- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Measurement of Secreted Embryonic Alkaline Phosphatase
Published: Vol 13, Iss 3, Feb 5, 2023 DOI: 10.21769/BioProtoc.4600 Views: 1962
Reviewed by: Laxmi Narayan MishraNityanand SrivastavaSashikantha Reddy Pulikallu

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Measurement of the Activity of Wildtype and Disease-Causing ALPK1 Mutants in Transfected Cells With a 96-Well Format NF-κB/AP-1 Reporter Assay
Tom Snelling
Nov 20, 2024 1602 Views

Fluorescence Polarization-Based High-Throughput Screening Assay for Inhibitors Targeting Cathepsin L
Keyu Guo [...] Shuyi Si
Jul 20, 2025 2277 Views

Detecting the Activation of Endogenous Small GTPases via Fluorescent Signals Utilizing a Split mNeonGreen: Small GTPase ActIvitY ANalyzing (SAIYAN) System
Miharu Maeda and Kota Saito
Jan 5, 2026 483 Views
Abstract
Secreted reporters have been demonstrated to be simple and useful tools for analyzing transcriptional regulation in mammalian cells. The distinctive feature of these assays is the ability to detect reporter gene expression in the culture supernatant without affecting the cell physiology or leading to cell lysis, which allows repeated experimentation and sampling of the culture medium using the same cell cultures. Secreted embryonic alkaline phosphatase (SEAP) is one of the most widely used reporter, which can be easily detected using colorimetry following incubation with a substrate, such as p-nitrophenol phosphate. In this report, we present detailed procedures for detection and quantification of the SEAP reporter. We believe that this step-by-step protocol can be easily used by researchers to monitor and measure molecular genetic events in a variety of mammalian cells due to its simplicity and ease of handling.
Graphical abstract

Schematic overview of the workflow described in this protocol
Background
Reporter gene assays have been important for biomedical and pharmaceutical studies to monitor cellular activities associated with gene expression, regulation, and signal transduction. An ideal secreted reporter system would provide a more simple, rapid, and sensitive method to detect gene expression in a quantitative manner compared to classical intracellular reporters. Secreted embryonic alkaline phosphatase (SEAP), a C-terminal truncated variant of human placental alkaline phosphatase (PLAP), is one of the most widely used secreted mammalian reporter enzymes, engineered by deleting the 30 amino acid anchoring domain of PLAP (Berger et al., 1988). The SEAP reporter assay has superior properties: it is a simple and non-invasive procedure that does not require cell lysis and allows for long-term monitoring of gene expression in vitro and in vivo (Yang et al., 1997; Schlatter et al., 2002; Jiang et al., 2008). Moreover, transfected cells are not disturbed and remain intact for further studies during measurement of SEAP activity (Hu et al., 2022). Furthermore, the heat stability of SEAP and resistance to the phosphatase inhibitor L-homoarginine allow the samples to be pretreated at 65°C or with this inhibitor, to eliminate endogenous alkaline phosphatase activity (Tannous and Teng, 2011). Here, we describe the experimental procedures of in vitro measurement of SEAP adapted from our previously published work (Wang et al., 2021). This method can be useful for real-time monitoring of gene expression patterns in various cells and animals.
Materials and Reagents
96-well plate, transparent (Corning, catalog number: 3365)
24-well plate, transparent (Corning, catalog number: 3537)
T25 flask (Corning, catalog number: 430639)
HEK-293T (ATCC, CRL-11268)
Dulbecco's modified Eagle's medium (DMEM) (Gibco, catalog number: 31600-083)
Fetal bovine serum (FBS) (Gibco, catalog number: 10270-106)
0.25% trypsin-EDTA
1% (vol/vol) penicillin and streptomycin solution (Beyotime Inc., catalog number: ST488-1/ST488-2)
p-nitrophenylphosphate (Sangon Biotech, CAS: 333338-18-4)
L-homoarginine (Sangon Biotech, CAS: 1483-01-8)
MgCl2·6H2O (Sangon Biotech, CAS: 7791-18-6)
Diethanolamine (Sangon Biotech, CAS: 111-42-2)
NaCl (Sangon Biotech, CAS: 7647-14-5)
KCl (Sangon Biotech, CAS: 7647-40-7)
Na2HPO4 (Sangon Biotech, CAS: 7558-79-4)
KH2PO4 (Sangon Biotech, CAS: 7778-77-0)
HCl (Sinopharm Chemical Reagent Co., Ltd., CAS: 7647-01-0)
Polyethyleneimine (PEI, molecular weight 40,000, stock solution 1 mg/mL in ddH2O) (Polysciences, catalog number: 24765)
1× PBS (see Recipes)
2× SEAP assay buffer (see Recipes)
Substrate solution (see Recipes)
Plasmids (see Table 1)
Table 1.Plasmids designed and used in this study
Plasmid Description and cloning strategy Reference pXY137 Constitutive mammalian BldD and BphS expression vector (ITR-PhCMV-p65-VP64-BldD-pA-PhCMV-BphS-P2A-YhjH-pA-ITR) Shao et al. (2017) pZQ5 Far-red light-inducible expression vector (2*crRNA binding site-PhCMVmin-SEAP-pA) This work pZQ28 Constitutive crRNA2SEAP expression vector (PU6-crRNASEAP-pA) This work pZQ113 Constitutive mammalian PhCMV-driven expression vector (PhCMV-scFv-45bp linker-p65-HSF1-pA) This work pZQ116 Far-red light-inducible expression vector (PFRL2-dAsCas12a-NLS-10×GCN4-pA; PFRL3, (OwhiG)2-PhCMVmin) This work
Equipment
Water bath
CO2 incubator
Constant temperature incubator (65°C)
Synergy H1 hybrid multi-mode microplate reader (BioTek Instruments, Inc.)
Multipass pipette
Reagent reservoirs
Hemocytometer (Merck, catalog number: Z359629)
Software
Excel (Microsoft)
Gen5 software (version: 2.04)
Procedure
Cell culture
Warm DMEM supplemented with 10% (vol/vol) FBS and 1% (vol/vol) penicillin and streptomycin solution (hereafter referred to as complete DMEM) and 1× PBS in a 37°C water bath for at least 15 min.
Wash ~90% confluent human embryonic kidney cells (HEK-293T) in a T25 flask with 3 mL of 1× PBS, aspirate the PBS, and add 0.5 mL of 0.25% trypsin-EDTA.
Incubate the cells at 37°C in a 5% CO2 incubator for 3–5 min, add 3 mL of prewarmed complete DMEM, and transfer the cell suspensions to a 15 mL sterile conical tube.
Spin down the cells at 300 × g for 3 min at room temperature, discard the supernatant, and resuspend the cell pellet in 15 mL of complete DMEM.
Dispense 4 mL of the cell suspension into a T25 flask and incubate it for three days at 37°C in a humidified atmosphere containing 5% CO2 before subculturing again.
Cell transfection
The 293T cell suspension should be prepared (as described in the Cell culture procedure) the day before transfection (Day 1) (Figure 1).
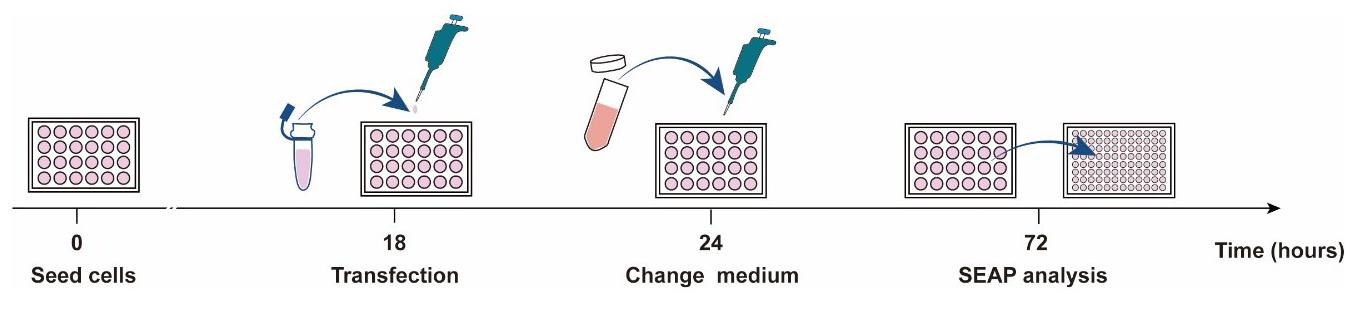
Figure 1. Schematic diagram of the schedule for cell transfection and SEAP analysisCount the cells using a hemocytometer and seed 6 × 104 HEK-293T cells per well in a 24-well cell culture plate; culture for 18 h.
The next day (Day 2), prepare the transfection mix as follows:
Add 500 ng of the corresponding plasmid (see Table 1) into 50 μL of FBS-free and antibiotic-free DMEM medium for each well of a 24-well plate.
Add 1.5 μL of PEI (1 μg/μL, PEI and DNA at a ratio of 3:1) into the above plasmid DNA solution.
To scale up, multiply the quantities by the number of replicates.
Vortex vigorously for 30 s.
Incubate the DNA-PEI mixture solution at room temperature for 15 min.
Add 50 μL of transfection mix dropwise to each well. Rock the slide gently a few times and incubate at 37°C in a humidified atmosphere containing 5% CO2.
Six hours after transfection, change the medium with fresh complete DMEM and incubate the 24-well plate at 37°C in a 5% CO2 incubator until Day 3.
On Day 3, the transfected slides can be used to perform SEAP reporter assay.
SEAP reporter assay
Aspirate 200 μL of cell culture supernatant from each sample and transfer into a chamber of a 96-well plate.
Heat-inactivate the cell culture supernatants at 65°C for 30 min.
Prewarm 2× SEAP assay buffer (see Recipes) at 37°C and protect from light.
Mix 100 μL of 2× SEAP buffer with 20 μL of substrate solution (see Recipes) in the dark.
Add 120 μL of the above-mentioned mixed substrate solution into 80 μL of heat-inactivated cell culture supernatant. At least three replicates should be performed for each group. The media will change color from pink to orange in the presence of SEAP (Figure 2).
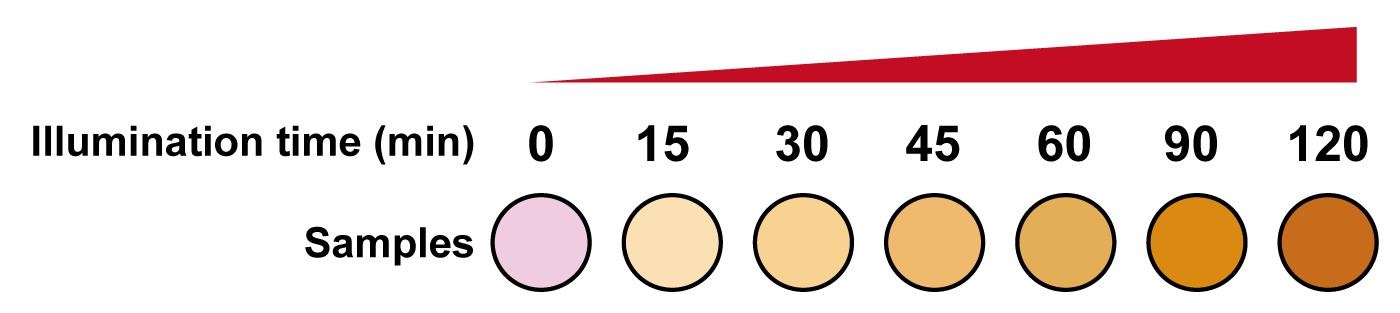
Figure 2. Detection of SEAP production at the indicated time points after illumination. In the presence of SEAP, the media changes from pink to orange. The yellow color intensity correlates with light illumination time.Measure light absorbance at 405 nm at 37°C for 15 min using a multi-mode microplate reader. See Figure 3 for the detailed procedure.
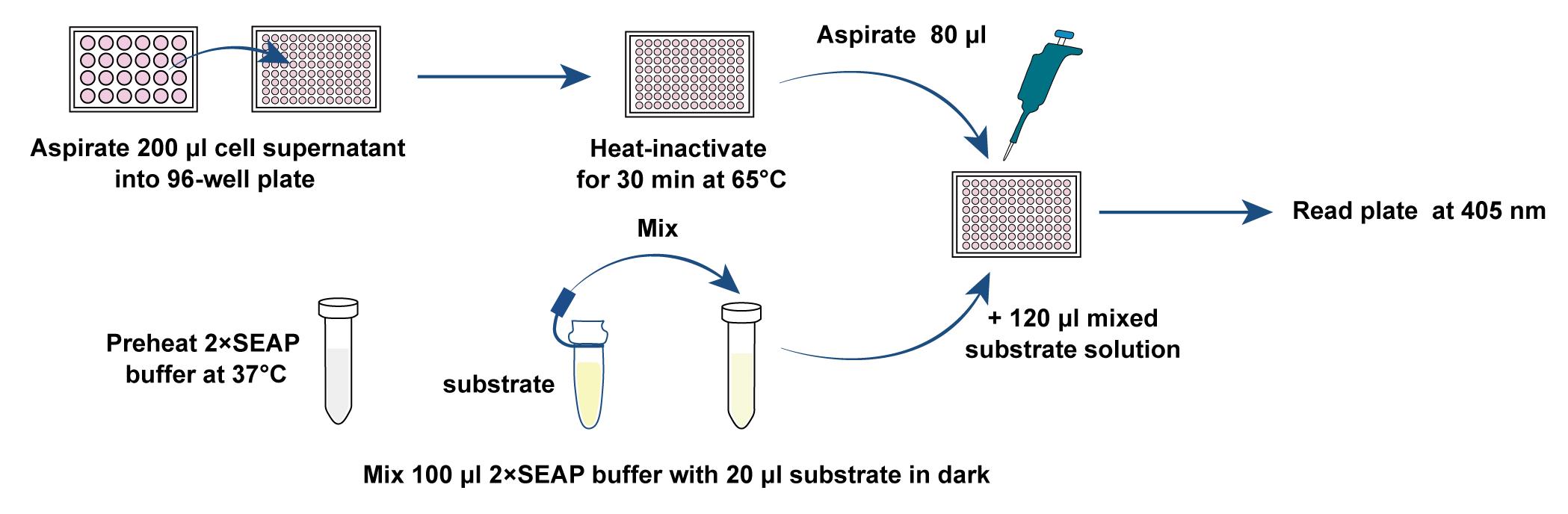
Figure 3. Schematic diagram of SEAP reporter assayCalculate the levels of SEAP activity using data points that yield a rate of change in light absorbance that is linear with respect to time. (If available, a computer-linked kinetic ELISA plate reader is most convenient, as this will precisely calculate the linear change in A405 in all 96 wells of a microplate over time.)
Data analysis
For each illumination time, data are expressed as mean ± standard deviation (SD); n = 3 independent experiments. The data can also be represented as fold change difference compared to non-stimulated cells and analyzed by one-way ANOVA followed by Tukey’s post-hoc tests. Perform statistical analysis using GraphPad Prism or other software.
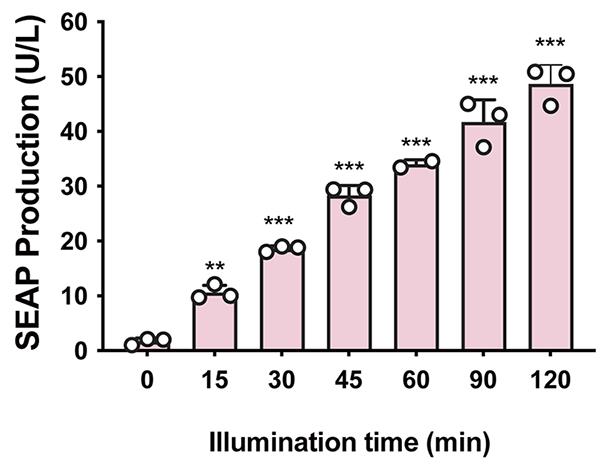
Figure 4. Quantification of far-red light-inducible SEAP expression kinetics. Transfected HEK-293T cells were illuminated with far-red light for different time periods (0–120 min). SEAP production in the culture supernatant was profiled at 24 h after illumination. **P < 0.01, ***P < 0.001 illumination groups vs. 0 min.
Notes
The mixture of 2× SEAP buffer and substrate solution should be protected from light, quickly added to cell culture supernatants, and detected immediately.
If SEAP production is too high, the samples should be diluted with PBS buffer solution.
Recipes
1× PBS buffer
Reagent Final concentration Amount NaCl 137 mM 8.00 g KCl 2.7 mM 0.20 g Na2HPO4 10 mM 1.42 g KH2PO4 1.8 mM 0.24 g H2O n/a 800 mL Adjust pH to 7.4 with HCl, then add ddH2O to final volume (1,000 mL) Total n/a 1,000 mL 2× SEAP buffer
Reagent Final concentration Amount L-homoarginine 20 mM 4.4938 g MgCl2 (3 M) 1 mM 0.334 mL Diethanolamine 21% (v/v) 210 mL Adjust pH to 9.8 with HCl, then add dH2O to final volume (1,000 mL) Total n/a 1,000 mL Store at 4°C and protect from light.
Substrate solution
Reagent Final concentration Amount p-nitrophenylphosphate 120 mM 2.226 g 2× SEAP buffer n/a 50 mL Total n/a 50 mL Aliquot, store at -20°C, and protect from light.
Acknowledgments
This protocol was adapted from previous work (Wang et al., 2021. DOI: 10.1126/sciadv.abh2358). This work was financially supported by grants from the National Key R&D Program of China (No. 2019YFA0904500, No. 2019YFA0110802), the National Natural Science Foundation of China (NSFC: No. 32171414, No. 31971346, No. 31861143016, No. 31870861), the Nature Science Foundation of Chongqing, China (No. CSTB2022NSCQ-MSX0461), the Open Project Program of State Key Laboratory of Dairy Biotechnology (No. SKLDB2021-001), and the Instruments Sharing Platform of the School of Life Sciences, East China Normal University.
Competing interests
All authors wish to declare no competing interests.
References
- Berger, J., Hauber, J., Hauber, R., Geiger, R. and Cullen, B. R. (1988). Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene 66(1): 1-10.
- Hu, Z., Zhang, T., Jiang, S. and Yin, H. (2022). Protocol for evaluation and validation of TLR8 antagonists in HEK-Blue cells via secreted embryonic alkaline phosphatase assay.STAR Protoc 3(1): 101061.
- Jiang, T., Xing, B. and Rao, J. (2008). Recent developments of biological reporter technology for detecting gene expression.Biotechnol Genet Eng Rev 25: 41-75.
- Schlatter, S., Rimann, M., Kelm, J. and Fussenegger, M. (2002). SAMY, a novel mammalian reporter gene derived from Bacillus stearothermophilus alpha-amylase. Gene 282(1-2): 19-31.
- Shao, J., Xue, S., Yu, G., Yu, Y., Yang, X., Bai, Y., Zhu, S., Yang, L., Yin, J., Wang, Y., Liao, S., Guo, S., Xie, M., Fussenegger, M. and Ye, H. (2017). Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci Transl Med 9(387): eaal2298.
- Tannous, B. A. and Teng, J. (2011). Secreted blood reporters: insights and applications.Biotechnol Adv 29(6): 997-1003.
- Wang, X., Dong, K., Kong, D., Zhou, Y., Yin, J., Cai, F., Wang, M. and Ye, H. (2021). A far-red light-inducible CRISPR-Cas12a platform for remote-controlled genome editing and gene activation. Science advances 7(50): eabh2358.
- Yang, T. T., Sinai, P., Kitts, P. A. and Kain, S. R. (1997). Quantification of gene expression with a secreted alkaline phosphatase reporter system.Biotechniques 23(6): 1110-1114.
Article Information
Copyright
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Wang, M., Wang, X. and Ye, H. (2023). Measurement of Secreted Embryonic Alkaline Phosphatase. Bio-protocol 13(3): e4600. DOI: 10.21769/BioProtoc.4600.
- Wang, X., Dong, K., Kong, D., Zhou, Y., Yin, J., Cai, F., Wang, M. and Ye, H. (2021). A far-red light-inducible CRISPR-Cas12a platform for remote-controlled genome editing and gene activation. Science advances 7(50): eabh2358.
Category
Biochemistry > Protein > Activity
Cell Biology > Cell-based analysis > Enzymatic assay
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









