- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Isolation and Expansion of Primary Conjunctival Stem Cells (CjSCs) from Human and Rabbit Tissues
Published: Vol 12, Iss 24, Dec 20, 2022 DOI: 10.21769/BioProtoc.4569 Views: 2236
Reviewed by: Vivien Jane Coulson-ThomasSudhir VermaAnonymous reviewer(s)
Abstract
Conjunctival disorders are multivariate degenerative ocular surface diseases that can jeopardize ocular function and impair visual capacity in severe cases. The recent development of stem cell technologies has shed a new light on the treatment of conjunctival disorders as the regenerative medicine using endogenous stem cells becomes a potential therapeutic strategy. However, the efficient in vitro expansion of the endogenous stem cells dominating the conjunctival regeneration, the conjunctival stem cells (CjSCs), remains challenging. Existing protocols largely adopted primary culture using feeder layers, which has limited efficiency and risk of contamination. Here, we report a protocol for the isolation and expansion of primary CjSCs derived from human or animal tissues. This protocol adopts collagenase-based enzymatic digestion to release the primary cells from conjunctival tissues and utilizes a feeder-free culture strategy based on a small molecule inhibitor cocktail that stimulates the expansion of CjSCs. The CjSCs generated with this method were rapidly dividing and highly homogeneous. They also expressed characteristic stem cell markers and exhibited differentiation potency. These findings marked an important step forward in building stable CjSCs in vitro expansion, which will help researchers better understand the biology of ocular surface stem cells and develop innovative regenerative medicine approaches for ocular surface diseases.
Graphical abstract
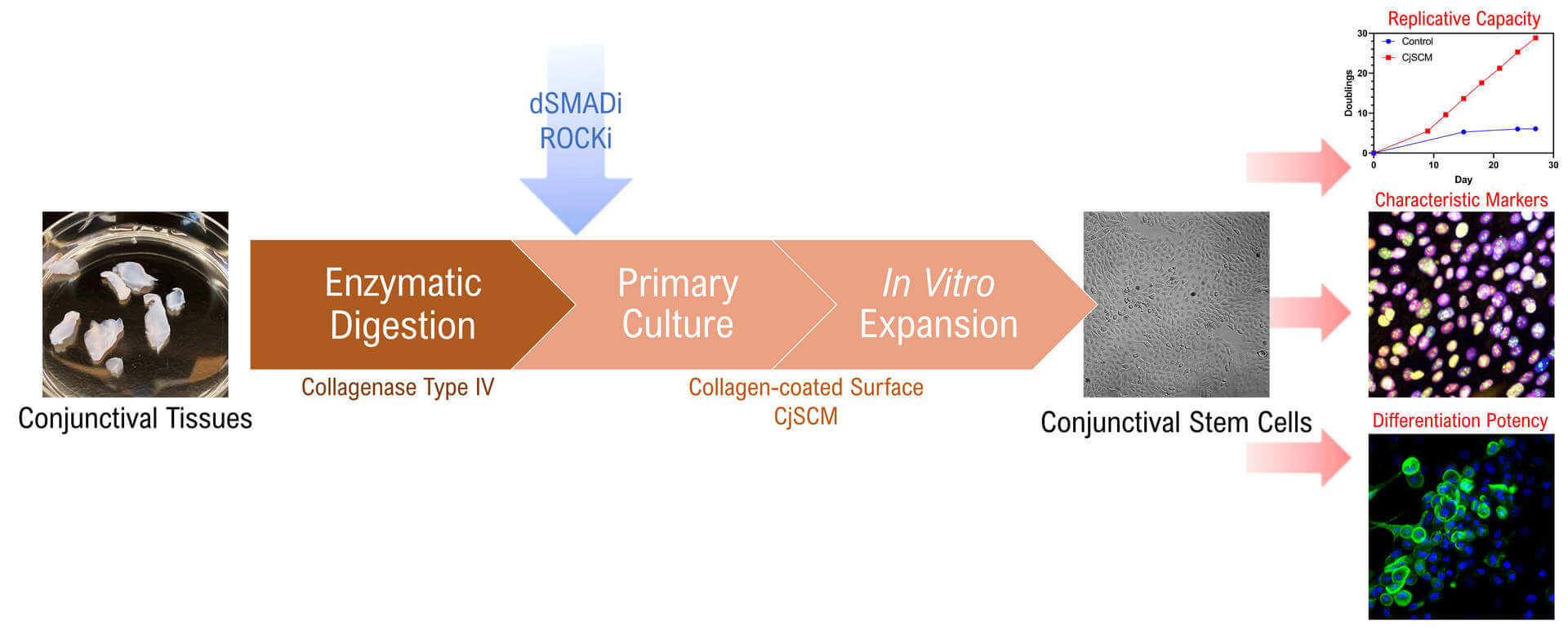
Background
The conjunctiva is a significant part of the ocular surface that functions as the immune barrier and protects the integrity of the eyes (Nguyen et al., 2011; Gipson, 2016). Similar to the involuted epithelium on gastrointestinal and airway internal surfaces, it is comprised of nonkeratinized mucosal epithelium containing mucin-secreting goblet cells, which provide the fundamental support of the tear film as well as the homeostasis of the ocular surface (Barabino et al., 2012). Disorders including cicatrizing conjunctivitis, dry eye diseases, and Stevens-Johnson syndrome can disrupt the normal function of the conjunctiva, which could further damage the ocular surface and jeopardize the vision (Barabino et al., 2003; Kohanim et al., 2016). Although these conditions are threatening millions of patients worldwide, existing treatments based on pharmaceutical therapy and amniotic membrane transplantation are limited by mediocre efficacy and insufficient regeneration (Liu et al., 2010; Tseng et al., 2016).
Recent advances in stem cell technology and regenerative medicine have made stem cell transplantation an alternative strategy for treating ocular surface diseases, and a huge interest has been raised in studying the endogenous stem cells residing in the conjunctiva, the conjunctival stem cells (CjSC) (Ramos et al., 2015; Nakamura et al., 2016; Williams et al., 2018; Zhong et al., 2021a). These are the bipotent progenitor that give rise to both conjunctival keratinocytes and conjunctival goblet cells, thus holding tremendous potential in conjunctival regeneration (Pellegrini et al., 1999; Majo et al., 2008; Nomi et al., 2021). However, as a critical premise in developing CjSC-based applications, the primary culture and in vitro expansion of CjSCs remains challenging. Early studies largely utilized feeder layers to support the primary culture of CjSCs, while the later ones tended to adopt the feeder-free culture system supplemented with cytokines and small molecules targeting key signaling pathways (Pellegrini et al., 1999; Stewart et al., 2015; Wu et al., 2020). The latest studies have demonstrated the efficacy of small molecule–based dual SMAD signaling inhibition (dSMADi) and ROCK signaling inhibition (ROCKi) in culturing epithelial stem cells derived from pulmonary alveoli, esophagus, and intestine (Mou et al., 2016; Zhang et al., 2018). Dual SMAD signaling (TGFβ and BMP signaling pathways) has been proved to regulate the maturation, self-renewal, and quiescence of epithelial stem cells, while ROCK signaling pathway contributes to the mechanotransduction and cell cycle progression (Kobielak et al., 2007; Amano et al., 2010; Tata et al., 2013). Therefore, we hypothesize that the combination of dSMADi and ROCKi can be applied to the CjSC culture and stimulate expansion.
Here, we established an isolation and feeder-free culture method for CjSCs derived from both human and animal conjunctival tissues. The primary conjunctival epithelial cells were first isolated through enzymatic digestion and seeded on a collagen-coated surface. Then, the cells were subjected to primary culture with the conjunctival stem cell medium (CjSCM) encompassing a small molecule cocktail of dSMADi and ROCKi, which stimulated the outgrowth of the stem cell population. CjSCM outperformed the control medium in generating the cells with a higher replicative capacity and shorter doubling time. The cells cultured with CjSCM also showed upregulated expression of stemness and lineage markers while retaining the differentiation potency. This method can be applied to produce functional CjSCs and support the development of regenerative medicine and stem cell therapy for ocular surface diseases.
Materials and Reagents
Sterilization pouches
BD syringe needle (BD, catalog number: 230-45094)
100 mm TC-treated culture dish (Corning, catalog number: 430167)
Costar® 6-well plates (Corning, catalog number: 3516)
Costar® 12-well plates (Corning, catalog number: 3513)
FalconTM 70 μm cell strainers (Corning, catalog number: 08-771-2)
Microcentrifuge tubes (ThermoFisher Scientific, catalog number: 3448PK)
15 mL conical centrifuge tubes (ThermoFisher Scientific, catalog number: 339651)
50 mL conical centrifuge tubes (ThermoFisher Scientific, catalog number: 339653)
Millicell EZ SLIDE 8-well glass, sterile (Sigma-Aldrich, catalog number: PEZGS0816)
Phosphate buffer solution (PBS) (ThermoFisher Scientific, catalog number: 10010023)
Dulbecco's modified Eagle medium (DMEM) (ThermoFisher Scientific, catalog number: 11885084)
DMEM/F12 medium (ThermoFisher Scientific, catalog number: 11330032)
Penicillin-streptomycin (pen-strep) (ThermoFisher Scientific, catalog number: 15140122)
0.25% trypsin-EDTA (ThermoFisher Scientific, catalog number: 25200056)
Fetal bovine serum (FBS) (ThermoFisher Scientific, catalog number: 10082147)
Keratinocyte serum-free medium (KSFM) with bovine pituitary extract (BPE) (ThermoFisher Scientific, catalog number: 17005042)
Collagen I, bovine (ThermoFisher Scientific, catalog number: A1064401)
Insulin-transferrin-selenium (ITS-G) (ThermoFisher Scientific, catalog number: 41400045)
4% paraformaldehyde solution (ThermoFisher Scientific, catalog number: J19943.K2)
Triton X-100 (ThermoFisher Scientific, catalog number: A16046.0F)
Fluoromount-GTM mounting medium (ThermoFisher Scientific, catalog number: 00-4958-02)
TRIzolTM reagent (ThermoFisher Scientific, catalog number: 15596026)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A9418)
DAPI (Sigma-Aldrich, catalog number: D9542)
Hydrocortisone (Sigma-Aldrich, catalog number: H0888)
Cholera toxin (Sigma-Aldrich, catalog number: C8052)
3,3′,5-Triiodo-L-thyronine sodium salt (Sigma-Aldrich, catalog number: T6397)
Recombinant human EGF protein (R&D Systems, catalog number: 236-EG)
Recombinant human BMP-4 (BioLegend, catalog number: 795606)
Recombinant human KGF (BioLegend, catalog number: 711702)
Recombinant human IL-13 (BioLegend, catalog number: 571106)
Recombinant human BMP-4 (BioLegend, catalog number: 795606)
Y-27632 dihydrochloride (Tocris, catalog number: 1254)
A83-01 (Tocris, catalog number: 2939)
DMH-1 (Tocris, catalog number: 4126)
Collagenase IV, powder (ThermoFisher Scientific, catalog number: 17104019)
Direct-zolTM RNA purification miniprep kit (Zymo Research, catalog number: R2050)
ProtoScript® II First Strand cDNA synthesis kit (New England Biolabs, catalog number: E6560L)
Luna® Universal qPCR master mix (New England Biolabs, catalog number: M3003L)
Conjunctival stem cell medium (CjSCM) (see Recipes)
Control medium (see Recipes)
Goblet cell differentiation medium (see Recipes)
DMEM/F12 + pen-strep (see Recipes)
DMEM/F12 + pen-strep + FBS (see Recipes)
0.5% type IV collagenase solution (see Recipes)
Equipment
Tweezers (Dumont, catalog number: 0203-54-PO)
Surgical scissors (FST, catalog number: 15011-12)
Hemocytometer (ThermoFisher Scientific, catalog number: 02-671-51B)
Pipette sets (Eppendorf, catalog number: 2231300004)
Centrifuge (Eppendorf, model: 5810R)
Pipet-aid (Drummond Scientific, catalog number: 4-000-101)
Biosafety cabinet (Labconco, model: 3460009)
Orbital shaker (Benchmark, model: BT4001)
Water bath (Fisher Scientific, model: 210)
Cell culture incubator (VWR, model: 51014992)
NanoDropTM 2000 spectrophotometer (ThermoFisher Scientific, catalog number: ND-2000)
Microcentrifuge (ThermoFisher Scientific, catalog number: 75002492)
StepOne M real-time PCR system (ThermoFisher Scientific, catalog number: 4376357)
Confocal microscope (Leica, model: SP8)
Autoclave machine (Tuttnauer, model: EZ9)
Procedure
Isolation of conjunctival epithelial cells from the human/rabbit conjunctival tissues
The human/animal tissues used in this study were acquired from certified third-party facilities. Therefore, the procurement of eye tissues is not described in this protocol.
Sterilize all forceps and tweezers in the autoclave before tissue processing.
Conjunctival tissues from human donor biopsy:
Transfer the human corneoscleral tissues from the preservation media to a sterile Petri dish and rinse with chilled PBS three times.
Keep the tissues in chilled DMEM/F-12 with pen-strep upon dissection.
Collect the conjunctival tissues from bulbar conjunctiva at 2–4 mm away from the limbus and keep the collected tissues in DMEM/F-12 with pen-strep. See Note 1.
Conjunctival tissues from New Zealand white rabbit (Oryctolagus cuniculus) eyeballs:
Transfer the rabbit eyeballs from the preservation media to a sterile Petri dish and rinse with chilled PBS multiple times.
Clean the tissues with forceps and surgical scissors to remove the residual blood clots, eye muscles, and hairs.
Immerse the cleaned eyeballs in chilled DMEM/F-12 with pen-strep upon dissection.
Gently pull up the conjunctival tissue away from the sclera with a tweezer. Inject chilled DMEM/F-12 with pen-strep into the subconjunctival region of the bulbar conjunctiva that is 3–5 mm away from the limbus for the blunt separation of epithelial tissues (Figure 1). After the injection, let the eyeball stand for a couple minutes on an ice block or in the fridge (maintain moisture), which allows the solution to spread in the subconjunctival region. See Note 2.
Cut and collect the conjunctival tissues with surgical scissors and keep the tissues in chilled DMEM/F-12 with pen-strep. See Note 3.
Transfer the tissues to a sterile 100 mm Petri dish and add a few drops of medium to maintain the tissue moisture. Mince the dissected tissues with a surgical blade until they are fine enough to be aspirated using a T-1000 pipette.
Transfer the minced tissues to a centrifuge tube (use a 15 mL or 50 mL tube depending on the number of tissues). Resuspend the minced tissues with 0.5% type IV collagenase solution.
Incubate the solution at 37 °C with 5% CO2 under agitation at 150 rpm for 30–60 min.
Stop the digestion when all sizable conjunctival tissues are digested (some white sclera tissues might be found in the solution; keep them in the solution and continue forward).
Dilute the cell-collagenase solution with DMEM/F-12 with pen-strep (at least 1:1) to facilitate the centrifugation.
Pellet the cells by centrifuging at 400 × g for 5 min. The centrifugation in this protocol was all performed at room temperature unless overwise stated.
Resuspend the pellet with DMEM/F-12 with pen-strep and repeat the centrifugation (the pellet might be loose depending on the number of undigested tissue residues).
Resuspend the pellet with 0.25% trypsin-EDTA and incubate the solution at 37 °C for 10 min, then quench the reaction by adding DMEM/F-12 with 10% FBS and pen-strep.
Pellet the cells by centrifuging at 200 × g for 5 min.
Proceed to Section B.
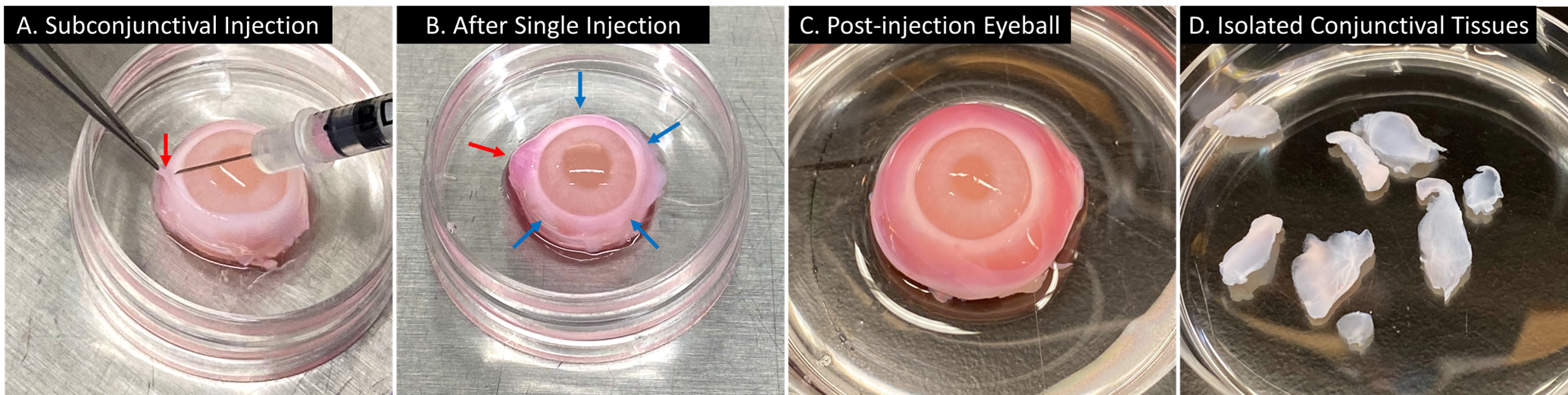
Figure 1. Isolation of conjunctival tissues from rabbit eyeballs. Representative image showing the subconjunctival injection of DMEM/F-12 (plus phenol red) with pen-strep on the rabbit eyeball for the blunt separation (from left to right). (A) The conjunctival tissue on the injection site (red arrow) was gently pulled up with a tweezer and the injection was performed using a 30-gauge syringe needle. (B) After one injection, a bulge formed on the injection site (red arrow); the injection would be repeated on multiple sites (blue arrows indicating the potential injection sites). (C) An eyeball received complete injection and the injected solution spread in the subconjunctival region. (D) Isolated conjunctival tissues.Primary culture
Collagen coating: incubate the plate/dish with a 5 µg/cm2 collagen I solution at room temperature for 1 h (the collagen-coated plate should be prepared freshly).
Resuspend the pellet with 5 mL pre-warmed culture media (CjSCM or control media).
Filter the cell solution with 70 μm cell strainers and measure the cell concentration with a hemocytometer (the straining can be skipped if the experiment does not require a precise seeding density; this will retain the residual microtissues in culture and allow them to grow as explants and increase the overall cell yield).
Seed the cells at a density of 1 × 104 –2 × 104 cells/cm2 on a collagen-coated surface.
Mark the cells as Passage 0 (P0).
Perform the primary culture in the incubator at 37 °C with 5% CO2 for three days (avoid unnecessary movement).
Change the culture media after three days and every other day from then on (pipette carefully and try to avoid disrupting the epithelial cell layer). See Note 4.
Proceed to the Section C.
Note: During the primary culture, CjSCs grow in homogeneous colonies. CjSCM can promote the formation of stem cell colonies and facilitate the outgrowth of CjSCs. The CjSC population can outgrow and dominate the total population during the primary culture phase.
In vitro expansion of CjSCs
Passage the P0 cells at 80%–90% confluence.
Aspirate the culture supernatant and rinse three times with PBS.
Add 0.25% trypsin-EDTA and incubate at 37 °C with 5% CO2 for 5 min.
Stop the digestion by adding an equal amount of culture media with 10% FBS.
Pellet the cells by centrifuging at 200 × g for 5 min.
Resuspend the pellet with pre-warmed culture media.
Filter the cell solution with 70 μm cell strainers and measure the cell concentration.
Seed the cells at a density of 2 × 104 cells/cm2 on a collagen-coated surface.
Mark the cells as P1.
Perform medium change every other day and passage the cells at 80%–90% confluence.
Mark the cells as P(n+1) after every subculture (n represents the number of passage times before the subculture).
Note: The cells expanded with CjSCM are uniform in size and show compacted, cuboidal, and homogeneous morphology. In contrast, the cells cultured with the control medium that contains no dSMADi or ROCKi display an elongated spindle shape (Figure 2).
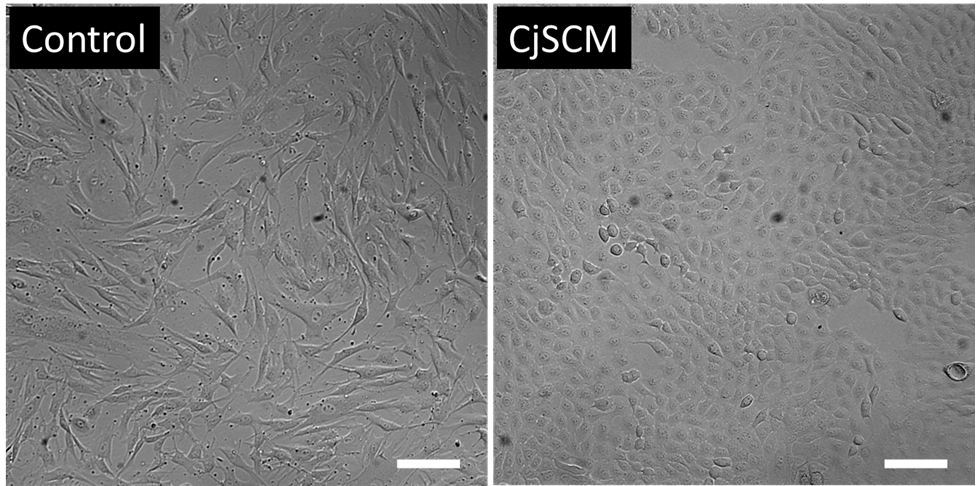
Figure 2. CjSCM-expanded cells displayed uniform cell morphology. Representative bright field images of primary human conjunctival epithelial cells cultured with control medium (left) or CjSCM (right) at P3. Scale bar = 100 μm.Basic characterization of CjSCs
Immunofluorescence staining and real-time quantitative PCR (qPCR) are performed to evaluate the expanded CjSCs in stemness, lineage, and proliferation by measuring corresponding markers on the transcriptional and post-transcriptional levels.
Immunofluorescence staining
Passage the cells at a density of 1 × 104 –2 × 104 cells/cm2 on a collagen-coated slide chamber and culture for 12–24 h (start the staining at 60%–80% confluence). See Note 5.
Wash once with PBS and fix the cells with 100 μL of 4% (w/v) paraformaldehyde for 20 min at room temperature, avoiding light.
Wash the samples three times with 100 μL of PBS (10 min incubation each time).
Perform permeabilization and blocking by incubating the samples with 100 μL of 5% (w/v) BSA solution containing 0.3% Triton X-100 for 1 h at room temperature.
Incubate the samples with the primary antibodies diluted in 100 μL of 5% (w/v) BSA solution at 4 °C overnight (antibodies are listed inTable 1). In this protocol, we adopted an epithelial stem cell marker (∆NP63), an ocular lineage marker (PAX6), and a proliferation marker (KI67) to evaluate the stem cell properties of the CjSCs.
Wash the samples three times with 100 μL of PBS (10 min incubation each time).
Incubate the samples with the secondary antibodies (Table 1) diluted in 100 μL of 5% (w/v) BSA solution for 1 h at room temperature, avoiding light.
Wash the samples three times with 100 μL of PBS (10 min incubation each time), avoiding light.
Incubate the samples with 100 μL of 1 mg/mL DAPI diluted in PBS for 10 min at room temperature, avoiding light. Make sure to wear personal protective equipment when working with DAPI. See Note 6.
Remove the DAPI solution and rinse the sample with PBS.
Aspirate all the solutions and disassemble the slide chamber.
Air-dry the slides for 30–60 s.
Mount the samples by adding Fluoromount-GTM mounting medium and seal the samples with coverslips.
Proceed to imaging with the fluorescent microscope or confocal microscope.
Table 1. Antibody list for immunofluorescence staining
Primary Antibody Catalog Vendor Dilution Secondary Antibody Catalog Vendor Dilution Purified anti-p63 (∆N) Antibody 699501 BioLegend 1:500 Anti-rat IgG Alexa Fluor ® 647 Conjugate 4418S Cell Signaling Technologies 1:500 Purified anti-Pax-6 Antibody 901301 BioLegend 1:100 Anti-rabbit IgG Alexa Fluor ® 555 Conjugate 4413S Cell Signaling Technologies 1:500 Purified Mouse Anti-Ki-67 550609 BD Pharmagin 1:500 Anti-mouse IgG Alexa Fluor ® 488 Conjugate 4408S Cell Signaling Technologies 1:500 MUC5AC Monoclonal Antibody (45M1) 12178 ThermoFisher Scientific 1:100 Anti-mouse IgG Alexa Fluor ® 488 Conjugate 4408S Cell Signaling Technologies 1:500 Anti-MUC1 antibody [HMFG1 (aka 1.10.F3)] AB70475 Abcam 1:100 Anti-mouse IgG Alexa Fluor ® 488 Conjugate 4408S Cell Signaling Technologies 1:500 Anti-MUC16 antibody [X75] AB1107 Abcam 1:100 Anti-mouse IgG Alexa Fluor ® 488 Conjugate 4408S Cell Signaling Technologies 1:500
RNA extraction and real-time qPCR
Digest the cells with 0.25% trypsin-EDTA, neutralize the digestion with culture media, and pellet the cells by centrifuging at 200 × g for 5 min.
Add chilled TRIzol® reagent and lyse the cells by repeated pipetting. To ensure complete lysis, use at least 300 µL of TRIzol® per million cells. Immediately subject the lysed samples to RNA extraction or store at -80 °C.
Extract the RNA using the Direct-zolTM RNA purification kit, following the manufacturer’s instructions. Measure the RNA concentration with NanoDropTM .
Perform reverse transcription with ProtoScript® First Strand cDNA synthesis kit, following the manufacturer’s instructions, on the StepOneTM real-time PCR system.
Perform qPCR using the Luna® Universal qPCR master mix according to the manufacturer’s instructions (primers are listed inTable 2) on the StepOneTM real-time PCR system. The PCR program was composed of a 60 s initial denaturation at 95 °C and 40 thermal cycles with a 15 s denaturation at 95 °C and a 60 s extension (signal capturing) at 60 °C. For quantitative analysis, GAPDH was used as an internal control.
Table 2. Primer list for qPCR
Human Gene 5'→3' KI67 Forward CTTTGGGTGCGACTTGACG Reverse GTCGACCCCGCTCCTTTT PAX6 Forward GTATTCTTGCTTCAGGTAGAT Reverse GAGGCTCAAATGCGACTTCAGCT P63 Forward CAGGAAGACAGAGTGTGCTGGT Reverse AATTGGACGGCGGTTCATCCCT VIM Forward GGACCAGCTAACCAACGACA Reverse TCCTCCTGCAATTTCTCCCG GADPH Forward CGACCACTTTGTCAAGCTCA Reverse AGGGGTCTACATGGCAACTG The immunofluorescence staining confirmed the expression of CjSC markers, highlighting the stemness, lineage, and proliferative activity of the cells cultured with CjSCM (Figure 3A). Real-time qPCR showed that the mRNA expression of epithelial stem cell marker and proliferation marker was upregulated in the cells cultured with CjSCM, while the expression of the mesenchymal marker was significantly downregulated (Figure 3B).
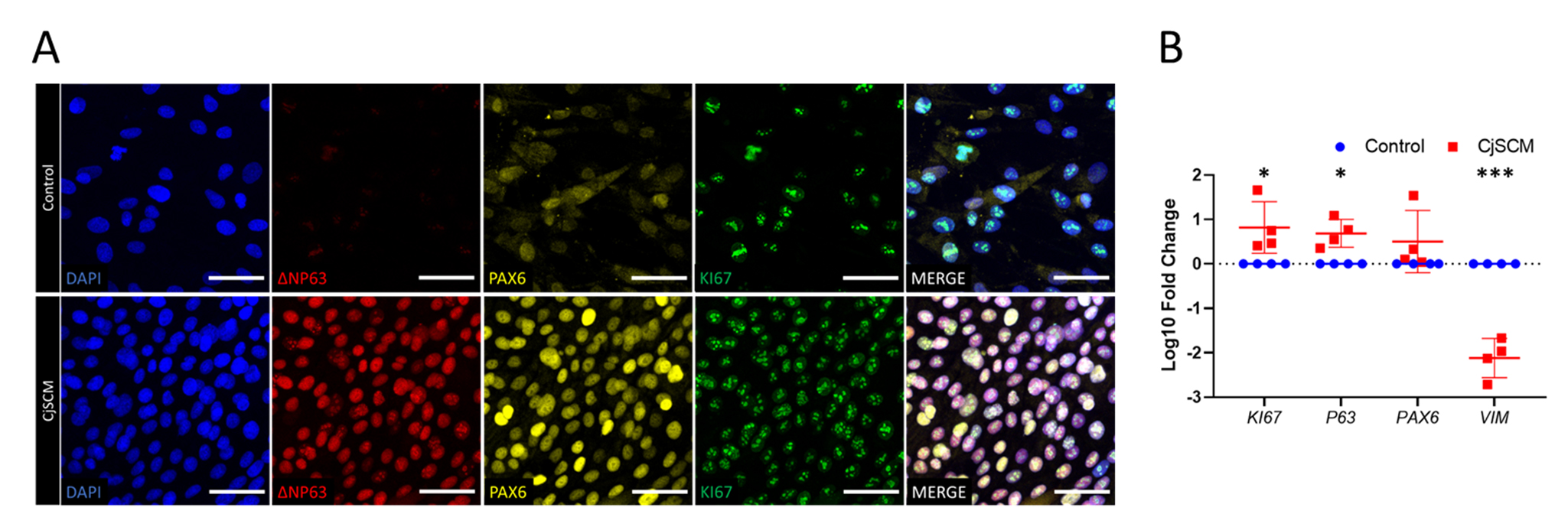
Figure 3. Immunofluorescence and mRNA profiling of CjSCM-expanded cells. (A) Representative immunofluorescence images of ∆NP63, PAX6, and KI67 in the cells expanded in CjSCM or control medium at passage 3. Scale bar = 50 μm. (B) Real-time qPCR showing the relative mRNA expression of KI67, P63, PAX6 , and VIM in the cells expanded in CjSCM or control medium (mean ± SD, n = 4, *: P < 0.05, ***: P < 0.001).Cell doubling quantification
Cell doubling quantification is an optional experiment to measure the cell doubling time and replicative potential. We performed this experiment to validate the efficacy of the CjSCM in expanding CjSCs. The experiment was performed with fresh P0 cells that had not been subjected to any culture.
Resuspend the pre-cultured P0 cells with pre-warmed media and seed the cells on a collagen-coated 12-well plate with 2 × 104 –4 × 104 cells per well (the number should be fixed among different groups for comparison).
Perform medium change every other day.
Passage the cells at 90% confluence.
Aspirate the culture supernatant and rinse three times with PBS.
Add 0.25% trypsin-EDTA and incubate at 37 °C with 5% CO2 for 5 min.
Stop the digestion by adding an equal amount of culture medium with FBS.
Pellet the cells by centrifuging at 200 × g for 5 min.
Resuspend the pellet with a pre-warmed culture medium.
Filter the cell solution with 70 μm cell strainers and measure the cell concentration.
Keep the cell count as a record for later calculation.
Seed the cells in a density of 1 × 105 cells per well on a collagen-coated 6-well plate.
Repeat the culture until desired passage number is met.
Draw the cumulative cell expansion curve by plotting cell doubling with time. Calculate the cell doubling time using the formula: DT=∆T∙ln 2/ln(Q2/Q1)(DT : doubling time; ∆T : culture time; Q1, Q2 : the cell counts of two passages).
Quantification of cell doubling in long-term culture showed that the cells cultured with CjSCM exhibited faster dividing and significantly shorter doubling time compared to those cultured with the control medium (Figure 4).
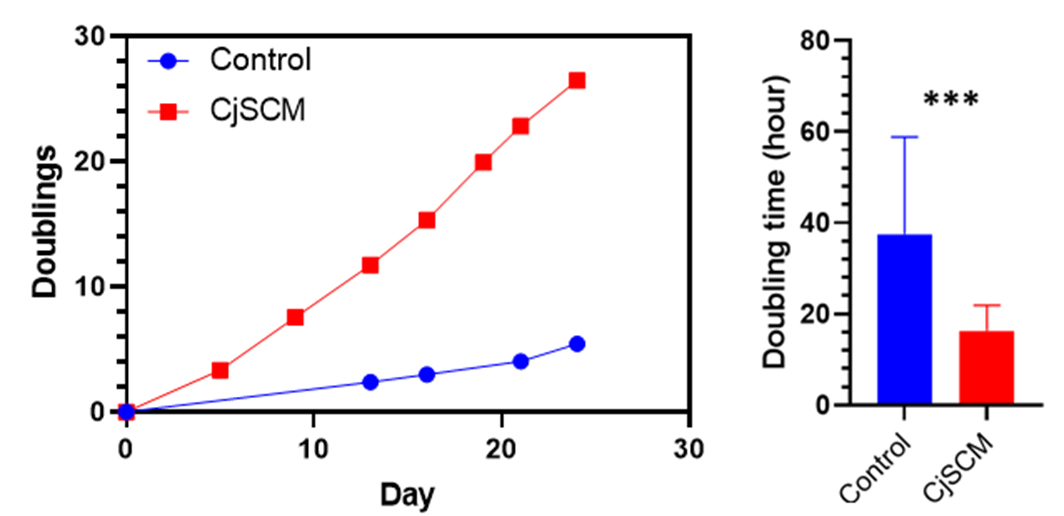
Figure 4. Cell doubling quantification. Representative cumulative curve of cell doublings and the average cell doubling time (P1–8) of the human primary conjunctival epithelial cells in culture with CjSCM or control medium (mean ± SD, n = 3; ***: P < 0.001).
Potency test: goblet cell differentiation
The potency is an optional experiment to validate the goblet cell differentiation potency of the expanded CjSCs. The test should be performed with cells after P1 to ensure homogeneity.
Seed the cells in a density of 2 × 104 cells/cm2 on a collagen-coated surface and culture the cells with CjSCM (the efficiency will be higher if the differentiation is performed on a 3D hydrogel matrix or collagen-coated Transwell membrane).
Initiate the goblet cell differentiation when the cell confluence reaches 90%–100% by switching the culture medium to the goblet cell differentiation medium.
Perform medium change every other day for 5–10 days (pipette carefully and try to avoid disrupting the epithelial cell layer).
Examine the goblet cell differentiation efficiency by immunofluorescence staining of the characteristic mucins expressed in the conjunctival goblet cells. See Note 7.
Note: The goblet-like cells with granules will start to appear after 3–5 days of differentiation; the number of these cells will increase over time. However, because the survival of goblet cells requires the support of surrounding cells, the number of viable goblet cells might drop in an extended differentiation after 10 days. In our test, we detected the expression of mucin proteins (MUC1, MUC5AC, and MUC16) in the cells after a seven-day differentiation, suggesting that the CjSCs expanded with CjSCM retained their differentiation potency (Figure 5).
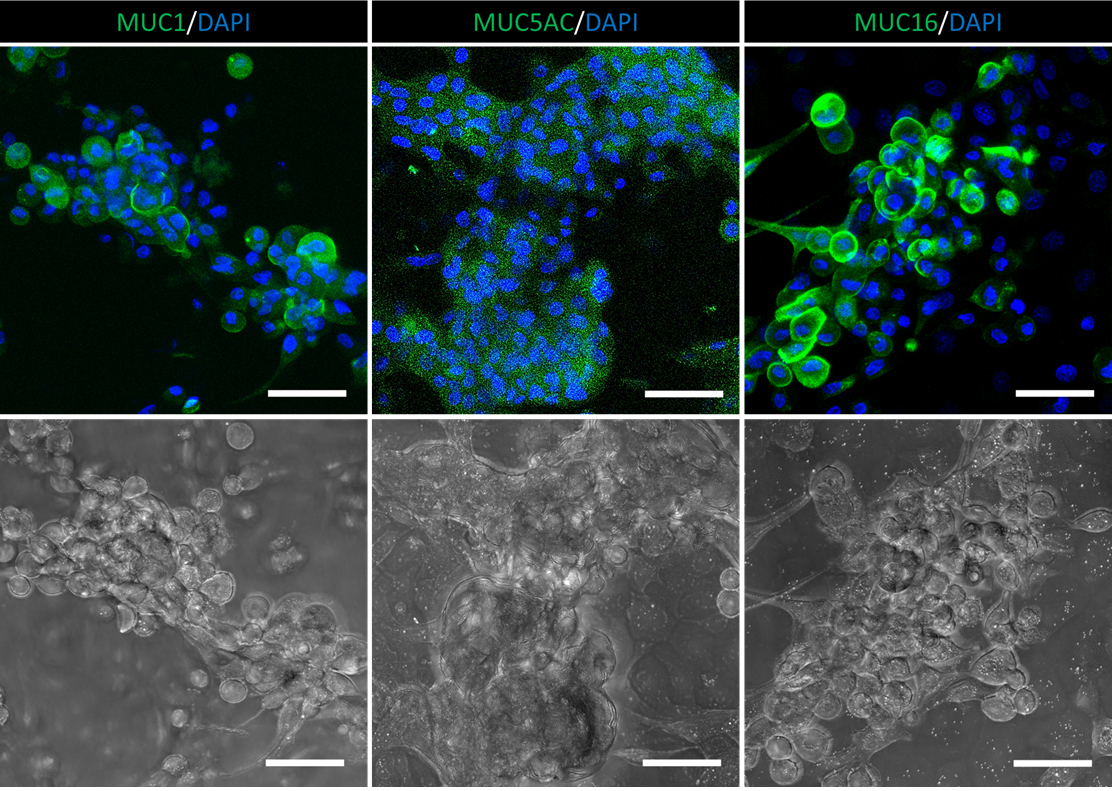
Figure 5. Goblet cell differentiation. Representative immunofluorescence staining of conjunctival goblet cell markers, MUC1, MUC5AC, and MUC16, and the corresponding bright field images of the post-differentiation cells. Scale bar = 50 μm.
Notes
For the purpose of growing human CjSCs, the starting materials can be whole human eyeball, ocular surface, or other type of corneoscleral tissues, as long as sufficient viable conjunctival tissues are present. Based on our experience, a minimal amount of 3–5 mm2 conjunctival tissues would be enough to establish the line but more tissue input would further ensure the success of the procedure. To ensure the viability of the isolated cells, the tissue should be processed within 72 h of the primary dissection (isolation from the donor). Using tissues with insufficient quantity or quality could significantly compromise the experiment results. Furthermore, this procedure was designed for isolating cells from normal/healthy donors. Modification of the procedure would be necessary for specific needs.
The conjunctiva and limbus are adjacent anatomical structures on the ocular surface. Limbus is formed by the ring-shaped junction of corneal epithelium and conjunctival epithelium, which also separates the transparent cornea and opaque sclera (VanBuskirk, 1989). The conjunctiva is a thin mucous membrane that covers the outer surface of the sclera and the inner surface of the eyelids (Shumway et al., 2018). For the blunt dissection of the bulbar conjunctiva, the subconjunctival injection sites should be 3–5 mm away from the edge of the cornea, where the limbus is located. Our protocol mainly applies to bulbar conjunctiva, but may be modified accordingly for isolating other conjunctival regions (fornix, palpebral). The use of a dissecting microscope is recommended for the process. This step requires training in tissue processing and basic surgical skills; please get professional help if needed.
The elastic nature of conjunctiva facilitates the blunt dissection. The subconjunctival injection will form bulges that separate a thin layer of the conjunctival epithelial tissues. The injection should be conducted slowly, and the syringe needle should be moved gently in the subconjunctival region to ensure a clear separation. Cut down the bulges from the bottom and ensure the collected tissues are outside of the limbus. The use of a dissecting microscope is recommended for the process. The size and shape of the collected tissues will depend on the blunt dissection and should not affect the overall yield. We tested the starting materials with only 200 viable P0 rabbit conjunctival cells and generated over a million cells in 10 days (unpublished data). In our experience, the conjunctival tissues collected from one rabbit eyeball could yield millions of cells.
In most cases, attached cells started to grow into small colonies 3–5 days after the primary seeding. If residual microtissues were seeded, cells would also grow out from the tissue at the same time. The seeded cells consist of a mixed population derived from the conjunctival tissue, and CjSCM can promote the formation of compact stem cell colonies, which could rapidly dominate the total population during the primary culture. Based on our data, the ratio of KRT14-positive cells (mitotically active epithelial stem cells) was less than 2% in the cells freshly isolated from the rabbit conjunctival tissues and increased to more than 90% in the primary culture with CjSCM (unpublished data). However, the CjSC expansion efficiency can be affected by donor tissue status and isolation practice.
CjSCs can grow into a compact cell sheet when they reach full confluence, which is ideal for exhibiting cell morphology. To better show cell morphology, the staining should be started with a confluence of 60%–80%. As the proliferation rate varies in cells from different donors or passages, the seeding density should be adjusted based on the cell status. Based on our experience, seeding around 10 thousand viable CjSCs per well in the Millicell EZ SLIDE 8-well glass chamber slide would result in 60%–80% confluence in less than 24 h.
Direct contact with DAPI may cause eye irritation and skin irritation. DAPI is also harmful by inhalation or ingestion. Rinse immediately with water for several minutes if direct contact occurs during the practice. Move to fresh air in case of inhalation. Professional medical help can be necessary if any symptoms persist.
The immunofluorescence staining of mucin follows the same procedures as described in section D, except for the permeabilization and blocking. For the staining of mucins, the fixed samples were permeabilized with PBS containing 0.2% Triton X-100 for 10 min, followed by a 1 h blocking with 5% (w/v) BSA.
Recipes
Conjunctival stem cell medium (CjSCM)
Reagent Final concentration Amount DMEM N/A 440 mL DMEM/F-12 (1:1) N/A 440 mL FBS 10% (v/v) 100 mL Pen-strep 1% (v/v) 10 mL ITS-G 1% (v/v) 10 mL Hydrocortisone 400 ng/mL N/A Cholera toxin 0.1 nM N/A Recombinant EGF 10 ng/mL N/A 3,3′,5′-Triiodo-L-thyronine 2 nM N/A Y27632 10 μM N/A A83-01 1 μM N/A DMH1 1 μM N/A Total N/A 1,000 mL Control medium
Reagent Final concentration Amount DMEM N/A 220 mL DMEM/ F-12 (1:1) N/A 220 mL FBS 10% (v/v) 50 mL Pen-strep 1% (v/v) 5 mL ITS-G 1% (v/v) 5 mL Hydrocortisone 400 ng/mL N/A Cholera toxin 0.1 nM N/A Recombinant EGF 10 ng/mL N/A 3,3′,5′-Triiodo-L-thyronine 2 nM N/A Total N/A 500 mL Goblet cell differentiation medium
Reagent Final concentration Amount KSFM N/A 490 mL BPE N/A 25 mg Pen-strep 1% (v/v) 5 mL Recombinant IL-13 100 ng/mL N/A Recombinant BMP4 10 ng/mL N/A Recombinant KGF 10 ng/mL N/A Recombinant EGF 10 ng/mL N/A Total N/A 500 mL DMEM/F12 + pen-strep
Reagent Final concentration Amount DMEM/ F-12 (1:1) N/A 495 mL Pen-strep 1% (v/v) 5 mL Total n/a 500 mL DMEM/F12 + pen-strep + FBS
Reagent Final concentration Amount DMEM/ F-12 (1:1) N/A 445 mL FBS 10% (v/v) 50 mL Pen-strep 1% (v/v) 5 mL Total n/a 500 mL 0.5% type IV collagenase solution
Reagent Final concentration Amount Type IV collagenase powder 0.5% 50 mg DMEM/ F-12 (1:1) N/A 9.9 mL Pen-strep 1% (v/v) 0.1 mL Total n/a 10 mL
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (R21 EY031122, R01 EB021857) and National Science Foundation (1937653, 2135720). We also acknowledge the University of California San Diego School of Medicine Microscopy Core, and they were supported by National Institutes of Health grant P30 NS047101.
This protocol was derived from “Rapid bioprinting of conjunctival stem cell micro-constructs for subconjunctival ocular injection” (Zhong et al., 2021b) and “Rapid 3D bioprinting of a multicellular model recapitulating pterygium microenvironment” (Zhong et al., 2022).
Competing interests
The authors declare no competing interests.
Ethics
The rabbit eyeballs were acquired from Sierra for Medical Science, Inc. (Whittier, CA) with the consent for biomedical research. The human corneoscleral tissue was provided by One Legacy or Saving Sight Eye Bank with consent for research use, and the corneoscleral tissue handling procedure was approved by the University of California, Los Angeles (UCLA) Institutional Review Boards (IRB#12-000363). The experimental work adhered to the tenets of the Declaration of Helsinki and the overall laboratory experimental procedure has been approved by the University of California, San Diego Institutional Biosafety Committee.
References
- Amano, M., Nakayama, M. and Kaibuchi, K. (2010). Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 67(9): 545-554.
- Barabino, S., Rolando, M., Bentivoglio, G., Mingari, C., Zanardi, S., Bellomo, R. and Calabria, G. (2003). Role of amniotic membrane transplantation for conjunctival reconstruction in ocular-cicatricial pemphigoid. Ophthalmology 110(3): 474-480.
- Barabino, S., Chen, Y., Chauhan, S. and Dana, R. (2012). Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease. Prog Retin Eye Res 31(3): 271-285.
- Shumway, C. L., Motlagh, M. and Wade, M. (2018). Anatomy, Head and Neck, Eye Conjunctiva. StatPearls.
- Gipson, I. K. (2016). Goblet cells of the conjunctiva: A review of recent findings. Prog Retin Eye Res 54: 49-63.
- Kobielak, K., Stokes, N., De La Cruz, J., Polak, L. and Fuchs, E. (2007). Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc Natl Acad Sci U S A 104(24): 10063-10068.
- Kohanim, S., Palioura, S., Saeed, H. N., Akpek, E. K., Amescua, G., Basu, S., Blomquist, P. H., Bouchard, C. S., Dart, J. K., Gai, X., et al. (2016). Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis--A Comprehensive Review and Guide to Therapy. I. Systemic Disease. Ocul Surf 14(1): 2-19.
- Liu, J., Sheha, H., Fu, Y., Liang, L. and Tseng, S. C. (2010). Update on amniotic membrane transplantation. Expert Rev Ophthalmol 5(5): 645-661.
- Majo, F., Rochat, A., Nicolas, M., Jaoude, G. A. and Barrandon, Y. (2008). Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456(7219): 250-254.
- McCauley, H. A. and Guasch, G. (2015). Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med 21(8): 492-503.
- Van Buskirk, E. M. (1989). The anatomy of the limbus. Eye (Lond) 3 (Pt 2): 101-8.
- Mou, H., Vinarsky, V., Tata, P. R., Brazauskas, K., Choi, S. H., Crooke, A. K., Zhang, B., Solomon, G. M., Turner, B., Bihler, H., et al. (2016). Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 19(2): 217-231.
- Nakamura, T., Inatomi, T., Sotozono, C., Koizumi, N. and Kinoshita, S. (2016). Ocular surface reconstruction using stem cell and tissue engineering.Prog Retin Eye Res 51: 187-207.
- Nguyen, P., Khashabi, S. and C, S. (2011). Ocular Surface Reconstitution. In: Progress in Molecular and Environmental Bioengineering - From Analysis and Modeling to Technology Applications.
- Nomi, K., Hayashi, R., Ishikawa, Y., Kobayashi, Y., Katayama, T., Quantock, A. J. and Nishida, K. (2021). Generation of functional conjunctival epithelium, including goblet cells, from human iPSCs. Cell Rep 34(5): 108715.
- Pellegrini, G., Golisano, O., Paterna, P., Lambiase, A., Bonini, S., Rama, P. and De Luca, M. (1999). Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol 145(4): 769-782.
- Ramos, T., Scott, D. and Ahmad, S. (2015). An Update on Ocular Surface Epithelial Stem Cells: Cornea and Conjunctiva. Stem Cells Int 2015: 601731.
- Stewart, R. M., Sheridan, C. M., Hiscott, P. S., Czanner, G. and Kaye, S. B. (2015). Human Conjunctival Stem Cells are Predominantly Located in the Medial Canthal and Inferior Forniceal Areas. Invest Ophthalmol Vis Sci 56(3): 2021-2030.
- Tata, P. R., Mou, H., Pardo-Saganta, A., Zhao, R., Prabhu, M., Law, B. M., Vinarsky, V., Cho, J. L., Breton, S., Sahay, A., et al. (2013). Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature 503(7475): 218-223.
- Tseng, S. C., He, H., Zhang, S. and Chen, S. Y. (2016). Niche Regulation of Limbal Epithelial Stem Cells: Relationship between Inflammation and Regeneration. Ocul Surf 14(2): 100-112.
- Williams, R., Lace, R., Kennedy, S., Doherty, K. and Levis, H. (2018). Biomaterials for Regenerative Medicine Approaches for the Anterior Segment of the Eye. Adv Healthc Mater 7(10): e1701328.
- Wu, N., Yan, C., Chen, J., Yao, Q., Lu, Y., Yu, F., Sun, H. and Fu, Y. (2020). Conjunctival reconstruction via enrichment of human conjunctival epithelial stem cells by p75 through the NGF-p75-SALL2 signaling axis. Stem Cells Transl Med 9(11): 1448-1461.
- Zhang, C., Lee, H. J., Shrivastava, A., Wang, R., McQuiston, T. J., Challberg, S. S., Pollok, B. A. and Wang, T. (2018). Long-Term In Vitro Expansion of Epithelial Stem Cells Enabled by Pharmacological Inhibition of PAK1-ROCK-Myosin II and TGF-beta Signaling. Cell Rep 25(3): 598-610 e595.
- Zhong, Z., Balayan, A., Tian, J., Xiang, Y., Hwang, H. H., Wu, X., Deng, X., Schimelman, J., Sun, Y., Ma, C., et al. (2021a). Bioprinting of dual ECM scaffolds encapsulating limbal stem/progenitor cells in active and quiescent statuses. Biofabrication 13(4). doi: 10.1088/1758-5090/ac1992.
- Zhong, Z., Deng, X., Wang, P., Yu, C., Kiratitanaporn, W., Wu, X., Schimelman, J., Tang, M., Balayan, A., Yao, E., et al. (2021b). Rapid bioprinting of conjunctival stem cell micro-constructs for subconjunctival ocular injection. Biomaterials 267: 120462.
- Zhong, Z., Wang, J., Tian, J., Deng, X., Balayan, A., Sun, Y., Xiang, Y., Guan, J., Schimelman, J., Hwang, H., You, S., Wu, X., Ma, C., Shi, X., Yao, E., Deng, S. X. and Chen, S. (2022). Rapid 3D bioprinting of a multicellular model recapitulating pterygium microenvironment. Biomaterials 282: 121391.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Zhong, Z. and Chen, S. (2022). Isolation and Expansion of Primary Conjunctival Stem Cells (CjSCs) from Human and Rabbit Tissues. Bio-protocol 12(24): e4569. DOI: 10.21769/BioProtoc.4569.
Category
Cell Biology > Cell isolation and culture
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









