- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
Human Auto-IgG Purification from High Volume Serum Sample by Protein G Affinity Purification
(*contributed equally to this work) Published: Vol 12, Iss 23, Dec 5, 2022 DOI: 10.21769/BioProtoc.4562 Views: 2833
Reviewed by: Anonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

ZnCl2 Precipitation-Assisted Sample Preparation for Proteomic Analysis
Qiqing He [...] Fuchu He
Jul 20, 2025 2726 Views

Protocol for the Preparation of a Recombinant Treacle Fragment for Liquid–Liquid Phase Separation (LLPS) Assays
Nadezhda V. Petrova [...] Artem K. Velichko
Sep 20, 2025 1814 Views

Optimized Secretome Sample Preparation From High Volume Cell Culture Media for LC–MS/MS Proteomic Analysis
Basil Baby Mattamana [...] Peter Allen Faull
Dec 20, 2025 1229 Views
Abstract
Immunoglobulins are proteins produced by the immune system, which bind specifically to the antigen that induced their formation and target it for destruction. Highly purified human immunoglobulins are commonly used in research laboratories for several applications, such as in vitro to obtain hybridomas and in vivo animal immunisation. Several affinity purification methods are used to purify immunoglobulins from human serum, such as protein A/G Sepharose beads, polyethylene glycol, and caprylic acid ammonium sulphate precipitation. Here, we provide a detailed protocol for purification of high-quality IgG from human serum, using affinity chromatography with protein G. The protocol is divided into four main steps (column preparation, serum running, wash, and elution) for IgG purification, and two extra steps (protein dialysis and sucrose concentration) that should be performed when buffer exchange and protein concentration are required. Several IgG affinity purification methods using protein A or G are available in the literature, but protein A has a higher affinity for rabbit, pig, dog, and cat IgG, while protein G has a higher affinity for mouse and human IgG. This affinity-based purification protocol uses protein G for a highly specific purification of human IgG for animal immunization, and it is particularly useful to purify large amounts of human IgG.
Graphical abstract
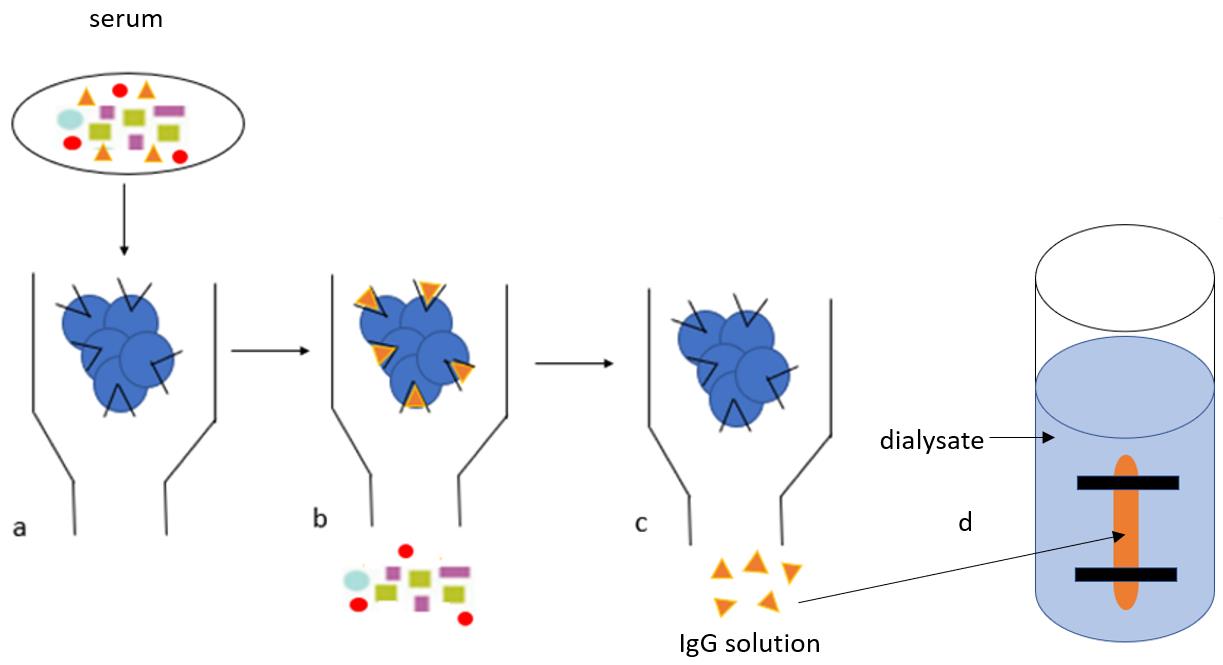
IgG purification protocol. The IgG purification protocol consists of four main steps (column preparation, serum running, wash, and elution) and two extra steps (protein dialysis and concentration). a. Diluted serum is added to the protein G beads and IgG binds to the Fc receptors on protein G beads. b. Beads are washed in Hartman’s solution to fully remove the complex protein mixture (multicolour shapes, as depicted in the graphical abstract). c. IgG (orange triangles, as depicted in the graphical abstract) are removed from protein G with glycine and collected in Tris buffer. d. The IgG is transferred into a semi-permeable membrane (‘snake skin’) and allowed to dialyse overnight for buffer exchange with a physiological solution (Hartmann’s).
Background
Immunoglobulins (Ig) or antibodies (Ab) are glycoproteins found in serum and tissue fluids, which are produced in large amounts after contact with immunogenic foreign molecules. They are Y-shaped and consist of five classes or isotypes: IgG, IgM, IgA, IgE, and IgD. Immunoglobulin G (IgG) is a type of antibody normally found in blood and extracellular fluid that is predominant in adaptive immune responses. The IgG isotype is 75% of normal serum immunoglobulins (14 mg/mL) and is divided into four sub-classes, called IgG1, IgG2, IgG3, and IgG4, in the following proportion: 70%, 20%, 8%, and 2%, respectively. All antibodies have the same basic structure divided into variable and constant regions; the variable region is antigen specific and contains two fragment antigen binding (Fab) sites, while the constant region, also known as fragment crystallizable (Fc), is isotype specific and stimulates antigen destruction. Human-derived IgGs are a subgroup of immunoglobulins that are commonly used for a variety of research methods or as effective therapeutics in inflammation, cancer, as well as autoimmune and infectious diseases. For in vitro methods, antibodies can be used directly in their crude form (serum) or in a pure form (IgG); however, purified antibodies are more advantageous than the whole serum because they reduce cross-reactivity, can be easily stored, and are stable for longer periods. Several autoimmune syndromes have been successfully "transferred" in animals immunized with highly purified human autoantibodies (Cuhadar et al., 2019; Goebel et al., 2021; Helyes et al., 2019; Pohoczky et al., 2022). Several protocols for antibody purification are available in the literature, and these are divided in two main groups: non chromatographic techniques, such as polyethylene glycol, and caprylic acid ammonium sulphate precipitation, and chromatographic techniques, such as affinity chromatography with protein A/G beads. Non-chromatographic techniques depend on the combination of several factors, and are therefore complex, laborious, and expensive, especially due to the usage of polyethylene glycol. Affinity chromatography with commercially available protein-A or protein-G beads is the standard methodology for purification of antibodies from serum, for the relative simplicity and reliability of results. While protein A has a higher affinity for rabbit, pig, dog, and cat IgG1, IgG2, and IgG4, protein G has a higher affinity for mouse and human IgG1, IgG2, IgG3, and IgG4. Furthermore, protein G beads have a wider binding range than protein A beads because they can bind to intact IgG as well as antibody fragments F(ab) 2 and Fc regions, while protein A binds strongly to the Fc part and weakly to the Fab region. Here, we provide a detailed and easy to follow protocol for purification of high-quality IgG from human serum, using affinity chromatography with protein G. Differently from previously published methods, this protocol is specifically designed for purification of large amounts of human serum (20 mL), whereby serum is diluted before purification, and Hartmann’s solution is used as a binding buffer instead of PBS, to keep neutral pH and physiological ionic strength(Cuhadar et al., 2019; Goebel et al., 2021; Helyes et al., 2019; Pohoczky et al., 2022). These steps significantly improved the human IgG purification. Furthermore, this protocol contains useful comments regarding factors affecting IgG binding and stability. This protocol could also be used for purification of antibodies from hybridoma culture supernatant or ascites.
Materials and Reagents
Nunc 96-Well U Bottom (Thermo Fisher, catalog number: 268200)
Syringes PlastipakTM 20 mL Luer-LokTM (BD, catalog number: 302237)
0.2 µm filters (Sigma-Aldrich, Millipore, catalog number: SLGP033RS)
50 mL centrifuge tubes (Sigma-Aldrich, Greiner, catalog number: T2318-500EA)
1.5 mL Eppendorf Safe-Lock Tubes (Eppendorfs, catalog number: 0030120086)
SnakeSkinTM Dialysis Tubing, 10K MWCO, 22 mm (Thermo Fisher, catalog number: 68100)
SnakeSkinTM Dialysis Clips (Thermo Fisher, catalog number: 68011)
Protein G Sepharose 4 Fast Flow, 5 mL (Sigma-Aldrich, catalog number: GE17-0618-01) storage at 4 °C
Econo-pac chromatography columns, package of 50 (Bio-Rad, catalog number: 7321010)
Two-way Stopcocks (Bio-Rad, catalog number: 7328102)
Compound Sodium Lactate Solution for Infusion BP (Hartmann's Solution for infusion) in Viaflo, 1,000 mL (Baxter, catalog number: FKE2324)
Bradford Reagent (Sigma-Aldrich, catalog number: B6916)
Ethanol, Absolute, Molecular Biology Grade, 500 mL (ThermoFisher, catalog number: 16606002)
Sucrose, Molecular Biology Grade (AlfaAesar by Thermo Fisher Scientific, catalog number: J65148.A1)
Trizima Base (ThermoFisher, catalog number: BP152-500)
Glycine (Sigma-Aldrich, catalog number: 50046)
HCl (Sigma-Aldrich, catalog number: H1758)
Neutralisation Buffer: Trizma base 1 M (pH 8.0) (see Recipes)
Elution Buffer: Glycine 100 mM (pH 2.3) (see Recipes)
Storage buffer: 20% Ethanol (see Recipes)
Washing buffer: Hartmann's Solution for Infusion (Compound Sodium Lactate) in Viaflo, 1,000 mL (see Recipes)
Equipment
pH Meter Mettler ToledoTM FiveEasyTM F20 pH/mV Meter (Mettler Toledo, catalog number: 15543360)
Centrifuge 5810R (Eppendorf, catalog number: 5811000465)
PIPETMAN Classic Starter Kit, P20, P200, P1000 (Gilson, catalog number: F167300)
Laboratory stand Model SBS-LS-100 (Expondo, catalog number: EX10030473)
Borosilicate Glass Tall Form Beakers 2 L (ThermoFisher, catalog number: 15499083)
Procedure
Preparation of the protein G column
Resuspend two vials of protein G Sepharose (5 mL each) in 50 mL of Hartmann’s solution in a 50-mL tube.
Centrifuge at 41 × g for 5 min, gently pour off the Hartmann’s solution by decantation, and add fresh Hartmann’s solution to top up the 50-mL tube; repeat twice.
Wash the column with 100% ethanol, and then with Hartmann’s solution.
Add protein G solution to the column with a plastic Pasteur pipette until the column is full. The two-way stopcock should be closed (horizontal) to avoid the loss of protein G. The length of the packed protein G column should be 6–8 cm (the empty colums is 12cm)".
Open the two-way stopcock (vertical) and let all the solution slowly run down (to allow the protein G to deposit at the bottom of the column). Do not permit protein G to dry, always leave at least 1 mL of Hartmann’s solution on top of the beads (Figure 1A).
Running the sample through the protein G column
Centrifuge the serum at 2,000 × g for 15 min to get rid of particulates before dilution. A second centrifugation may be required to achieve this.
Dilute the serum 1:3 in Hartmann’s solution: 20 mL of serum and 40 mL of Hartmann’s solution, for a total of 60 mL of diluted serum.
Add the diluted serum (60 mL) to the column with a plastic Pasteur pipette. Run the serum slowly through the column, adjusting the stopcock to half closed for 1 drop/second, and setting 1 min on a timer (1 mL of IgG should pass through the column in 1 min). Firstly, top up the column with serum, then open the stopcock and start the purification. Continue topping up the column until all serum is transferred into the column.
Run the diluted serum slowly (1 mL/min) through, and collect the flow through in a 50-mL tube.
Run the flow through through the column again, then discard or store the flow through for further analysis (Figure 1B).
Washing any non-specific binding with HS
Run 60–100 mL of Hartmann’s solution through the column, to wash away any non-specific binding (Figure 1C).
Check Hartmann’s flow through for IgG, using the Bradford reagent: Resuspend 20 μL of eluted flow through in 100 μL of Bradford reagent, using a well of a 96-well plate. Continue adding Hartmann’s solution until no blue colour is detected, then start eluting with glycine (Procedure D). The blue colour means that there are still other unbound protein in the column (not IgG) that should be removed before elution.
Elution with glycine pH 2.3 and Tris pH 8.0
Place 30 Eppendorf tubes (1.5 mL) on ice. Add 100 μL of 1 M Tris, pH 8.0 into each tube (Figure 1D).
Add 30 mL of 100 mM glycine pH 2.3 to the column, and elute 900 μL of flow through into each prepared tube. Run glycine slowly through the column, adjusting the stopcock to half closed for 1 drop/second and setting 1 min on a timer (1 mL of IgG should pass through the column in 1 min). Firstly, top up the column with glycine, then open the stopcock and start elution from the first tube. When the column needs to be topped up, close the stopcock and add glycine, as above. Shake each fraction, to neutralize glycine with Tris and to protect the sample. Usually, there is no IgG in the first four tubes.
Check the presence of IgG in all tubes using the Bradford assay: Resuspend 20 μL of eluted solution from each tube with 100 μL of Bradford reagent using 30 wells of a 96-well plate. The last tube should have no blue colour.
Pool the highly concentrated fractions, as indicated by the intense blue colour, into one 50-mL tube. For 20 mL of serum (diluted in 40 mL of Harmann’s = 60 mL of overall solution), the yield should be approximately 20 tubes.
Run 60 mL of Hartmann’s Solution (1 mL/min) to remove any residual glycine from the column.
Check if there is no glycine in the flow through with the Bradford assay, as in step D3.
Store the column at 4 °C in 20% ethanol to prevent contaminations. The same column can be used up to five times.

Figure 1. IgG purification steps. A. Column preparation. Wash the column with 20 mL of 100% ethanol and then 20 mL of binding buffer (HS), and then add the protein G Sepharose slurry from the vials to the column. B. Serum running. Slowly apply diluted serum to the column twice (1 mL/min). C. column washing. Wash protein G beads in 100 mL of binding buffer. D. Elute the bound IgG fraction by adding 20 mL of 100 mM glycine pH 2.3 to the column, and collect in 20 tubes (1.5-mL Eppendorf tubes) pre-filled with Tris buffer. E. IgG dialysis. Dialyse purified IgG at 4 °C overnight in Hartmann’s solution using a 10 kDa dialysis membrane. F. IgG concentration. Move the IgG from the beaker into a tray, covered with sucrose and left until has reached the right concentration; the sucrose starts to slowly liquefy after approximately 20 min and the sample is concentrated 2x after 1 h. G. IgG aggregates. IgG aggregates (red circle) may form during overnight dialysis, but these can be removed with a spin at 657 × g for 10 min.Dialysis
Fill a beaker with 1 L of Hartmann’s solution.
Cut approximately 10 cm of snakeskin dialysis tubing, fold over one end, and secure with a clip. Then, pipette the IgG solution into it, leaving approximately an extra third volume of air.
Fold over the top and secure with a clip.
Place the dialysis bag into the beaker and leave it on a stirrer at 4°C, allowing the sample to dialyse overnight (Figure 1E).
Sucrose concentration
Move the dialysis preparation from the beaker into a tray, cover with sucrose, and leave until the sample has reached the right concentration—the exact time will depend upon the starting concentration of the dialysed sample, as it may take a couple of hours and it may need sucrose changing after 1 h (Figure 1E).
Transfer the sample into a 50-mL tube.
Centrifuge the sample at 657 × g for 10 min and transfer the IgG solution into a fresh tube to remove aggregates. Aggregates may still form later but they can be removed with another spin at 657 × g for 10 min (Figure 1G).
Filter the sample through a 0.2 μm filter.
Store the IgG sample at 4 °C.
Data analysis
IgG concentration is measured using a Nanodrop 2000 after purification, after dialysis, and after sucrose concentration.
Open the Nanodrop 2000 software and select Protein 280. Then select "IgG" as sample type.
Pipette 1.5 μL of Hartmann’s solution onto the lower measurement pedestal, then click on "Blank" to measure the blank.
Clean residual Hartmann’s solution with a tissue. Pipette 1.5 μL of IgG solution onto the lower measurement pedestal, then click on "measure" to measure the sample.
An OD280 graph is then provided, together with IgG concentration (Figure 2).
Human IgG purified from serum has a concentration from 7 mg/mL to 10 mg/mL, while human IgG purified from plasma has a concentration between 5 mg/mL and 7 mg/mL.
The OD280 graph is used to assess the specificity and quantitative yield of the purification procedure. In particular, OD260/280 ratio is used to check sample purity and possible contamination by nucleic acids (absorbance at 260). An OD260/280 less than 0.60 indicate pure protein yield. Hartmann’s solution (salt buffer) is used as IgG buffer to help minimize the presence of nucleic acids.
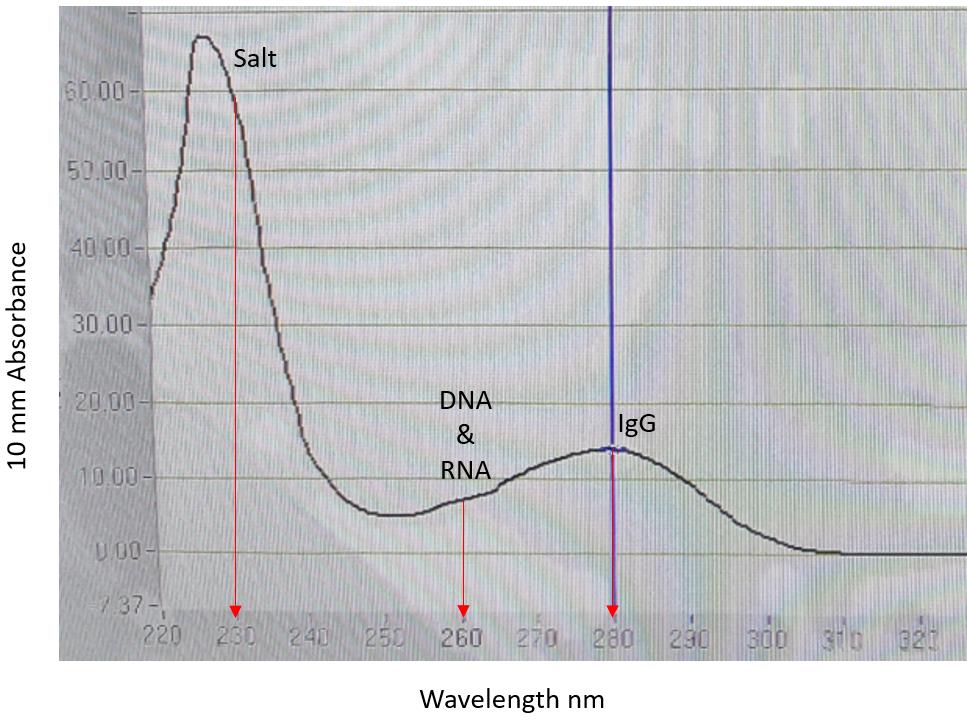
Figure 2. Purified IgG OD280 graph. Red arrows indicate salts (absorbance at 230), DNA and RNA (absorbance at 260), and IgG (absorbance at 280). The high salt peak is caused by salts in the Hartmann’s solution (IgG buffer). OD260/280 ratio is 0.51, IgG concentration is 10.10 mg/mL.
Notes
Factors affecting IgG binding and stability
Due to issues related to temperature fluctuations in the laboratory during summer (defect in the air-conditioning), serendipitously initially IgG were purified either at high room temperature (30 °C) or at standard room temperature (20 °C). Subsequently, the IgG solution purified at 30 °C was found not to be active by collaborating groups when used in in vivo experiments, suggesting that the very high room temperature may have negatively affected either IgG binding to protein G or IgG stability, as the manufacturer recommended room temperature (20 °C) as optimal.
Another factor that affects IgG binding and stability is pH. Low pH (2.5–3.0) is commonly used during protein G elution to break the ionic and hydrogen bonds between the antigen and antibody. IgGs used for this project were eluted at a lower pH 2.3, to achieve a more complete elution yield.
Other factors that may negatively affect IgG binding to protein G include protein G beads freezing (freezing may cause detachment of the protein G from the agarose beads) and column flow rate (high flow rate leading to IgG loss, as IgG would not have sufficient time to bind to Fc receptors on protein G). Finally, according to the manufacturer’s instructions, any element of drying of the protein G resin will result in a detachment of the protein G from agarose beads, with significant loss in IgG yield.
Recipes
Neutralisation Buffer: Trizma base 1 M (pH 8.0)
Dissolve 60.57 g of Trizma base in 400 mL of ddH2O placed on a beaker with a magnetic stirrer.
Adjust pH to 8.0 by adding HCl.
Adjust the volume to 500 mL using ddH2O.
Store at room temperature for up to 1 year.
Elution Buffer: Glycine 100 mM (pH 2.3)
Dissolve 3.785 g of glycine in 400 mL of ddH2O placed on a beaker with on a magnetic stirrer.
Adjust pH to 2.3 by adding HCl.
Adjust the volume to 500 mL using ddH2O.
Store at 4 °C for up to 3 months.
Storage buffer: 20% Ethanol
Mix 20 mL of 100% ethanol with 80 mL of ddH2O.
Store at 4 °C.
Washing buffer: Hartmann's Solution for Infusion (Compound Sodium Lactate) in Viaflo, 1,000 mL
Acknowledgments
This work was supported by grants from the Pain Relief Foundation, Liverpool, UK.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics
Plasma samples used for IgG purification were obtained after plasma exchange therapy (PTE) and no ethics approval was required for the use of waste plasma (15/NW/0467, North West – Haydock Research Ethics Committee).
Sera samples used for IgG purification were obatined under ethical permission and individual consent for autoantibody research (12/EE/0164, East of England).
References
- Cuhadar, U., Gentry, C., Vastani, N., Sensi, S., Bevan, S., Goebel, A. and Andersson, D. A. (2019). Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain 160(12): 2855-2865.
- Goebel, A., Krock, E., Gentry, C., Israel, M. R., Jurczak, A., Urbina, C. M., Sandor, K., Vastani, N., Maurer, M., Cuhadar, U., et al. (2021). Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest 131(13).
- Helyes, Z., Tekus, V., Szentes, N., Pohoczky, K., Botz, B., Kiss, T., Kemeny, A., Kornyei, Z., Toth, K., Lenart, N., et al. (2019). Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proc Natl Acad Sci U S A 116(26): 13067-13076.
- Pohoczky, K., Kun, J., Szentes, N., Aczel, T., Urban, P., Gyenesei, A., Bolcskei, K., Szoke, E., Sensi, S., Denes, A., et al. (2022). Discovery of novel targets in a complex regional pain syndrome mouse model by transcriptomics: TNF and JAK-STAT pathways. Pharmacol Res 182: 106347.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Sensi, S. and Goebel, A. (2022). Human Auto-IgG Purification from High Volume Serum Sample by Protein G Affinity Purification. Bio-protocol 12(23): e4562. DOI: 10.21769/BioProtoc.4562.
Category
Immunology > Antibody analysis > Antibody detection
Biochemistry > Protein > Isolation and purification
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link









