- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Synthesis of Novel MicroRNA-30c Analogs to Reduce Apolipoprotein B Secretion in Human Hepatoma Cells
Published: Vol 12, Iss 24, Dec 20, 2022 DOI: 10.21769/BioProtoc.4574 Views: 2359
Reviewed by: Chiara AmbrogioMatthew SwireAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

ACE-score-based Analysis of Temporal miRNA Targetomes During Human Cytomegalovirus Infection Using AGO-CLIP-seq
Sungchul Kim and Kwangseog Ahn
Apr 20, 2016 13179 Views

Biotinylated Micro-RNA Pull Down Assay for Identifying miRNA Targets
Pornima Phatak and James M Donahue
May 5, 2017 19848 Views
Abstract
Atherosclerosis, a condition characterized by thickening of the arteries due to lipid deposition, is the major contributor to and hallmark of cardiovascular disease. Although great progress has been made in lowering the lipid plaques in patients, the conventional therapies fail to address the needs of those that are intolerant or non-responsive to the treatment. Therefore, additional novel therapeutic approaches are warranted. We have previously shown that increasing the cellular amounts of microRNA-30c (miR-30c) with the aid of viral vectors or liposomes can successfully reduce plasma cholesterol and atherosclerosis in mice. To avoid the use of viruses and liposomes, we have developed new methods to synthesize novel miR-30c analogs with increasing potency and efficacy, including 2’-O-methyl (2’OMe), 2’-fluoro (2’F), pseudouridine (ᴪ), phosphorothioate (PS), and N-acetylgalactosamine (GalNAc). The discovery of these modifications has profoundly impacted the modern RNA therapeutics, as evidenced by their increased nuclease stability and reduction in immune responses. We show that modifications on the passenger strand of miR-30c not only stabilize the duplex but also aid in a more readily uptake by the cells without the aid of viral vectors or lipid emulsions. After uptake, the analogs with PS linkages and GalNAc-modified ribonucleotides significantly reduce the secretion of apolipoprotein B (ApoB) without affecting apolipoprotein A1 (ApoA1) in human hepatoma Huh-7 cells. We envision an enormous potential for these modified miR-30c analogs in therapeutic intervention for treating cardiovascular diseases.
Keywords: LipidsBackground
Atherosclerosis, the aggregation of fibrofatty plaques in the artery wall, has been implicated in the pathogenesis of cardiovascular diseases with high morbidity and mortality rates in the United States and worldwide. High levels of plasma cholesterol is a risk factor leading to myocardial infarctions and strokes. Cholesterol is insoluble and, therefore, is packaged into lipoproteins for transport and delivery. As the major carriers, lipoproteins consist of fatty components such as free cholesterol, triglycerides, and phospholipids, enclosed by apolipoproteins (Apo) on the surface (Jialal and BartonDuell, 2016). These serum lipoproteins, synthesized in the liver, mainly include apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB). Aside from endogenous lipids production, the liver is also a key player in lipid metabolism (Yan et al., 2019). Abnormal lipid metabolism is one of the most important indications of cancer cell formation (Liu et al., 2017). Hepatocellular carcinoma (HCC), the most common form of liver cancer, accounts for approximately 80%–90% of all liver cancer types diagnosed worldwide. Studies have found that the ApoA1 level was substantially higher in curative resection HCC patients with overall improved survival. This suggested that ApoA1 can inhibit tumor proliferation and induce apoptosis, possibly by the mitogen-activated protein kinase (MAPK) pathway (Ma et al., 2016). Moreover, another study noticed that elevated ApoB is an alarming sign of tumor size greater than 5 cm at diagnosis and is associated with poor prognosis in HCC patients (Yan et al., 2019). Taken together, these findings indicate that both ApoA1 and ApoB are potential biomarkers for prognosis in post-surgery HCC patients. Additionally, ApoB is the main driver in atherogenesis, as it is the primary transporter for low density lipoprotein (LDL) (Libby et al., 2019). On the other hand, ApoA1 is a constituent of high density lipoprotein, known as the good cholesterol, playing a role in the elimination of excess cholesterol from peripheral tissues by the process of reverse cholesterol transport to the liver (Frank and Marcel, 2000). Deregulation in LDL cholesterol levels has also been linked to risk of myocardial infarction and cardiovascular disease (Mortensen and Nordestgaard, 2020).
Statins and proprotein convertase subtilis/kexin type 9 inhibitors (PCSK9) lower the plasma levels of low-density lipoprotein cholesterol (LDL-C) in hyperlipidemia and hypercholesterolemia patients (Blumenthal, 2000; Stoekenbroek et al., 2015); however, roughly 1–2 out of 10 patients develop statin intolerance with adverse symptoms (Ahmad, 2014). Moreover, cholesterol absorption inhibitors are ineffective in patients with genetic conditions caused by loss-of-function mutations in the LDL receptor or gain-of-function mutations in PCSK9, making homozygous familial hypercholesterolemia (FH) especially challenging to treat (Rader and Kastelein, 2014). Therefore, there is a need for more robust therapies that would encompass FH patients with lifelong drug durability. Additional approaches could involve reducing assembly and secretion of ApoB-containing lipoproteins. Biosynthesis of lipoproteins requires two components: a chaperone protein, the microsomal triglyceride transfer protein (MTP), and a structural protein, the ApoB. Both of these proteins are target candidates for therapeutic intervention in treating premature coronary diseases (Hussain and Bakillah, 2008). Lomitapide, a MTP inhibitor, is the current available therapy for patients with FH; however, this drug is known to cause hepatic steatosis and increase plasma transaminases, markers of liver injury (Rizzo and Wierzbicki, 2011). Hence, new avenues must be considered to reduce plasma LDL-C levels.
MicroRNAs (miRs) are short, non-coding RNA species, approximately 22–23 nucleotides in length that regulate gene expression post transcriptionally (Bartel, 2009). miRs are also known to be involved in various biological processes of cancerous cells, such as cell cycle and apoptosis, and were therefore further defined as either proto-oncogenes or tumor suppressors (Gong et al., 2015). The overexpression of tumor suppressor miRs can be used as a gene silencing tool that inhibits cell proliferation and metastasis, which ultimately leads to tumor cells arrest (Khare et al., 2013).
MiRs interact with the 3′-untranslated regions of target mRNAs by base pair complementarity to signal for their degradation and/or translational repression (Iwakawa and Tomari, 2015). Since their discovery, miR therapeutics have been an area of active research for their potential in treating different diseases. However, these developments have encountered difficulties. Chemical modifications result in loss of potency. Safe and targeted delivery is also challenging (Rupaimoole and Slack, 2017). To address these shortcomings, we synthesized an azido-modified N-acetylgalactosamine (GalNAc) moiety (GalNAcαProN3) that can be attached to any position of the RNA oligonucleotide modified with an alkyne group. In addition, phosphorothioate linkages were also introduced for the best action. Of course, this design could be extended to other miR systems, such as miR-122, which has been shown to inhibit growth and proliferation of HepG2 cells (Li et al., 2018). In our study, miR-30c was the best candidate in lowering plasma lipids and testing the effect on cell penetrating capacity when modifying the passenger strand. MiR-30c is a short, double-stranded non-coding RNA that belongs to the miR-30 family (miR-30a-e), highly conserved in the seed sequence (Irani and Hussain, 2015). We have previously reported that miR-30c interacts with the 3′-untranslated region of the MTP mRNA, leading to mRNA degradation and thereby reducing the secretion of apolipoprotein B by liver cells (JamesSoh, 2018. The successful synthesis of modified miR-30c passenger analogs with increased duplex stability and enhanced uptake by hepatoma cells, resulting in significant ApoB reductions without affecting the level of ApoA1(Yadav et al., 2022), have proven the feasibility of this approach. By introducing GalNAc residues at either end, we eliminated the need for lipid emulsion delivery as it might be more cost effective. Here, we detail the synthesis of modified miR-30c analogs. We envision this approach to be versatile as it can be applied to any tumor suppressor miR systems, to facilitate the development of their analogs as possible therapeutic drugs for lipid-lowering therapies and for the treatment of other diseases.
Materials and Reagents
U-shape cell culture flask, canted neck, 75 cm2 (Corning, catalog number: 430641U)
96-well ELISA plates (Fisher Scientific, Corning, catalog number: 07-200-640)
6-well plate (Fisher Scientific, Corning, catalog number: 07-200-80)
PrecisionGlideTM 25G needle (BD, catalog number: 305125)
Thin layer chromatography (TLC) plates pre-coated with silica gel F254 (Sigma-Aldrich, catalog number: 717185)
WatersTM Corp Sep-Pak C18 3 cc Vac cartridge (Fisher Scientific, catalog number: 50785823)
Protected RNA phosphoramidites [ChemGenes, catalog numbers: ANP-5671 (rA-CE), ANP-5673 (rG-CE), ANP-6676 (Ac-rC-CE), and ANP-5674 (rU-CE)]
Oligonucleotide synthesizer empty column (Alibaba, catalog number: HJ-H-818)
CPG-500 Å solid support (ChemGenes, catalog number: N-6103-05)
Anhydrous acetonitrile or ACN (CH3CN), HPLC grade (Sigma-Aldrich, catalog number: 34851-4L)
3% trichloroacetic acid in dichloromethane (ChemGenes, catalog number: RN-1462)
5-Ethylthio-1H-tetrazole (ETT) in acetonitrile solution (0.25 M) (ChemGenes, catalog number: RN-1466)
Tetrahydrofuran (THF) (Sigma-Aldrich, catalog number: 186562-1L)
CapA (80% THF/10% acetic anhydride/10% 2,6-lutidine) (ChemGenes, catalog number: RN-1458)
CapB (16% N-methylimidazole in THF) (ChemGenes, catalog number: RN-7776)
3-((Dimethylamino-methylidene)amino)-3H-1,2,4-dithiazole-3-thione) (DDTT) (ChemGenes, catalog number: RN-1588)
Ammonium hydroxide (Sigma-Aldrich, catalog number: 221228-500mL)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418-100mL)
Triethylamine trihydrogen fluoride (Et3N·3HF) (Sigma-Aldrich, catalog number: 344648-25g)
Sodium acetate (Invitrogen, catalog number: AM9740)
N-Acetyl-D-galactosamine (Thermo Fisher Scientific, catalog number: J66095.06)
Acetyl chloride (Thermo Fisher Scientific, catalog number: 219472500)
Sodium azide (NaN3) (Thermo Fisher Scientific, catalog number: J21610.A1)
Sodium iodide (NaI) (Thermo Fisher Scientific, catalog number: 203182500)
2'-O-propargyl adenosine (n-bz) CED phosphoramidite (ChemGenes, catalog number: ANP-7751)
2'-O-propargyl cytidine (n-bz) CED phosphoramidite (ChemGenes, catalog number: ANP-7752)
2'-O-propargyl guanosine (n-ibu) CED phosphoramidite (ChemGenes, catalog number: ANP-7753)
2'-O-propargyl uridine CED phosphoramidite (ChemGenes, catalog number: ANP-7754)
Copper(I) bromide (CuBr) (Thermo Fisher Scientific, catalog number: 047211.30)
Tert-butanol (tBuOH) (Sigma-Aldrich, catalog number: 471712)
Sodium phosphate, monobasic (NaH2PO4) (Fisher Scientific, catalog number: 567545500GM)
Sodium phosphate dibasic (Na2HPO4) (Fisher Scientific, catalog number: 7558794)
Sodium chloride (NaCl) (Fisher Scientific, catalog number: AC447300010)
Modified Eagle’s medium (DMEM) (Fisher Scientific, catalog number: MT10027CV)
Fetal bovine serum (FBS) (Fisher Scientific, catalog number: MT35010CV)
L-glutamine (Fisher Scientific, catalog number: AAJ60573A1)
LipofectamineTM RNAiMAX transfection reagent (Fisher Scientific, Invitrogen, catalog number: 13778075)
Human ApoB ELISA development kit (HRP) (MABTECH lnc., catalog number: 3715-1H-6)
3,3′,5,5′ tetramethylbenzidine substrate (Thermo Fisher Scientific, catalog number: J61325.EQE)
Coomassie (Bradford) protein assay kit (Thermo Fisher Scientific, catalog number: 23200)
Human apolipoprotein A-I/ApoA1 DuoSet ELISA kit (R&D Systems, catalog number: DY3664)
Substrate reagent pack (R&D Systems, catalog number: DY999)
Stop solution 2 N sulfuric acid (R&D Systems, catalog number: DY994)
Protease inhibitor cocktail (Fisher Scientific, catalog number: PIA32965)
Anti-MTTP/MTP antibody (Abcam, catalog number: ab63467)
β-Actin (D6A8) rabbit mAb (Cell Signaling, catalog number: 8457)
Anti-rabbit IgG, HRP-linked antibody (Cell Signaling, catalog number: 7074)
Phosphate buffer saline (PBA 10×) (Thermo Fisher Scientific, catalog number: AAJ62851AP)
Oxidation solution (0.02 M iodine in THF/pyridine/water, 7:2:1 v/v) (ChemGenes Corporation, catalog number: RN14552L)
Screw cap tube (Thermo Scientific, catalog number: AB1389500)
Solid phase synthesis column frit (BioAutomation, catalog number: FR-1502-1)
Molecular Biology Grade (200 Proof) Ethanol (Fisher Scientific, catalog number: BP2818100)
RNase/DNase free water (MP BiomedicalsTM, catalog number: MP112450204)
3-chloroproanol (Thermo ScientificTM, catalog number: C0270100G)
TEAAC buffer (see section B, #14 for detailed preparation)
8 M urea (see section B, #15 for detailed preparation)
30% acrylamide/bis stock (Thermo ScientificTM, catalog number: HBGR329500)
10× TBE running buffer (Invitrogen, catalog number: AM9864)
TEMED (Thermo ScientificTM, catalog number: 17919)
Ammonium Persulfate (APS) (Thermo ScientificTM, catalog number: 17874)
Loading buffer (Thermo ScientificTM, catalog number: J62832S0)
Ethidium Bromide 10mg/ml (InvitrogenTM, catalog number: 15585011)
Tm Buffer or sodium phosphate buffer pH 6.5 (see Recipes)
Buffer K containing one tablet of protease inhibitor cocktail (see Recipes)
Equipment
Nuclear magnetic resonance NMR (Brucker, Ascend 500 MHz spectrometer)
Automated solid-phase oligonucleotide synthesizer (BIOSSET Ltd, ASM800)
DNA/RNA speed-vac concentrator (Thermo Electron Corporation, Savant DNA 120 SpeedVac Concentrator)
Mass spectrometer (Agilent Technologies, Accurate-Mass Q-TOF LC/MS 6530)
UV-visible spectrometer (Perkin Elmer, Lambda 35 UV/VIS Spectrometer)
Circular Dichromism (CD) Spectropolarimeter (JASCO, J-815 spectropolarimeter)
ChemiDocTM touch imaging system (Bio-Rad, 1708370)
Eppendorf centrifuge (Eppendorf, 5424)
UV lamp (MilliporeSigmaTM SupelcoTM, Z16964114EA)
Tube revolver rotator (Thermo ScientificTM, 88881001)
Heating Blot (Thermo SchientificTM, 88870003)
Procedure
Synthesis of chemically modified novel miR-30c-3p analogs (Figure 1)
Note: Standard phosphoramidites and reagents were available from ChemGenes. Modified antisense miR-30c oligonucleotides were synthesized at one micromole scale using Oligo-800 DNA/RNA solid-phase synthesizer. The system is protected under helium gas. The synthesis proceeds in 3′ to 5′ direction with four main steps per synthesis cycle: detritylation, coupling, capping, and oxidation that adds one nucleotide at the completion of one cycle. All synthesis steps are performed on control-pore glass (CPG-500) that has the first nucleotide base immobilized through succinate linker inside the column.
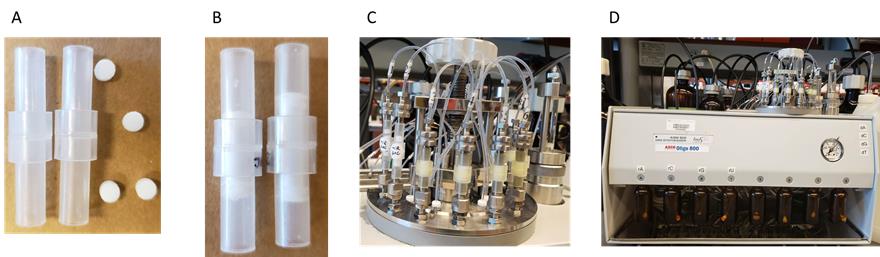
Figure 1. Preparation for RNA synthesis. (A) Solid phase synthesis empty columns with frits. (B) Column assembled with tow frits that hold the CPG-500 beads in the center, where the synthesis takes place. (C) Securely mound the columns onto the system. (D) Screw on each nucleotide AUCG reservoir, measured and dissolved in anhydrous acetonitrile (ACN).Dissolve the modified and native RNA phosphoramidites separately in anhydrous acetonitrile to a concentration of 0.07 M.
Note: 0.07 M is the final concentration for each type of phosphoramidite (modified and native); the volume of anhydrous acetonitrile is determined by the length, sequence, and molecular weight of commercial AUCG phosphoramidites.
Assemble the column with two frits that hold the CPG-500 Å beads in the center, where the synthesis takes place.
Employ the Oligo-800 synthesizer in DMTr-off mode to perform automated solid-phase oligonucleotide synthesis of modified miR-30c analogs on a one micromole scale. All the necessary phosphoramidite solutions, including A, U, C, and G, are pre-filled in the synthesis lines and pressure-checked before the synthesis can begin.
Note: This is an automatic system; the input sequence is the final synthesized product. The synthesis adds one nucleotide per synthesis cycle, beginning with detritylation and ending with oxidation. The average time for eight -mers of RNA is 4 h under working pressure of 1.5 bars.
Detritylation is the removal of the 5′ DMT-protecting group on the 3′-end of the oligonucleotide, using 3% trichloroacetic acid in dichloromethane.
Coupling is carried out with ETT (0.25 M in acetonitrile) for 12 min.
Capping of the 5′-OH is performed using CapA (80% THF/10% acetic anhydride/10% 2,6-lutidine) and CapB (16% N-methylimidazole in THF) solutions
Phosphate backbone oxidation is performed with 20 mM iodine solution in pyridine/THF/water; the synthesis of phosphorothioate backbones is performed with the sulfurization reagent, DDTT.
After the completion of the synthesis cycle, transfer the RNA product into a screw cap tube.
Cleave the RNAs from the solid support and deprotect using 800 µL of concentrated aqueous ammonium hydroxide at room temperature for 18 h.
Dry the resulting RNA solution in a speed vacuum concentrator (approximately 3–4 h).
The Savant DNA/RNA 120 speed-vac concentrator operates at three drying rates—low, medium, and high heat—with temperature ranging from approximately 43°C to 65°C, a displacement capacity of 31 L/min at 50 Hz and 36 L/min at 60 Hz, and maximum vacuum at 7 Torr or 9 mbar. Low or medium heat is preferred for drying to prevent sample denaturing.
Redissolve the solid residue with 100 µL of DMSO by vortexing, and desilylate by incubating with 125 µL of Et3N·3HF solution at 65 °C for 2.5 h using a heating block. Desilylation is the removal of silyl moiety.
Precipitate RNAs by adding 25 µL of 3 M sodium acetate solution and 1 mL of 100% ethanol and keep at -80 °C for at least 3 h.
Centrifuge at 15,000 × g and 4 °C for 45 min to recover the RNA. Completely dry the RNA under vacuum.
Dissolve the RNA in 500 µL of RNase-free water and assess its integrity if necessary. Perform desalting twice prior to storage at -20 °C.
Synthesis of GalNAcαProN3 3 (Figure 1) and 2′-GalNAc-modified RNA strands (Figure 2)
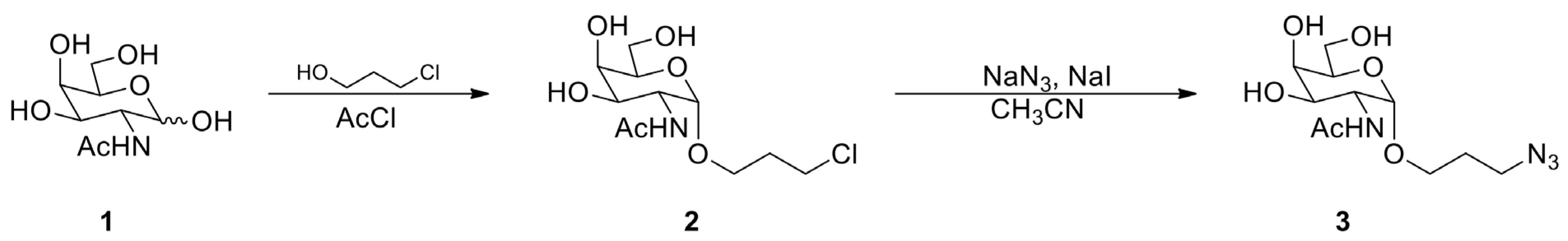
Figure 2. Synthesis of 3-chloropropyl GalNAc 2 and GalNAcαProN3Dissolve 330 mg (1.5 mmol) of N-acetyl-D-galactosamine in 5 mL of 3-chloropropanol.
Add 0.13 mL (1.8 mmol) of acetyl chloride to the above solution while on ice (approximately 0 °C).
Heat the resulting solution at 70 °C for 15 h using a hot plate.
Concentrate the solution by speed vacuum using medium heat and purify the residue by silica gel chromatography to get 3-chloropropyl GalNAc 2 .
Typical yield of approximately 45% for 200 mg white solid with the following parameters:
TLC Rf = 0.5 (20% MeOH in CH2Cl2)
1H NMR (500 MHz, D2O) δ 4.92 (d, J = 4.0 Hz, 1H), 4.15 (dd, J = 4.4, 12.8 Hz, 1H), 4.00–3.84 (m, 4H), 3.77–3.72 (m, 4H), 3.62–3.56 (m, 1H), 2.10–2.02 (m, 5H)
Dissolve 200 mg (0.671 mmol) of 3-chloropropyl GalNAc 2 in 6 mL of CH3CN by heating the solution.
Add 436 mg (6.71 mmol) of NaN3 and 101 mg (0.671 mmol) of NaI to the solution and stir at 60 °C for 15 h.
Concentrate the solution by speed vacuum and purify the residue by silica gel chromatography to obtain GalNAcαProN3 3.
Approximately 54% yield for approximately 110 mg white solid with the following parameters:
TLC Rf = 0.4 (20% MeOH in CH2Cl2)
1H NMR (500 MHz, D2O) δ 4.94 (m, 1H), 4.21–4.18 (m, 1H), 4.03-3.94 (m, 3H), 3.85–3.78 (m, 3H), 3.59–3.47 (m, 3H), 2.08 (d, 3H), 1.95–1.91 (m, 2H).
Note: The synthesis of GalNAc-modified RNA strands begins with synthesizing propargyl-modified RNA oligonucleotides, following protocol A, with commercially available 2′-(O-propargyl)-phosphoramidite building blocks from ChemGenes. The chemically synthesized azido-GalNAc moiety is conjugated to the propargyl RNA via click chemistry with copper as catalyst.
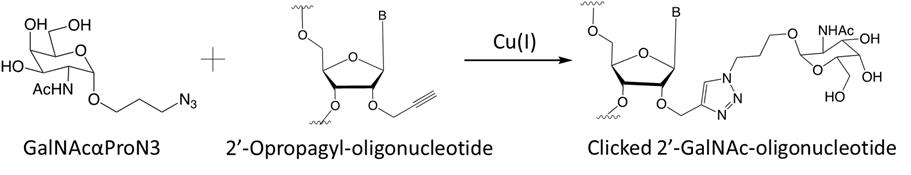
Figure 3. Synthesis of GalNAc-modified RNA strands through Cu(I)-catalyzed click reactionTo make GalNAc-modified RNA strands (sequences shown in Tables 1 and 2), add 100 equivalents of azido-modified GalNAc (GalNAcαProN3) to the freshly synthesized propargyl RNA (1 equivalent) in a 1.5 mL Eppendorf tube (i.e., for every microgram of propargyl RNA use 100 µg of azido-modified GalNAc (GalNAcαProN3) to react) (Yadav et al., 2022).
Table 1. MiR-30c analogs and sequences containing GalNAc modification
Compound Short form Strand Sequence (5’ to 3’) miR30c-B1 B1 miR-30c-1-3p (pC)UgGgAgAgGgUuGuUuAcUcC miR30c-B2 B2 miR-30c-1-3p CuGgGaGaGgGuUgUuUaCuC(pC)u miR30c-B3 B3 miR-30c-1-3p (pC)uGgGaGaGgGuUgUuUaCuC(pC)u miR30c-B4 B4 miR-30c-2-3p (pC)UgGgAgAaGgCuGuUuAcUcU miR30c-B5 B5 miR-30c-1-3p CuGgGaGaGgGuUgUuUa(pC)U(pC)(pC)u miR30c-B6 B6 miR-30c-1-3p (pC)UgGgAgAgGgUuGuUuA(pC)U(pC)(pC)u miR30c-B7 B7 miR-30c-2-3p CuGgGaGaAgG(pC)uGuUuA(pC)u(pC)U miR30c-B8 B8 miR-30c-2-3p (pC)UgGgAgAaGg(pC)UgUuUa(pC)U(pC)u miR30c-B9 B9 miR-30c-1-3p (pC)(pC)(pC)UgGgAgAgGgUuGuUuAcUcC miR30c-B10 B10 miR-30c-2-3p (pC)(pC)(pC)UgGgAgAaGgCuGuUuAcUcU miR30c-B11 B11 miR-30c-2-3p (pC)(pC)(pC)ugggagaaggcuguuuacucu Upper case letters: 2′-deoxy-2′-fluoro (2′-F) ribosugar-modified nucleosides; lower case letters: 2′-O-methyl (2′-OMe) ribosugar-modified nucleosides; (pC): 2′-GalNAc clicked cytidines.
Table 2. MiR-30c analogs and sequences containing GalNAc and phosphorothioate linkages
Compound Short form Strand Sequence (5' to 3') miR30c-C1 C1 miR-30c-1-3p (pC)•U•gGgAgAgGgUuGuUuAcUc•C miR30c-C2 C2 miR-30c-1-3p (pC)•UgGgAgAgGgUuGuUuAc•U•c•C miR30c-C3 C3 miR-30c-1-3p (pC)•U•gGgAgAgGgUuGuUuAc•U•c•C miR30c-C4 C4 miR-30c-1-3p GgGaGaGgGuUgUuUaCuC(pC)•u miR30c-C5 C5 miR-30c-1-3p GgGaGaGgGuUgUuUaCu•C•(pC)•u miR30c-C6 C6 miR-30c-1-3p C•u•GgGaGaGgGuUgUuUaCuC(pC)u Upper case letters: 2′-deoxy-2′-fluoro (2′-F) ribosugar modifications; lower case letters: 2′-O-methyl (2′-OMe) ribosugar modifications; (pC): 2′-GalNAc clicked cytidine; •: phosphorothioate linkages
In a separate tube, dissolve 22 equivalents of CuBr (100 mM in 25% tBuOH/75% DMSO) in 20% acetonitrile and mix well.
Transfer the mixture to the RNA solution and rotate at approximately 20 rpm using the tube revolver rotator at room temperature for 12 h.
Precipitate the RNA with 3 M sodium acetate and 1 mL of cold ethanol and store at -80 °C for at least 3 h.
Centrifuge the RNA at 18,400 × g for 15 min.
Resuspend the RNA pellet in 500 µL of RNase-free water.
Desalt the RNA solution using Sep-Pak C18 cartridges.
First, condition the cartridge with 1 mL of ACN and equilibrate with 1 mL of RNase free water and 1 mL of 2 M TEAAC buffer. Discard the flow through each time. Load RNA samples dropwise and allow for RNA to bind. Perform three washes with RNase free water, 1 mL each wash, and elute the desalted RNA with 1 mL of 50% ACN.
Note: 2 M TEAAC pH 8: in the fume hood, prepare 1 L of stock with 619 mL of distilled water, 268 mL of triethylamine, and 114 mL of glacial acetic acid. Recipe is adopted from the Cold Spring Harbor laboratory.
Concentrate the eluted RNA using the speed vac and check the resulting products by analytical gel electrophoresis with 15% polyacrylamide containing 8 M urea.
Make the 15% polyacrylamide gel containing 8 M urea stock solution (500 mL) with 50 mL of 10× TBE, 250 mL of 30% acrylamide/bis stock, and 240 g of 8 M urea. A small analytical gel is made with 6.5 mL of 15% 8 M urea stock solution, 37 µL of 10% APS, and 6.8 µL of TEMED. Heat the samples at 95 °C for 5 min and cool down on ice for another 5 min. Use 10 µM sample as final concentration after adding loading buffer. Perform the gel electrophoresis under 1× TEB as running buffer at 250 V for approximately 30 min depending on the percentage of the gel. Following electrophoresis, stain the gel with EthBr for 15 min prior to imaging.
Cell culture studies with the focus on the effects of GalNAc-modified miR-30c analogs on ApoB, ApoA1, and MTP in human hepatoma Huh-7 cell line
Maintain the human hepatoma Huh-7 cells in DMEM containing 10% FBS and 1% L-glutamine in a 75 cm2 culture flask with vent cap at 37 °C and 5% CO2 in a humidified incubator.
Seed the Huh-7 cells in a 6-well plate with 2 mL of aforementioned media at the concentration of approximately 100,000 cells/well.
Mix the non-GalNAc-modified microRNAs with RNAiMAX at a ratio of 3:1 and incubate for 30 min at room temperature prior to cell transfection.
Add GalNAc-modified microRNAs directly into the cells without the need for liposome-assisted delivery.
Change into 1 mL of fresh DMEM (10% FBS) media 72 h post transfection and incubate overnight.
Collect media for ApoB and ApoA1 measurements.
Wash and harvest the cells in the presence of protease inhibitor cocktail for MTP protein level measurements.
Determine the ApoB and ApoA1 levels in the collected media using specific human ApoB/ApoA1 ELISA development kit with 3,3′,5,5′ tetramethylbenzidine substrate. Later, stop solutions in 96-well ELISA plates.
Calculate each concentration using ApoB/ApoA1 standard curve prepared in parallel with the experimental samples using the standard provided by the manufacturer.
Normalize ApoB/ApoA1 levels to total protein in the respective wells, i.e., set the control microRNA–transfected cells to 100% and modified miR-30c normalized to this value.
Determine the MTP protein levels within the cells, wash the transfected Huh-7 cells with ice-cold PBS, and scrap off the wells in ice-cold buffer K (see Recipes).
Lyse the cells by passing them through a PrecisionGlideTM 25G needle.
Measure the total protein concentration using the Coomassie (Bradford) protein assay kit prior to MTP detection on western blot.
Data analysis
UV thermal denaturation and circular dichroism (CD) spectroscopy studies with 2′-OMe-modified miR-30c strands
Note: To study the biophysical properties, the synthesized 2’-OMe-modified miR-30c-1-3p and miR-30c-2p antisense strands were annealed with sense unmodified native miR-30c-5p in sodium phosphate buffer (10 mM, pH 6.5) containing 100 mM NaCl.
Heat the solution at 95 °C for 3 min and slowly cool down at the rate of 1 °C /min at room temperature for approximately 2 h. Store at 4 °C overnight prior to running on the UV-visible spectrometer.
For the UV melting study, collect all data points at 260 nm with two cycles of heating and cooling from 5 °C to 85 °C (4 ramps total) at the rate of 0.5 °C/min.
Record the CD spectra over a wavelength range of 200–300 nm using a 1 cm path length quartz cuvette.
Collect all data points with a scanning speed of 100 nm/min, bandwidth of 1.0 nm, and integration time of 1.0 s.
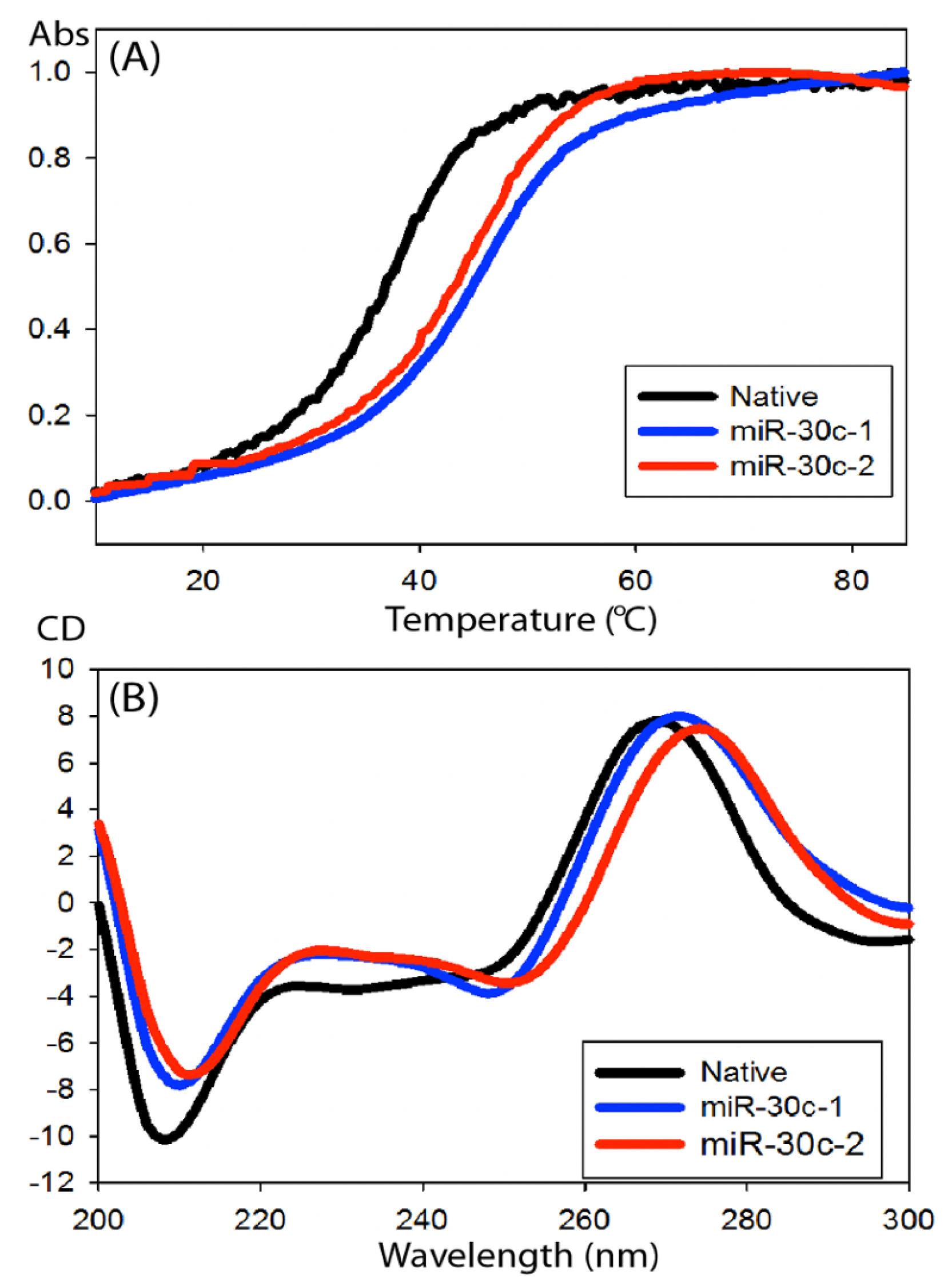
Figure 4. CD spectra of modified miR-30c duplexes. Compared to the native miR-30c-1-3p annealed with miR-30c-5p, the modified duplexes showed an overall increase in thermal stability by approximately 7 °C, indicating enhanced duplex stability (A). The CD spectra showed similar conformation indicating that the modification of miR-30c-1-3p does not impair the interaction with the miR-30c-5p (B) (Yadav et al., 2022).
- Confirmation check for the synthesized compounds
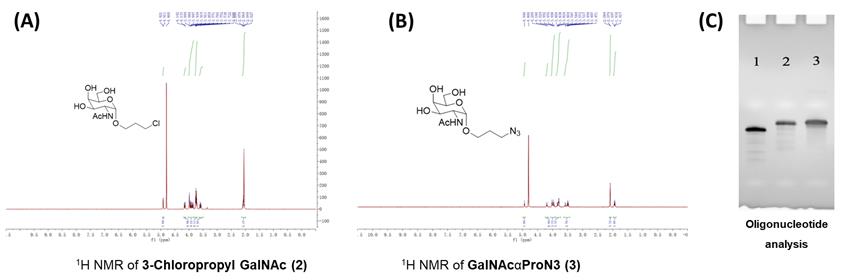
Figure 5. 1H NMR spectra of 3-chloropropyl GalNAc 2 and GalNAcαProN3 3, and analysis of antisense-oligonucleotide in 15% PAGE gel. Correct synthesis of both compounds 3-Chloropropyl GalNAc 2 (A) and GalNAcαProN3 3 (B) was confirmed by 1H NMR spectroscopy and (C) 15% polyacrylamide analytical 8 M urea gel electrophoresis. Lane 1: antisense-oligonucleotide (ASO), lane 2: post-clicked GalNAc-ASO, and lane 3: reference GalNAc-ASO (Yadav et al., 2022). Effect of GalNAc-modified miR-30c analogs on ApoB and ApoA1 secretion and cellular MTP protein levels in human hepatoma Huh-7 cells
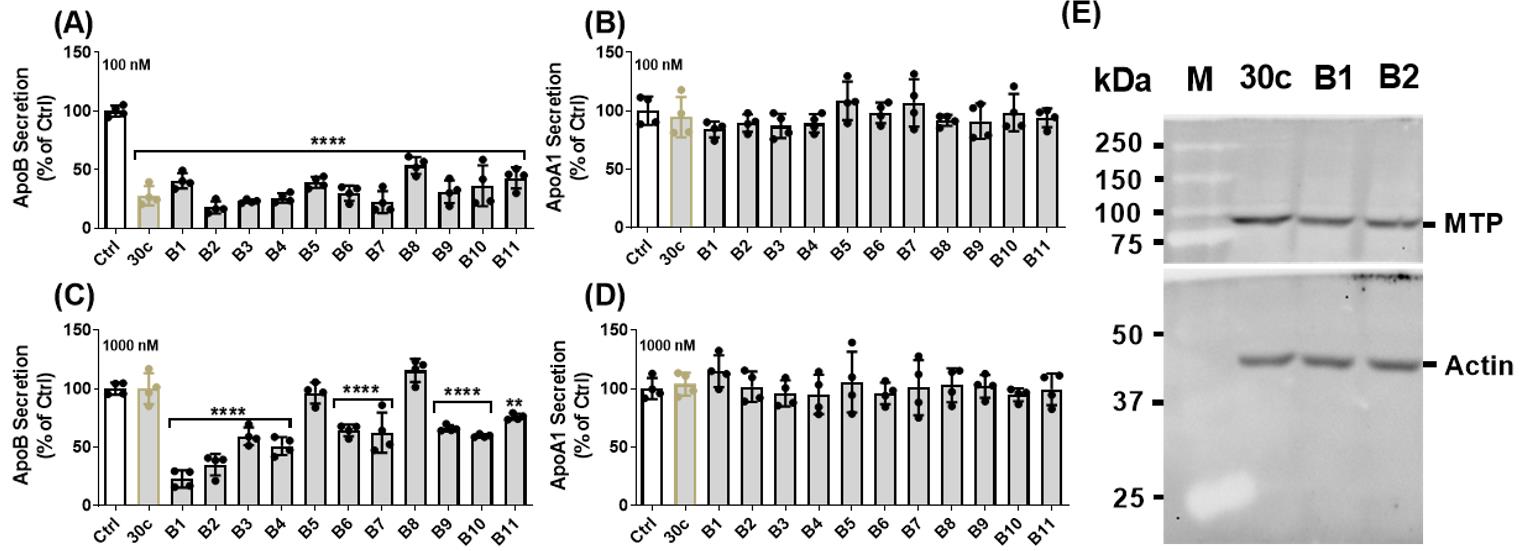
Figure 6. Effects of various GalNAc-modified miR-30c analogs on apoB secretion in Huh-7 human hepatoma cells. All analogs from Table 1 were transfected into cells using lipofectamine RNAiMAX. All potently inhibited ApoB secretion (A) without affecting ApoA1 secretion (B). However, when these analogs were given to cells without lipofectamine RNAiMAX, only analogs B5 and B8 had no effect on ApoB, while all other analogs reduced secretion, ranging from 40% to 80% (C). Any of the analogs had an effect on ApoA1 (D). These results indicate that analogs B1 and B2 containing one copy of GalNAc have better biological activity compared to analogs with multiple GalNAc-modified nucleotides B5 and B8. The level of MTP protein is detected by western blot resolved on an SDS-PAGE (10%) gel. Briefly, 50 µg of total proteins were used in this experiment. A polyclonal rabbit primary antibody to human MTP and a monoclonal rabbit antibody to β-actin were used at 1:1,000 dilution. Anti-rabbit IgG and HRP-linked secondary antibody were used at 1:2,000 dilution. The blots were imaged using ChemiDocTM touch imaging system. Analysis on western blot also showed a decrease in MTP protein expression (Yadav et al., 2022).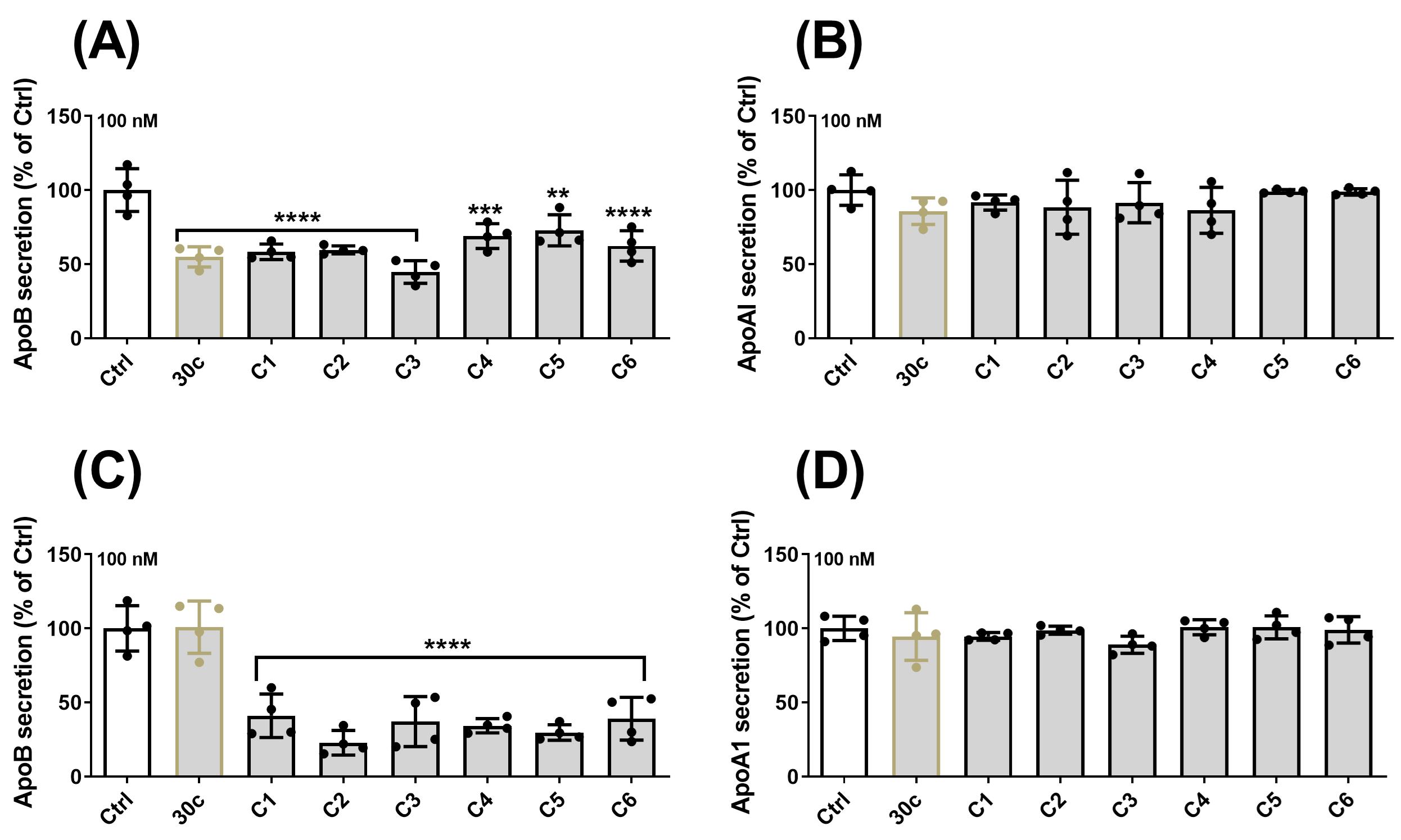
Figure 7. Effects of various phosphorothioate-linked miR-30c analogs on apoB secretion in Huh-7 human hepatoma cells. Since B1 and B2 analogs have shown potent inhibition towards ApoB secretion, we further modified the analogs (Table 2) by introducing phosphorothioate linkages, which have been suggested to improve specificity and silencing activity. The analogs synthesized with phosphorothioate linkages showed potent inhibition towards ApoB secretion (A) without having an effect on ApoA1 when cells were transfected with RNAiMAX. More importantly, all the analogs with phosphorothiorate linkages reduced ApoB secretion (C) but had no effect on ApoA1 (D) when cells were transfected without the lipofectamine-assisted delivery (Yadav et al., 2022).
Recipes
Tm Buffer or sodium phosphate buffer pH 6.5
Reagent Final concentration Amount NaCl 100 mM 294.4 mg Na2HPO4 10 mM 70.95 mg NaH2PO4 10 mM 59.99 mg Dissolve in total of 50 mL of Milli-Q H2O
Buffer K contains 1 tablet of protease inhibitor cocktail
Reagent Final concentration Amount Tris-HCl 1 mM 7.88 mg EGTA 1 mM 19.02 mg MgCl2 1 mM 4.76 mg Dissolve in a total of 50 mL of Milli-Q H2O
Acknowledgments
We thank U.S. National Institutes of Health (NIH) DK121490, HL137202, and HD094778 and VA Merit Award BX004113 to MMH, NSF CHE1845486 to JS, and MRI grant CHE1726724 for the financial support. The content of this protocol is based on the published work (Yadav et al., 2022).
Competing interests
The authors declare no conflict of interests.
Ethics
No human and/or animal subjects were used in this study.
References
- Ahmad, Z. (2014). Statin intolerance. Am J Cardiol 113(10): 1765-1771.
- Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136(2): 215-233.
- Blumenthal, R. S. (2000). Statins: effective antiatherosclerotic therapy. Am Heart 139(4): 577-583.
- Frank, P. G. and Marcel, Y. L. (2000). Apolipoprotein A-I: structure-function relationships. J Lipid Res 41(6): 853-872.
- Gong, J., He, X. X. and Tian, A. (2015). Emerging role of microRNA in hepatocellular carcinoma (Review). Oncol Lett 9(3): 1027-1033.
- Hussain, M. M. and Bakillah, A. (2008). New approaches to target microsomal triglyceride transfer protein. Curr Opin Lipidol 19(6): 572-578.
- Irani, S. and Hussain, M. M. (2015). Role of microRNA-30c in lipid metabolism, adipogenesis, cardiac remodeling and cancer. Curr Opin Lipidol 26(2): 139-146.
- Iwakawa, H. O. and Tomari, Y. (2015). The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends Cell Biol 25(11): 651-665.
- James Soh, J., Iqbal, J., Queiroz, J., Fernandez-Hernando, C. and Hussain, M. M. (2018). MicroRNA-30c reduces hyperlipidemia and atherosclesrosis by decreasing lipid synthesis and lipoprotein secretion. Nat Med 19: 892-900.
- Jialal, I. and Barton Duell, P. (2016). Diagnosis of Familial Hypercholesterolemia. Am J Clin Pathol 145(4): 437-439.
- Khare, S., Zhang, Q. and Ibdah, J. A. (2013). Epigenetics of hepatocellular carcinoma: role of microRNA. World J Gastroenterol 19(33): 5439-5445.
- Li, S., Yao, J., Xie, M., Liu, Y. and Zheng, M. (2018). Exosomal miRNAs in hepatocellular carcinoma development and clinical responses. J Hematol Oncol 11(1): 54.
- Libby, P., Buring, J. E., Badimon, L., Hansson, G. K., Deanfield, J., Bittencourt, M. S., Tokgozoglu, L. and Lewis, E. F. (2019). Atherosclerosis. Nat Rev Dis Primers 5(1): 56.
- Liu, Q., Luo, Q., Halim, A. and Song, G. (2017). Targeting lipid metabolism of cancer cells: A promising therapeutic strategy for cancer. Cancer Lett 401: 39-45.
- Ma, X. L., Gao, X. H., Gong, Z. J., Wu, J., Tian, L., Zhang, C. Y., Zhou, Y., Sun, Y. F., Hu, B., Qiu, S. J., et al. (2016). Apolipoprotein A1: a novel serum biomarker for predicting the prognosis of hepatocellular carcinoma after curative resection. Oncotarget 7(43): 70654-70668.
- Mortensen, M. B. and Nordestgaard, B. G. (2020). Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet 396(10263): 1644-1652.
- Rader, D. J. and Kastelein, J. J. (2014). Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 129(9): 1022-1032.
- Rizzo, M. and Wierzbicki, A. S. (2011). New lipid modulating drugs: the role of microsomal transport protein inhibitors. Curr Pharm Des 17(9): 943-949.
- Rupaimoole, R. and Slack, F. J. (2017). MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16(3): 203-222.
- Stoekenbroek, R. M., Kastelein, J. J. and Huijgen, R. (2015). PCSK9 inhibition: the way forward in the treatment of dyslipidemia. BMC Med 13: 258.
- Yadav, P. K., Haruehanroengra, P., Irani, S., Wang, T., Ansari, A., Sheng, J. and Hussain, M. M. (2022). Novel efficacious microRNA-30c analogs reduce apolipoprotein B secretion in human hepatoma and primary hepatocyte cells. J Biol Chem 298(4): 101813.
- Yan, X., Yao, M., Wen, X., Zhu, Y., Zhao, E., Qian, X., Chen, X., Lu, W., Lv, Q., Zhang, L., et al. (2019). Elevated apolipoprotein B predicts poor postsurgery prognosis in patients with hepatocellular carcinoma. Onco Targets Ther 12: 1957-1964.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zheng, Y. Y., Haruehanroengra, P., Yadav, P. K., Irani, S., Mao, S., Wang, T., Hussain, M. M. and Sheng, J. (2022). Synthesis of Novel MicroRNA-30c Analogs to Reduce Apolipoprotein B Secretion in Human Hepatoma Cells. Bio-protocol 12(24): e4574. DOI: 10.21769/BioProtoc.4574.
- Yadav, P. K., Haruehanroengra, P., Irani, S., Wang, T., Ansari, A., Sheng, J. and Hussain, M. M. (2022). Novel efficacious microRNA-30c analogs reduce apolipoprotein B secretion in human hepatoma and primary hepatocyte cells. J Biol Chem 298(4): 101813.
Category
Drug Discovery > Drug Design
Medicine > Cardiovascular system
Biochemistry > RNA > miRNA targeting
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








