add 2 mL of distilled water into each tube again, vortex, and centrifuge at 2,380× g for 10 min at room temperature.
Remove the aqueous layer using an aspirator.
Transfer the chloroform phase (which contains non-saponified lipids, including sterols) to a new glass vial (Mighty Vials) using a Pasteur pipette and dry under nitrogen gas stream.
Derivatization of extracted cellular sterols
Add 50 μL of pyridine and 50 μL of MSTFA into each vial and mix gently.
Incubate the vials at 80 °C for 1 h with agitation at 300 rpm using an Eppendorf ThermoMixer C.
Transfer the derivatized sample to a new TORAST vial with a TORAST vial insert using a Hamilton micro syringe and close the tube with a TORAST vial cap.
Preparation of standard sterol mixtures
Prepare two types of standard sterol mixtures in separate vials, as follows: the standard mixtures of non-hydroxysterols contain cholesterol, cholesterol-d7, desmosterol, 7-dehydrocholesterol, lathosterol, lanosterol, and 24,25-dihydrolanosterol; those of oxysterols contain 4β-hydroxycholesterol, 7α-hydroxycholesterol, 7β-hydroxycholesterol, 24(S)-hydroxycholesterol, 25-hydroxycholesterol, 25-hydroxycholesterol-d6, 27-hydroxycholesterol, 7α,25-dihydroxycholesterol, 7α,27-dihydroxycholesterol, and 24,25-epoxycholesterol. The quantities of each sterol added to each vial are 0.1, 1, 5, 10, 50, and 100 ng for the non-hydroxysterol mixtures and 0.01, 0.1, 0.5, 1, 5, 10, and 20 ng for the oxysterol mixtures.
Dry up under nitrogen gas stream at room temperature.
Dissolve sterols in 50 μL of pyridine and 50 μL of MSTFA.
Note: The final concentrations are 1, 10, 50, 100, 500, and 1000 ng/mL for the non-hydroxysterol mixtures and 0.1, 1, 5, 10, 50, 100, and 200 ng/mL for the oxysterol mixtures.
Incubate the vials at 80 °C for 1 h with agitation at 300 rpm using an Eppendorf ThermoMixer C.
Transfer the derivatized sample to a new TORAST vial with a TORAST vial insert using a Hamilton micro syringe and close the tube with a TORAST vial cap.
GC–MS/MS analysis
Inject 1 μL of sample or the standard mixture into a GCMS-TQ8040 NX equipped with a BPX5 GC column using an AOC-20i autosampler in splitless mode. Detailed conditions for the analysis are described in Table 1.
Table 1. GC–MS/MS conditions
GC parameters Injection mode Spitless Column BPX5 GC column (30 m × 0.25 mm, 0.25 mm) Rxi Guard Column (5 m × 0.25 mm) Carrier gas control Linear velocity (49.5 cm/s) High pressure injection 250 kPa (1 min) Injection temperature 275 °C Column oven temperature 200 °C (1 min) → 25 °C/min → 250 °C → 15 °C/min → 290 °C → 5 °C/min → 320 °C (2 min) MS parameters Interface temperature 280 °C Ion source temperature 230 °C Loop time 0.3 s Measurement mode Multiple reaction monitoring (MRM) Detect the 17 sterols by MS/MS under multiple reaction monitoring (MRM) mode. Retention time, collision energies, quantification ions, and confirmation ions for each sterol are shown in Table 2. Figure 2 shows MRM chromatograms for respective sterols.
Table 2. Retention time, quantification ions, confirmation ions, and collision energy (CE) for the sterol analysis
Sterols Retention time (min) Quantification ions Confirmation ions MRM transition (m/z) CE (eV) MRM transition (m/z) CE (eV) 7α-hydroxycholesterol 8.48 456 > 208.3 18 456 > 119, 456 > 95.2 30, 33 Cholesterol-d7 8.89 336 > 121.1 18 375 > 145.1, 336 > 109.2 18, 18 Cholesterol 8.94 368 > 145.1 21 329 > 95.1, 329 > 81.1 27, 24 Desmosterol 9.24 129 > 73 15 129 > 57.9, 129 > 127.1 30, 15 7-dehydrocholesterol 9.28 351 > 145.1 30 351 > 128.1 42 7β-hydroxycholesterol 9.38 456 > 233.2 18 233 > 73.1 24 Lathosterol 9.41 213 > 157.2 12 213 > 81.1 15 4β-hydroxycholesterol 9.61 366 > 158.3 18 366 > 129.1 36 24,25-dihydrolanosterol 10.01 395 > 145.2 30 395 > 107 30 7α,25-dihydroxycholesterol 10.21 131 > 73.1 18 544 > 73.2 39 Lanosterol 10.36 393 > 95.2 24 393 > 187.4 12 24,25-epoxycholesterol 10.44 143 > 73.1 18 129 > 73.1, 143 > 128.1 15, 18 24(S)-hydroxycholesterol 10.60 159 > 69.1 9 159 > 73.1 18 7α,27-dihydroxycholesterol 10.60 103 > 73.1 9 544 > 233.2 33 25-hydroxycholesterol-d6 10.75 137 > 73.2 15 137 > 58.2 30 25-hydroxycholesterol 10.79 131 > 73.2 9 131 > 58.1 30 27-hydroxycholesterol 11.26 129 > 73.1 15 456 > 131.2, 417 > 69 42, 42 Notes:
Cholesterol-d7 and 25-hydroxycholesterol-d6 serve as internal standards for non-hydroxylated sterols and hydroxylated sterols, respectively.
The quantification ions are used to identify and calculate the amount of each sterol. The confirmation ions are for assisting in the identification of respective sterols. Several sterols share the same precursor ions, quantification ions, and confirmation ions. When these sterols are not resolved, distinct precursor ions should be selected for the identification and quantification.
Precursor ions for quantification and confirmation ions are carefully determined by the following criterion: higher m/z and stronger intensity. Collision energy is automatically calculated by the instrument.
Retention times for each sterol become shorter after a column is cut for maintenance because it depends on the column length.
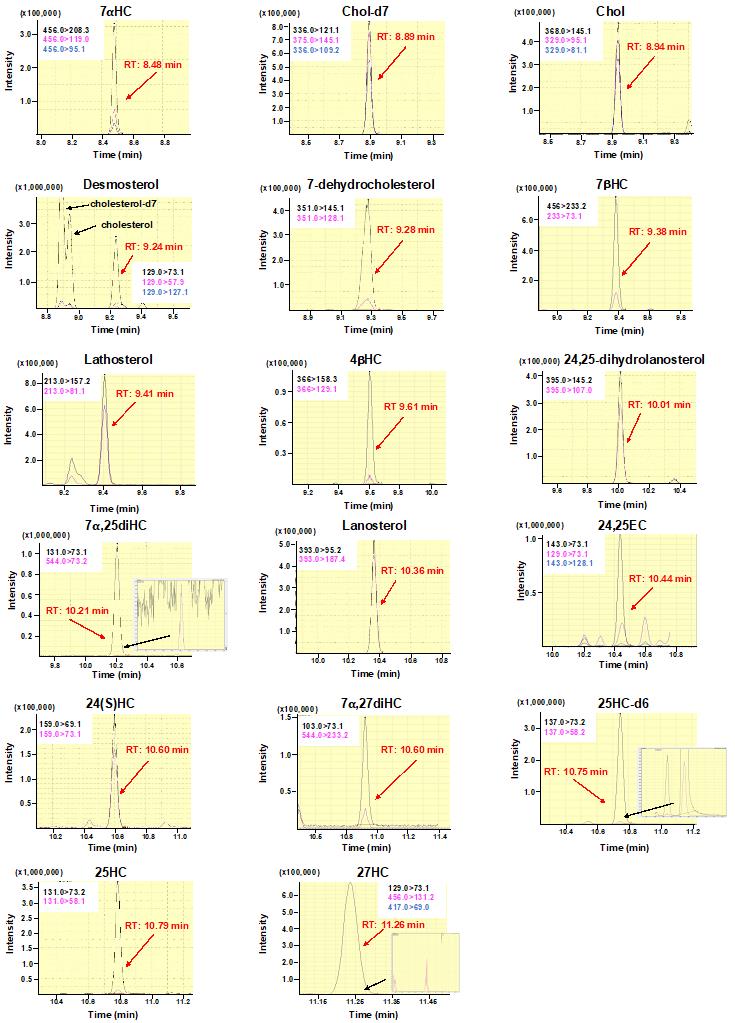
Figure 2. Multiple reaction monitoring (MRM) chromatograms of sterols analyzed in this protocol. MRM chromatograms for each sterol are shown according to retention time. Quantification ions (black) and confirmation ions (pink and blue) are presented in each chromatogram. Black lines and pink and blue lines denote quantification ions and confirmation ions, respectively.
Cellular protein assay
After cellular lipid extraction (step B5), add 1 mL of 0.1 N NaOH (see Recipes) into each well and place at room temperature for 20–24 h to solubilize cellular protein.
Mix solubilized proteins using a pipette and transfer 10 μL of a sample to a well of a 96-well plate.
Add 150 μL of BCA assay reagent to each well, incubate the plate for 30 min at 37 °C, and record absorbance at 562 nm using a plate reader (SpectraMax M2e).
Determine the protein concentration of samples with bovine serum albumin as a standard.
Data analysis
Obtain each sterol peak area using GC-MSsolution v4 software.
Create a standard curve for each sterol using GC-MSsolution. Typical standard curves for individual sterols are shown in Supplementary Figure 1.
Determine the concentration of each sterol in a sample using GC-MSsolution based on peak areas and the standard curve.
Note: As described in Procedure D, the ranges of the two standard curves are as follows: 1 ng/mL to 1 μg/mL for non-hydroxysterols and 0.1 ng/mL to 200 ng/mL for oxysterols.
Find the extraction efficiency of cholesterol-d7 and 25-hydroxycholesterol-d6 with the following equation:
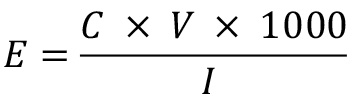
where E is extraction efficiency, C is the concentration (μg/mL) of cholesterol-d7 or 25-hydroxycholesterol-d6 detected by GC–MS/MS, V is the final volume (mL) of the sample (0.1 mL in this protocol), and I is the quantity (ng) of the internal standard added at the step B6 (100 ng of cholesterol-d7 or 10 ng of 25-hydroxycholeterol-d6, in this protocol).
Calculate the original amounts of each sterol per sample using the appropriate extraction efficiency.
Note: The extraction efficiencies of cholesterol-d7 and 25-hydroxycholesterol-d6 are used to calculate the original amounts of non-hydroxysterols and oxysterols, respectively.
Normalize the amounts of each sterol to cell protein per well (Figure 3).
All data are represented by the mean ± S.D. of at least three independent biological replicates. Statistical analyses are performed with RStudio software by Student’s t-test or one-way ANOVA with Dunnett or Tukey-Kramer post-hoc test. p < 0.05 is considered statistically significant.
Advantages and limitations
LC–MS/MS, GC–MS, and GC–MS/MS have been employed for sterol analysis, with GC–MS as the most traditional technique (Krone et al., 2010; McDonald et al., 2012; Griffiths et al., 2013; Saito et al., 2023). Although each method has advantages and limitations, the current GC–MS/MS protocol described above has several advantages over other methods. Since our method is highly sensitive, as 1 pg of sterols can be detected, lipids extracted from cells in a well of a 6-well plate (approximately 0.5–1 × 106 cells) are sufficient to measure a series of sterols, including oxysterols and intermediate sterols. The current GC–MS/MS analysis of cellular sterol is much more sensitive than our previous analysis with GC–MS, in which cellular lipids were extracted from a 100 mm dish or a whole 6-well plate (Yamauchi et al., 2007), while the amounts of cellular sterol contents detected are equivalent. Therefore, many samples can simultaneously be handled for studying multiple experimental conditions in a single assay. In addition, the run time for a sample in our method is approximately 15 min. Typical run times for sterol analysis by GC–MS and LC–MS/MS are 20–30 min and 12–20 min, respectively, depending on the columns and conditions used. Thus, our GC–MS/MS method is comparable to LC–MS/MS analysis concerning run time.
Furthermore, GC-based methods generally show better chromatographic resolution than LC–MS/MS. The previous LC–MS/MS method was unable to resolve 7α-HC and 7β-HC (McDonald et al., 2012), whereas our GC–MS/MS protocol distinguished between these two oxysterols (Saito et al., 2023). As such, GC–MS/MS is the superior technology for analyzing small amounts of isomeric oxysterols, while a major limitation of LC–MS/MS methods is that such oxysterols tend to provide similar spectra.
However, GC–MS/MS-based sterol analysis also has disadvantages. In contrast to LC–MS/MS methods, which do not often require derivatization (McDonald et al., 2012), sterol needs to be derivatized for GC–MS/MS analysis, which is a laborious and time-consuming process. In addition, since GC–MS/MS detects a compound of interest with MRM mode like LC–MS/MS, only target molecules with available standards can be measured.
In summary, the current GC–MS/MS protocol provides a rapid and highly sensitive method for quantification of a series of oxysterols synthesized endogenously within cells, determining cholesterol hydroxylase activity quantitatively.
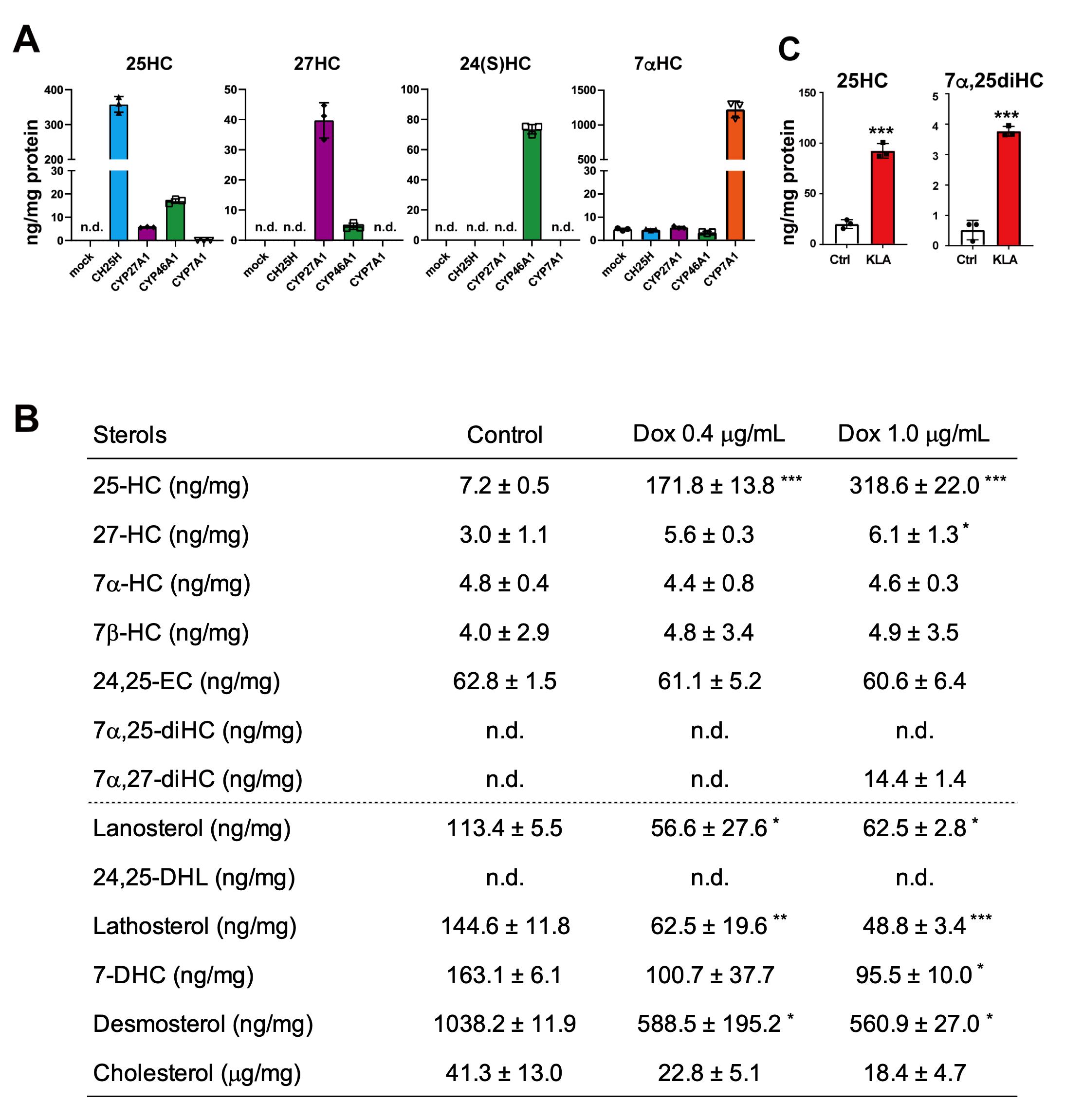
Figure 3. Comprehensive and quantitative determination by GC–MS/MS of oxysterols synthesized by cholesterol hydroxylases in living cells. (A) Oxysterol contents in CHO-K1 cells forcedly expressing CH25H, CYP27A1, CYP46A1, or CYP7A1. The data show that CH25H and CYP7A1 are very specific to synthesizing 25-HC and 7α-HC, respectively, while CYP27A1 and CYP46A1 exhibit broader specificities; CYP27A1 produces not only 27-HC but also 25-HC, and CYP46A1 synthesizes 25-HC and 27-HC in addition to 24(S)-HC. (B) CH25H expression level–dependent production of 25-HC and its effect on intermediate sterol contents in CHO-CH25Htet-on cells. Doxycycline (Dox) induces 25-HC synthesis in a dose-dependent manner, which results in the reduction in intermediate sterol contents. 24,25-DHL, 24,25-dihydrolanosterol; 7-DHC, 7-dehydrocholesterol. (C) 25-HC and 7α,25-diHC contents in J774.1 murine macrophages treated with or without 100 ng/mL of Kdo2-Lipid A (KLA) for 20 h. Stimulation of the cells with KLA induces the expression of CH25H and the production of 25-HC and 7α,25-diHC. Statistical analyses were performed by one-way ANOVA with Dunnett post-hoc test (B) or Student’s t-test (C) (*p < 0.05, **p < 0.01, ***p < 0.001). Data presented in this figure were reproduced from the original paper (Saito et al., 2023).
Acknowledgments
This is a detailed protocol of the GC–MS/MS analysis reported in Saito et al. (2023). We thank Ms. Misato Takata for her assistance with the maintenance of our GC–MS/MS machine. The study was supported by the Japan Agency for Medical Research and Development (AMED)-CREST grants 20gm091008h and 21gm091008h (to Y. Y. and R. S.) from the Japan Agency for Medical Research and Development, KAKENHI grants 19H02908 and 22H02281 (to Y. Y.) and 20H00408 (to R. S.) from the Japan Society for the Promotion of Science, and a research grant from Asahi Group Foundation (to Y. Y.). H.S. was supported by the Japan Society for the Promotion Science Research Fellowship for Young Scientist (20J10181).
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
No human or animal subjects were included in this study.
References
- Bauman, D. R., Bitmansour, A. D., McDonald, J. G., Thompson, B. M., Liang, G. and Russell, D. W. (2009). 25-Hydroxycholesterol secreted by macrophages in response to Toll-like receptor activation suppresses immunoglobulin A production. Proc Natl Acad Sci U S A 106(39): 16764–16769.
- Brown, A. J., Sharpe, L. J. and Rogers, M. J. (2021). Oxysterols: From physiological tuners to pharmacological opportunities. Br J Pharmacol 178(16): 3089–3103.
- Brown, M. S., Radhakrishnan, A. and Goldstein, J. L. (2018). Retrospective on Cholesterol Homeostasis: The Central Role of Scap. Annu. Rev. Biochem. 87(1): 783–807.
- Chang, T. Y., Chang, C. C., Ohgami, N. and Yamauchi, Y. (2006). Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol 22: 129–157.
- Cyster, J. G., Dang, E. V., Reboldi, A. and Yi, T. (2014). 25-Hydroxycholesterols in innate and adaptive immunity. Nat Rev Immunol 14(11): 731–743.
- Gill, S., Chow, R. and Brown, A. J. (2008). Sterol regulators of cholesterol homeostasis and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res 47(6): 391–404.
- Griffiths, W. J., Crick, P. J. and Wang, Y. (2013). Methods for oxysterol analysis: Past, present and future. Biochem. Pharmacol. 86(1): 3–14.
- Horton, J. D., Goldstein, J. L. and Brown, M. S. (2002). SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Invest. 109(9): 1125–1131.
- Krone, N., Hughes, B. A., Lavery, G. G., Stewart, P. M., Arlt, W. and Shackleton, C. H. (2010). Gas chromatography/mass spectrometry (GC/MS) remains a pre-eminent discovery tool in clinical steroid investigations even in the era of fast liquid chromatography tandem mass spectrometry (LC/MS/MS). J Steroid Biochem 121(3-5): 496–504.
- Liu, C., Yang, X. V., Wu, J., Kuei, C., Mani, N. S., Zhang, L., Yu, J., Sutton, S. W., Qin, N., Banie, H., et al. (2011). Oxysterols direct B-cell migration through EBI2. Nature 475(7357): 519–523.
- Liu, S. Y., Aliyari, R., Chikere, K., Li, G., Marsden, M. D., Smith, J. K., Pernet, O., Guo, H., Nusbaum, R., Zack, J. A., et al. (2013). Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38(1): 92–105.
- Luo, J., Yang, H. and Song, B.-L. (2020). Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 21(4): 225–245.
- McDonald, J. G., Smith, D. D., Stiles, A. R. and Russell, D. W. (2012). A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J. Lipid Res. 53(7): 1399–1409.
- Mutemberezi, V., Guillemot-Legris, O. and Muccioli, G. G. (2016). Oxysterols: From cholesterol metabolites to key mediators. Prog Lipid Res 64: 152–169.
- Nelson, E. R., Wardell, S. E., Jasper, J. S., Park, S., Suchindran, S., Howe, M. K., Carver, N. J., Pillai, R. V., Sullivan, P. M., Sondhi, V., et al. (2013). 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342(6162): 1094–1098.
- Russell, D. W. (2009). Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res 50 Suppl: S120-125.
- Russell, D. W., Halford, R. W., Ramirez, D. M., Shah, R. and Kotti, T. (2009). Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem 78: 1017–1040.
- Saito, H., Tachiura, W., Nishimura, M., Shimizu, M., Sato, R. and Yamauchi, Y. (2023). Hydroxylation site-specific and production-dependent effects of endogenous oxysterols on cholesterol homeostasis: Implications for SREBP-2 and LXR. J Biol Chem 299(1): 102733.
- Tontonoz, P. (2011). Transcriptional and Posttranscriptional Control of Cholesterol Homeostasis by Liver X Receptors. Cold Spring Harbor Symp. Quant. Biol. 76: 129–137.
- Yamauchi, Y., Reid, P. C., Sperry, J. B., Furukawa, K., Takeya, M., Chang, C. C. and Chang, T. Y. (2007). Plasma Membrane Rafts Complete Cholesterol Synthesis by Participating in Retrograde Movement of Precursor Sterols. J. Biol. Chem. 282(48): 34994–35004.
- Yamauchi, Y. and Rogers, M. A. (2018). Sterol Metabolism and Transport in Atherosclerosis and Cancer. Front Endocrinol (Lausanne) 9: 509.
- Zhou, Q. D., Chi, X., Lee, M. S., Hsieh, W. Y., Mkrtchyan, J. J., Feng, A. C., He, C., York, A. G., Bui, V. L., Kronenberger, E. B., et al. (2020). Interferon-mediated reprogramming of membrane cholesterol to evade bacterial toxins. Nat Immunol 21(7): 746–755.
Supplementary information
The following supporting information can be downloaded here:
- Supplementary Figure 1.
Article Information
Copyright
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Saito, H., Nishimura, M., Sato, R. and Yamauchi, Y. (2024). Quantitative Determination of Cholesterol Hydroxylase Specificities by GC–MS/MS in Living Mammalian Cells. Bio-protocol 14(2): e4924. DOI: 10.21769/BioProtoc.4924.
- Saito, H., Tachiura, W., Nishimura, M., Shimizu, M., Sato, R. and Yamauchi, Y. (2023). Hydroxylation site-specific and production-dependent effects of endogenous oxysterols on cholesterol homeostasis: Implications for SREBP-2 and LXR. J Biol Chem 299(1): 102733.
Category
Biochemistry > Lipid > Lipid measurement
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









