- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
In vitro Assay to Evaluate Cation Transport of Ionophores
Published: Vol 12, Iss 22, Nov 20, 2022 DOI: 10.21769/BioProtoc.4552 Views: 2254
Reviewed by: David PaulJohn P PhelanAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

Detection and Quantification of Calcium Ions in the Endoplasmic Reticulum and Cytoplasm of Cultured Cells Using Fluorescent Reporter Proteins and ImageJ Software
Shunsuke Saito and Kazutoshi Mori
Aug 20, 2023 2850 Views

A Protocol for Custom Biomineralization of Enzymes in Metal–Organic Frameworks (MOFs)
Zoe Armstrong [...] Zhongyu Yang
Feb 5, 2024 1885 Views
Abstract
Ion homeostasis is a fundamental regulator of cellular processes and depends upon lipid membranes, which function as ion permeability barriers. Ionophores facilitate ion transport across cell membranes and offer a way to manipulate cellular ion composition. Here, we describe a calcein quenching assay based on large unilamellar vesicles that we used to evaluate divalent cation transport of the ionophore 4-Br-A23187. This assay can be used to study metal transport by ionophores and membrane proteins, under well-defined conditions.
Graphical abstract:
Background
Ion homeostasis is a fundamental requirement for all living cells, as it controls diverse biological processes, including cell signaling, maintenance of cell volume and osmotic pressure, and regulation of cellular pH. Ions also act as enzyme cofactors, providing force through concentration gradients, and serve as corepressors for protein regulators orchestrating, for instance, the oxidative stress response. Ion homeostasis in cells depends upon lipid membranes, which function as ion permeability barriers. This allows cells to maintain highly asymmetric concentrations of cations and anions across both plasma membranes and intracellular organelles. Major ionic gradients across the plasma membrane include sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl-). Ionophores, which transport ions across biological membranes, offer a way to manipulate cellular ion composition. Examples of commonly used ionophores include valinomycin, which transports potassium (K+) (Su et al., 2019), or calcimycin (A23187) and ionomycin, which transport zinc (Zn2+), manganese (Mn2+), and Ca2+ (Erdahl et al., 1994, 1995, 1996), or 4-bromocalcimycin (4Br-A23187), which is highly selective for the transport of Zn2+ and Mn2+, compared to Ca2+ (Erdahl et al., 1996). Ion transport and specificity of ionophores are typically characterized in vitro, in model systems comprising model membranes and a single ion species available for transport. A common cell membrane model is based on large unilamellar vesicles (LUVs) formed from one lipid type or lipid mixtures of different compositional complexity. The vesicles are typically prepared from a lipid solution in a volatile organic solvent. A thin lipid film on the inside of a glass flask is formed by slow evaporation of the organic solvent. The subsequent hydration of the lipid film results in the formation of large multilamellar vesicles, which are converted to unilamellar vesicles of defined size by a freeze-thaw and extrusion method (Olson et al., 1979; Hope et al., 1986; Mayer et al., 1986; Kunding et al., 2008). This approach has been used to encapsulate radioactive ions (e.g., 22Na+, 45Ca2+, and the K+ mimic 86Rb+) inside the liposomes to study their efflux upon dilution of the liposomes into an isotope-free solution (e.g., Hamilton and Kaler, 1987 and 1990). A drawback to this procedure is the inconvenience associated with handling radioactive reagents. Alternative assays are based on the encapsulation of water-soluble ion-sensitive dyes into the vesicle. Ion permeability is then monitored by fluorescence spectroscopy. The protocol presented here utilizes calcein as a fluorescent dye, which can be quenched by Cu2+, Ni2+, Co2+, and Fe3+ at neutral pH (Černiauskas et al., 2017; Picard et al., 2000), and was recently used by us to reveal 4-Br-A23187 as a potent copper ionophore (Senges et al., 2022). Given the broad range of ions that can quench calcein fluorescence, the assay should be useful for determining the selectivity and mechanisms of ionophore-mediated cation transport. Furthermore, the impact of ionophores on membrane integrity can be tested using a calcein leakage assay by loading the vesicles with a self-quenching concentration of calcein (> 750 µM), as described previously (Maherani et al., 2013; Dutta et al., 2020; Bae et al., 2021; Senges et al., 2022). This versatile protocol can be fitted to other ion-sensitive dyes, although encapsulation efficiency should be tested for each dye. The assay can also be adapted to study metal transport by reconstituted membrane proteins (Billesbølle et al., 2020).
Materials and Reagents
Ice bucket (e.g., Magic Touch 2TM ice bucket with lid; Sigma-Aldrich, catalog number: BAM168072002)
Glass bead, 3 mm (Merck, catalog number: 104015)
Microcentrifuge tubes of 1.5 mL capacity (SARSTEDT AG & Co. KG, catalog number: 72.690.001)
Polyethersulfone membrane with a pore size of 0.2 μm (Filtropur, SARSTEDT AG & Co. KG, catalog number: 83.1826.001)
Polypropylene tubes of 15 mL and 50 mL capacity (e.g., Falcon tubes, SARSTEDT AG & Co. KG, catalog numbers: 62.554.502 and 62.547.254)
Round bottom glass tubes (16 × 150 mm, Carl Roth, catalog number: NY90.1)
Magnetic bars ROTILABO® Micro (diameter 2 mm, length 5 mm, Carl Roth, catalog number: 0955.2)
Syringe filter, Filtropur S, membrane: Polyethersulfone (PES), filtration surface: 6.2 cm2, pore size: 0.2 µm, for sterile filtration (Sarstedt, catalog number: 83.1826.001)
Syringe sterile dimethyl sulfoxide (DMSO; Thermo Scientific, catalog number 036480.AP)
1,2-dioleoyl-sn-glycerophosphatidylcholine (DOPC; Avanti Polar Lipids, catalog number: 850375C)
Aluminum foil
Calcein (Merck, catalog number: 1461-15-0)
Calcium chloride (Grüssing, catalog nummer: 102331000U)
Chloroform, ethanol-stabilized and certified for absence of phosgene and HCl (VWR, catalog number: 22711.290)
Deionized water
HEPES (Carl Roth, catalog number: 6763.3)
Liquid nitrogen
Methanol (VWR, catalog number: 20834.291)
N2 gas (ALPHAGAZ 1 N2, 99.999%, Air Liquide, Germany)
Potassium chloride (Sigma-Aldrich, catalog number: 7447-40-7)
Potassium hydroxide (Fisher Scientific, catalog number: P250-1)
SephadexTM G-50 fine (GE Healthcare Bio-Sciences AB, catalog number: 17-0042-01)
Triton X®-100, extra pure (Carl Roth, catalog number: 3051.3)
4-Br-A23187 (Cfm Oskar Tropitzsch, Marktredwitz, catalog number: 76455-48-6)
Buffer A (200 mL) (see Recipes)
Calcein stock in buffer A (16.6 mM, 1 mL) (see Recipes)
CuCl2 stock (2 mM) (see Recipes)
G50-Gel in buffer A (see Recipes)
HEPES, pH 7.4 (0.5 M, 200 mL) (see Recipes)
Ionophore stock (6 mM) (see Recipes)
KCl (0.5 M, 200 mL) (see Recipes)
KOH (1 M) (see Recipes)
Lipid stock in chloroform (see Recipes)
Loading buffer (1 mL) (see Recipes)
Triton X-100 solution (20% w/v) (see Recipes)
Equipment
Forceps (e.g., VWR, catalog number: 232-0032)
Analytical balance (e.g., Sartorius Entris-I II, 220 g/0.1 mg; Buch Holm, catalog number: 4669128)
Centrifuge with rotor for 15 mL polypropylene tubes (e.g., Eppendorf 5810 R)
Flow cabinet to work with organic solvents
Fluorometer (PTI QuantaMaster 800 fluorometers) with integrated FelixGX software, equipped with single cuvette Peltier K-155-C temperature control and magnetic stirrer (Horiba)
Freezer (-20 °C)
Hamilton 700 Series Syringes of 10 µL, 100 µL, 250 µL, 500 µL, and 1,000 µL (Hamilton® syringe, 700 and 1000 Series)
Macro polystyrene cuvettes (SARSTEDT AG & Co. KG, Germany, maximum 4.5 mL volume, catalog number: 67.745)
Magnets 5 × 2 mm (Merck, catalog number: Z328839)
Mini-Extruder Set (Avanti Inc., catalog number: 610023)
1 mL gas-tight syringes (Avanti no: 610017)
10 mm filter supports (Avanti no: 610014)
19 mm Nucleopore tracketch membrane with a pore size of 0.2 μm (Schleicher & Schuell)
pH-meter (pH-Meter 761 Calimatic, Knick)
Pipettes P20, P200, P1000 (GILSON®, catalog numbers: FD10001, FD10005, and FD10006)
Pipette tips 2 µL, 20 µL, 200 µL, and 1,000 µL (SARSTEDT AG & Co. KG, catalog numbers: 70.1130.212, 70.3021, 70.760.002, and 70.3050.020)
Refrigerator (4 °C)
Scissors
Rotavapor® R-100 Evaporator with I-100 Controller and V-100 vacuum pump (Flawil, Switzerland)
Vortexer (Vortex Genie 2TM, BENDER & HOBEIN AG, Switzerland)
Water bath (e.g., WPE45 Memmert, Germany)
Software
FelixGX software for the control of the PTI QuantaMaster 8000 fluorometer and accessories
Microsoft Excel for Microsoft 365 MSO (Version 2205)
Procedure
Preparation of the lipid film
Clean the Hamilton syringes by flushing them five times with chloroform:methanol (1:1, volume/volume) under a fume hood.
Note: Chloroform is a hazardous solvent. Conduct all work in a fume hood, while wearing appropriate personal protective equipment.
Using Hamilton syringes, transfer 200 µL of a 25 mg mL-1 DOPC stock solution (see Recipe 9) into a round bottom glass tube placed on ice.
Note: Avoid any use of plasticware when handling organic solvents.
Evaporate the organic solvent at room temperature (RT) under in a rotary evaporator at the reduced pressure of 250 mbar overnight, followed by evaporation at ~10 mbar for 15 min, see Figure 1.
The dried lipid film can be stored at -20 °C.
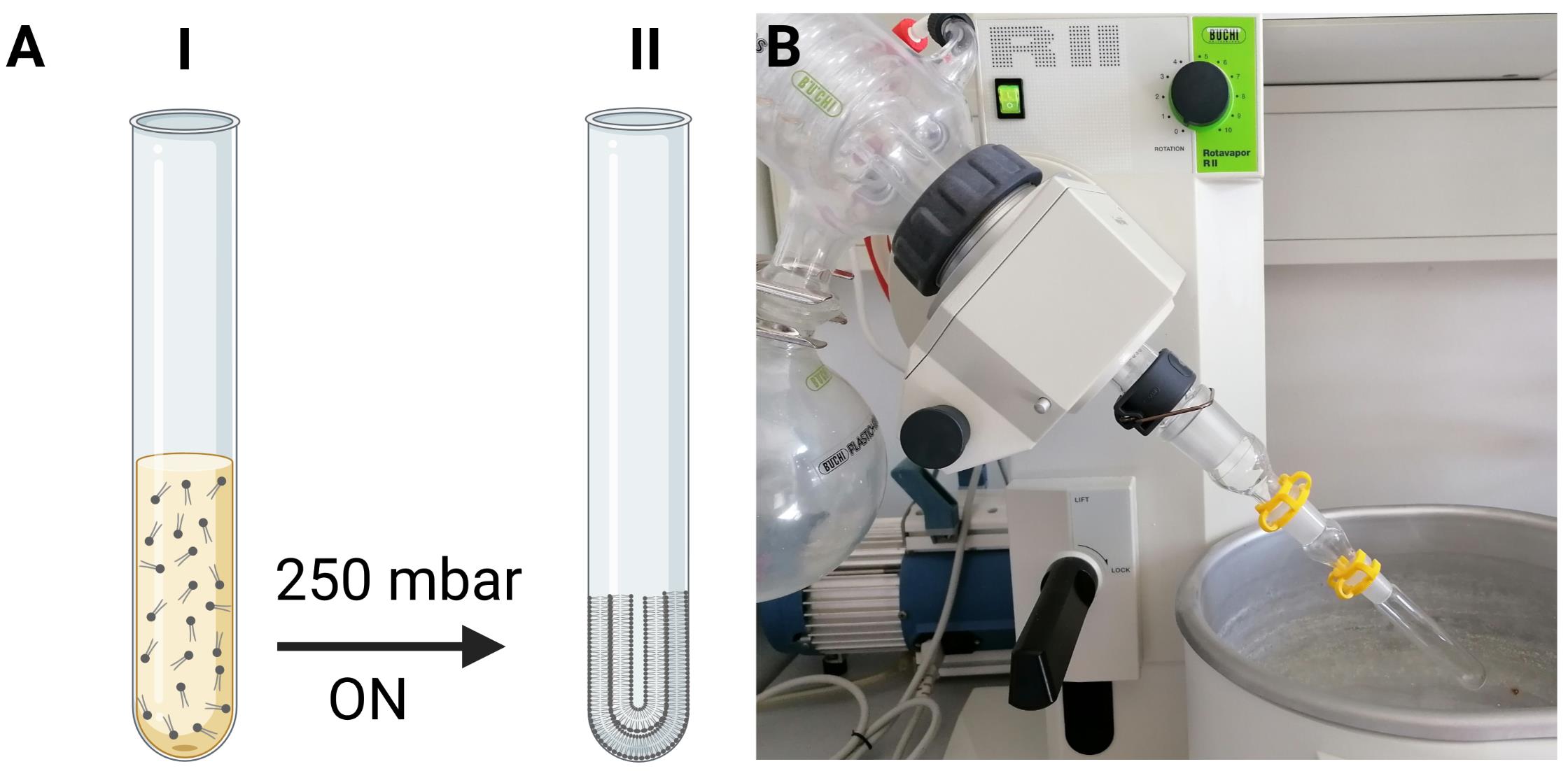
Figure 1. Scheme of lipid film preparation. (A) The desired amount of lipid (I) in chloroform is transferred to a glass tube. Subsequently, the solvent is evaporated in a rotary evaporator under the reduced pressure of 250 mbar overnight (ON), resulting in a thin lipid film on the sides of the glass tube (II). (B) The glass tube containing the desired lipids in chloroform is connected to the rotary evaporator.Preparation of calcein-loaded LUVs
Add 1 mL of loading buffer (see Recipe 10) and a 3-mm glass bead to the lipid film. Vortex for 10 min (see Figure 2A).
Transfer the lipid suspension to a new glass tube, without the glass bead.
Subject the lipid suspension to five freeze-thaw cycles by placing the tube alternatively into liquid nitrogen for 10 min for freezing and in a water bath at 50 °C for 5 min for thawing.
Note: Wear a face shield and insulating gloves when handling liquid nitrogen. Use glass tubes with high thermal shock resistance.
Assemble the mini-extruder, consisting of two Hamilton glass syringes, a Teflon cylinder with two drain discs, and a 200-nm polycarbonate membrane in between them, see Figure 2B.
Note: We routinely use polycarbonate membranes of 200-nm pore size and perform the extrusion at RT. However, for lipids containing saturated, long-chain fatty acids, which have a high lipid phase transition temperature (Tm), the extruder needs to be heated up to a temperature 5–10 °C above the Tm. This temperature can vary greatly between lipid formulations. A useful table of lipid phase transition temperatures is provided by the manufacturer at https://avantilipids.com/tech-support/physical-properties/phase-transition-temps/.
Check the tightness of the mini-extruder by flushing with 1 mL of buffer A (see Recipe 1), i.e., pass back and forth between the syringes three to four times. Continue only if the buffer volume stays the same for each passing.
Fill one Hamilton glass syringe with ~1 mL of lipid/calcein solution, and place the filled syringe into one end of the mini-extruder.
Carefully place an empty syringe into the opposite end of the mini-extruder. The plunger of the empty syringe should be depressed completely into the syringe barrel.
Extrude lipid suspension by passing through the filters a minimum of 11 times, starting in syringe 1 and finishing in syringe 2.
Note: The number of passages through the extruder needs to be uneven so that the final liposome sample is collected in syringe 2, which is uncontaminated by residual multilamellar vesicles that have never passed the extruder.
Inject the final lipid solution into a 1.5-mL microcentrifuge tube, and store it at 4 °C.
Note: Size distribution of the resulting liposomal preparations can be evaluated by dynamic light scattering. When using polycarbonate membranes of 200-nm pore size, we typically obtain a preparation with an hydrodynamic diameter in the range of 168.5 ± 2.5 nm.
Immediately wash all parts of the extruders with Milli-Q water, then with 70% ethanol, and dry it thoroughly before storing. Solvent-rinse syringes before storing. When extruding additional vesicles, disassemble and clean all parts of the extruder, and replace the membrane and filter supports.
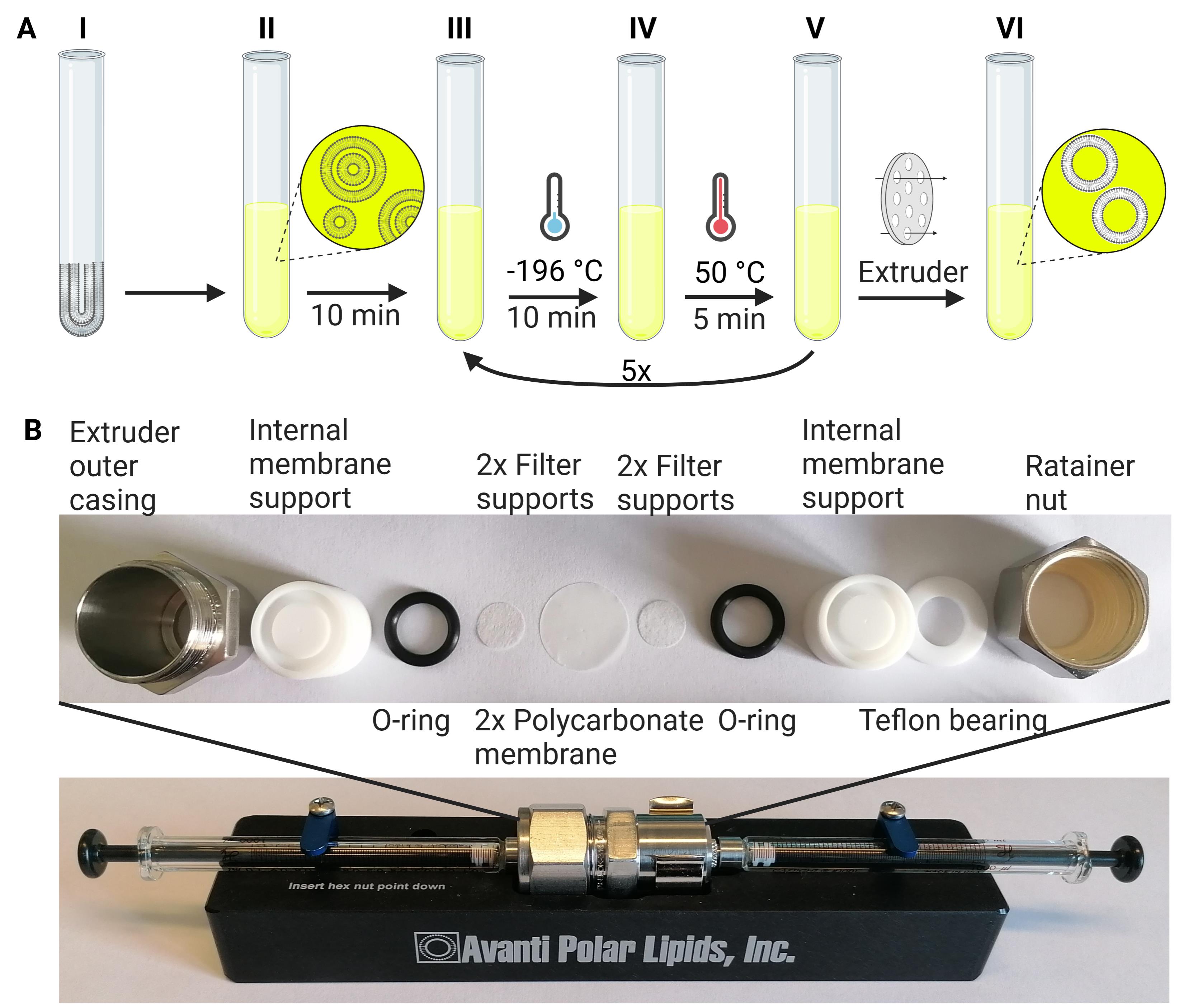
Figure 2. Scheme for preparation of calcein-loaded LUVs. (A) The dry lipid film is hydrated in a loading buffer containing the fluorescent dye calcein (step I) by vortexing for 10 min (step II). The lipid suspension (step III) is subjected to five freeze-thaw cycles using liquid nitrogen (-196 °C, step IV) and a water bath at 50 °C (step V). Next, the suspension is passed 11 times through 0.2-μm nucleopore polycarbonate membranes mounted in a mini-extruder to form LUVs (step VI). (B) Assembly of mini-extruder. Use four filter supports, two on each side. Use two polycarbonate membranes, with blank sides towards each other. Further directions and guidance on proper extruder assembly are provided on the manufacturer’s web site (https://avantilipids.com/divisions/equipment-products/mini-extruder-assembly-instructions; https://www.youtube.com/watch?v=WT6WPvGv5eY).Separation of calcein-loaded LUVs from free dye
Prepare two G50 columns, using 2-mL plastic syringes without a plunger, and filter supports as a stopper, see Figure 3A.
Place the syringes into disposable 15-mL reaction tubes, add 3 mL of Sephadex G50 fine slurry (see Recipe 4) using a Pasteur pipette, centrifuge at 180 × g for 5 min, and transfer the columns to a new 15-mL reaction tube.
Load the sample on the top of one of the G50 columns.
Centrifuge at 180 × g and RT for 5 min (see Figure 3B).
Transfer the eluate to the top of the second G50 column.
Centrifuge again at 180 × g and RT for 5 min (see Figure 3C).
Note: Repeat the size exclusion chromatography of the eluate with a new G50 column, if you do not achieve a separation of the free dye (intense yellow part of the column) from the vesicles (colorless lower part of the column).
Collect the eluate containing the calcein-loaded LUVs in a new 15-mL Falcon tube.
Cover the tube with aluminum foil.
Note: Calcein-loaded LUVs could be stored at 4 °C in the dark until the next day.
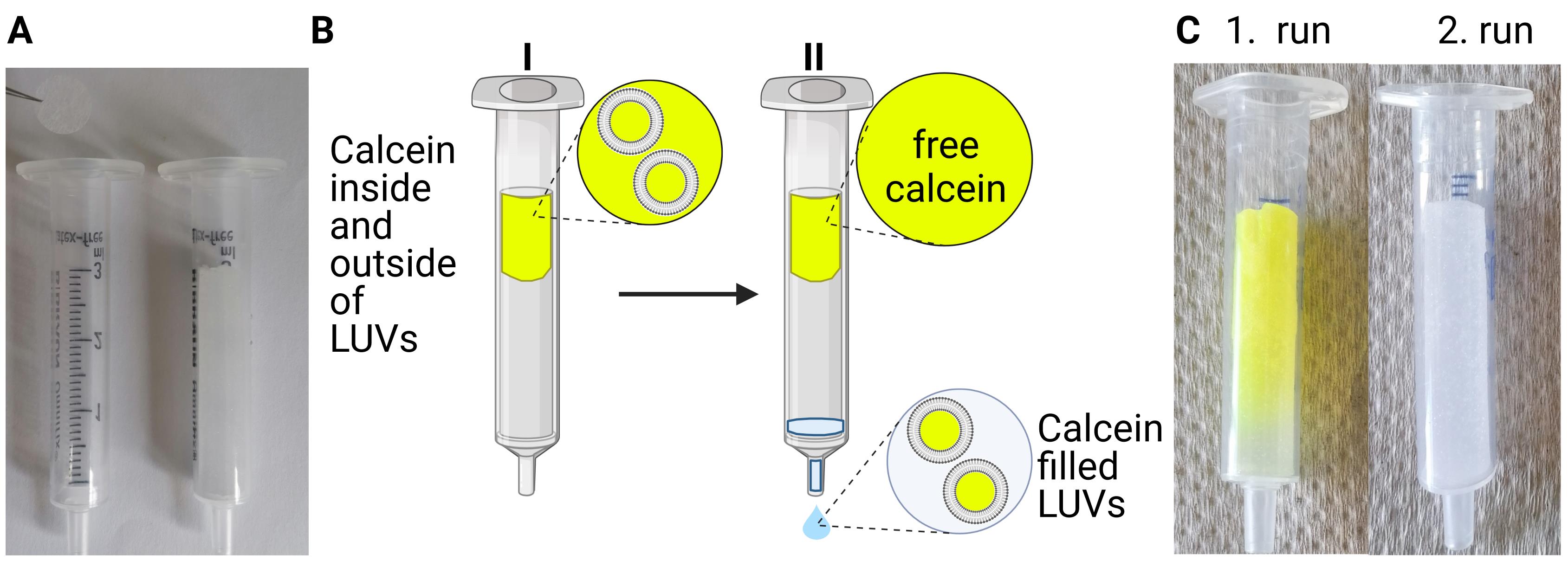
Figure 3. Separation of calcein-loaded LUVs by size exclusion chromatography. (A) For size exclusion chromatography, two filter supports are placed as a stopper in a 3-mL plastic syringe without a plunger, and 3 mL of Sephadex G50 fine slurry is added. (B) Calcein-loaded LUVs are separated from free calcein during the size-exclusion chromatography. The LUVs will elute with the void volume in the early fractions, whereas the non-liposome-associated calcein will elute in later fractions. (C) Image showing a G50 filtration column after the filtration with the trapped free calcein. Free calcein is in the first G50 filtration column after the first separation. After the next separation, the second G50 filtration column contains no free calcein.Monitoring ion permeability
Cool buffer A (see Recipe 1), the cuvette, and the calcein-loaded LUV at 10 °C (e.g., by storing in a fridge at 10 °C).
Note: To suppress the rate of uncatalyzed transport in the absence of the ionophore, the assay is performed at 10 °C. Liposomes of other lipid compositions might exhibit lower passive permeability and thus allow higher assay temperatures.
Turn on the fluorometer and set up the parameters as follows: excitation wavelength 480 nm, emission wavelength 520 nm, and measurement duration approximately 10 min, with 1 s resolution. Adjust slits as necessary; a bandpass of 3 nm is usually sufficient.
Cool down the sample holder of the fluorometer to 10 °C.
Note: We use Peltier-based temperature control with magnetic stirring, providing temperature stability and full control software during the measurements.
Add 20 µL of calcein-loaded LUV in the fluorometer cuvette, and top up to 2 mL using precooled buffer.
For probing the impact of ionophores on ion permeability, add 1 µL of ionophore (see Recipe 6, final concentration 3 µM) into the cuvette, and incubate in the fridge for a further 5 min.
As a control for quenching of calcein in the presence of ions, prepare one sample by adding 10–20 µL of 20% Triton X-100 (see Recipe 11) into a cuvette, and incubate in the fridge for 5 min.
Introduce the cuvette into the fluorometer (remember to include the magnetic stir bar), and start monitoring the emission intensity.
Wait until fluorescence is stable (90 s).
Measure the fluorescence intensity. After 1.5 min, add 3 µM CuCl2 (3 µL of 2 mM CuCl2, see Recipe 3), and record the fluorescence for another 8.5 min.
Note: To ensure your setting and the calcein-loaded LUVs are working properly, run an excitation scan from 450 to 500 nm (emission 520 nm) and an emission scan from 500 to 550 nm (excitation 480 nm) at the start of the measurements. For calcein, the excitation and emission maxima should be at 480 nm and 520 nm, respectively (see Figure 4).
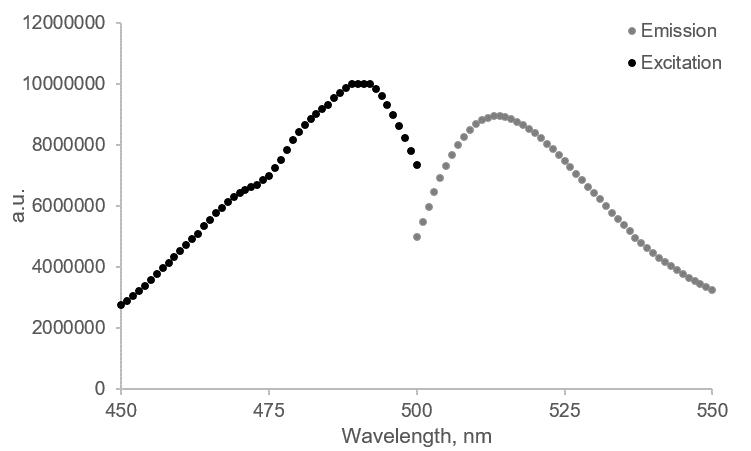
Figure 4. Excitation and emission scans of the calcein-loaded LUVs were measured using a fluorometer. In the fluorometer cuvette, add 20 µL of calcein-loaded LUVs, and fill up to 2 mL using precooled buffer A. An excitation scan (black trace) was performed from 450–500 nm (emission 520 nm, slit 3 nm) and an emission scan (grey trace) from 500–550 nm (excitation 480 nm, slit 3 nm).
Data analysis
Save the measured data as a text document (txt file).
Export the data from the text document to Microsoft Excel.
Find the maximum measured fluorescence signal (Fmax).
For normalization, divide the measured fluorescence signal by Fmax and multiply by 100 (see formula 1).
Fnorm =F⁄Fmax*100 (1)
F: measured fluorescence signal
Fmax: maximum of measured fluorescence signal
Fnorm: normalized fluorescence signal
Plot Fnorm against time (see Figure 5).
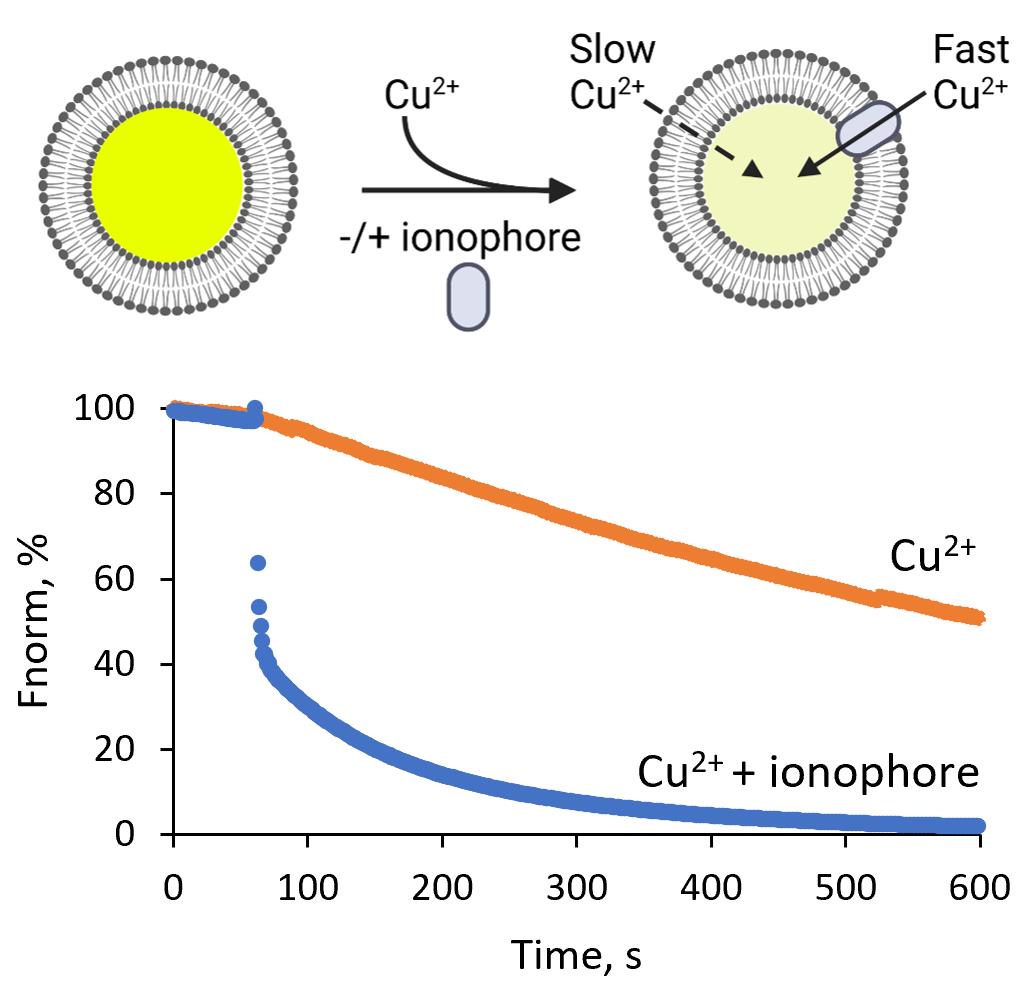
Note for data interpretation: In this in vitro quenching assay, insertion of ionophores (here 4-Br-A23187) into LUVs allows the transport of Cu2+ ions into the vesicles, which then quench the encapsulated calcein fluorophore (Figure 5). In the absence of an ionophore, fluorescence intensity decreases linearly with time, indicating a slow permeation of Cu2+ ions into the vesicles (Figure 5B, orange trace). 4-Br-A23187 accelerates the influx of Cu2+ ions into vesicles, as indicated by rapid quenching of the calcein fluorescence (Figure 5B, blue curve). The addition of Triton X-100 disrupts the liposomes and thus serves as a control for ~100% quenching (Figure 5B, grey trace; Figure 5B, blue curve).
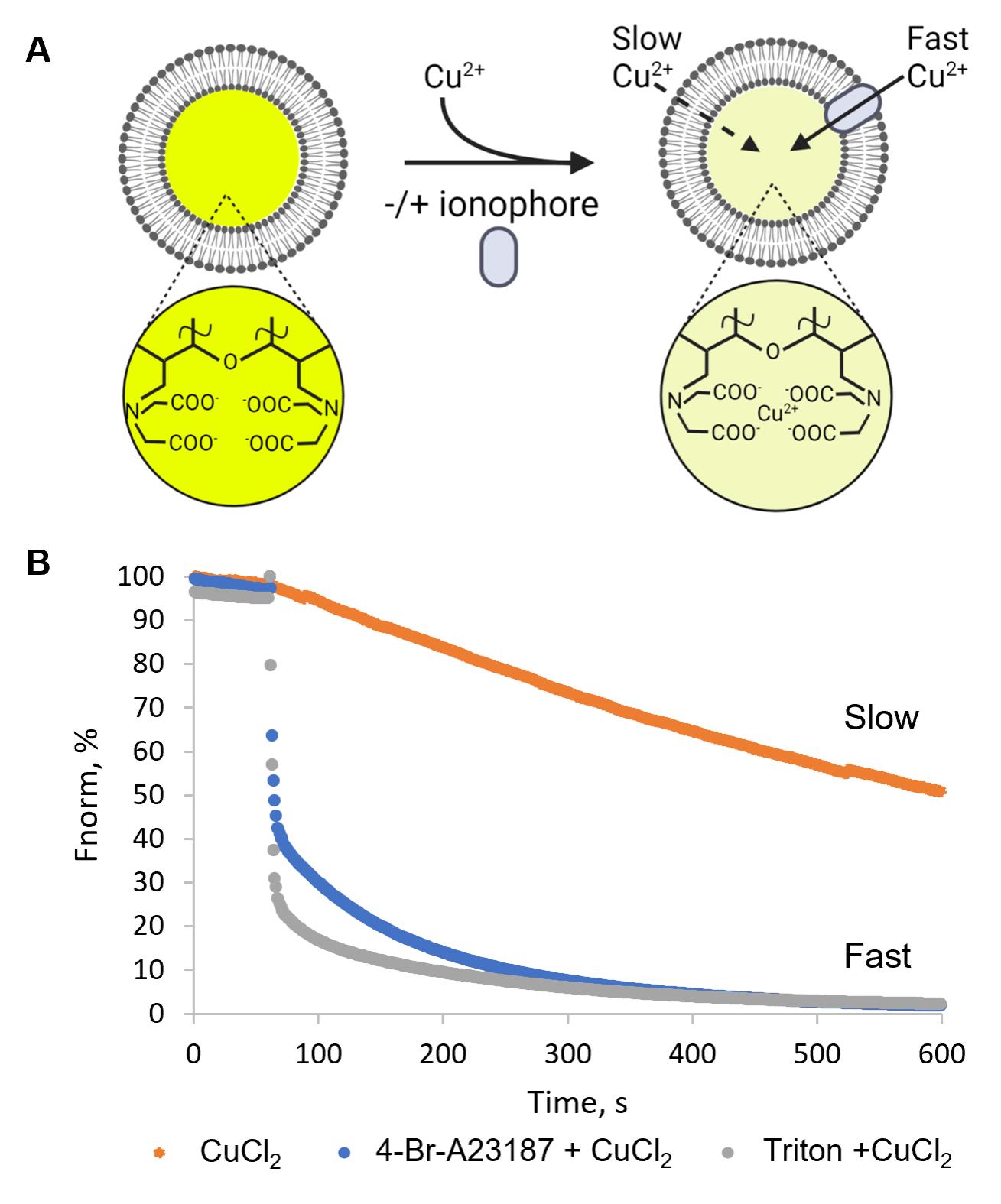
Figure 5. Copper transport by the ionophore 4-Br-A23187. (A) Schematic of the assay based on calcein-loaded LUVs to measure the transport of divalent cations. Transport of divalent cations into the LUVs leads to time-dependent quenching of calcein fluorescence inside the liposomes. (B) Calcein-loaded LUVs were treated with 3 µM CuCl2 alone (orange trace), or in the presence of 3 µM 4-Br-A23187 (blue trace), or 0.1% Triton X-100 (grey trace). Higher ion permeability in the presence of the ionophore leads to faster calcein fluorescence quenching rates than in the absence of the ionophore (orange trace compared to blue trace). Disruption of the liposomes by Triton X-100 makes all ions accessible to calcein, resulting in ~100% quenching (grey trace). Fluorescence intensity was normalized to the fluorescence before copper addition.
Notes
All figures were prepared using Biorender.com.
Recipes
Buffer A (200 mL)
Mix 4 mL of 0.5 M HEPES pH 7.4 (final 10 mM) with 60 mL of 0.5 M KCl (final 150 mM).
Add 100 mL of deionized water and adjust pH with KOH to 7.4.
Top up to a final volume of 200 mL with deionized water, and filter-sterilize with a 0.22 µm filter (Millipore).
Calcein stock in buffer A (16.6 mM, 1 mL)
Dissolve 10.334 mg calcein to a final volume of 1 mL in buffer A by vortexing.
Cover the tube with aluminum foil to protect it from daylight.
CuCl2 stock (2 mM)
Dissolve 110.98 mg CuCl2 to a final volume of 1 mL in deionized water, to obtain1 M CuCl2.
Then, add 2 µL of 1 M CuCl2 into 998 µL of deionized water.
G50-Gel in buffer A
Add 2.5 g Sephadex G-50 to 50 mL of buffer A, and dissolve it by mixing.
The gel has to swell at RT ON, and is then stored at 4 °C.
HEPES, pH 7.4 (0.5 M, 200 mL)
Dissolve 9.532 g HEPES to a final volume of 170 mL in deionized water.
Adjust the pH with 1 M KOH to 7.4, and top it up to 200 mL with deionized water.
Ionophore stock (6 mM)
Prepare an initial stock of 10 mg 4-Br-A23187 in 1 mL of DMSO.
To reach 6 mM 1-Br-A23187, 36.15 µL of the stock solution is diluted with 63.85 µL of filter-sterilized DMSO, resulting in a final volume of 100 µL.
Before the experiments, the DMSO is filter-sterilized using a 0.22-μm syringe filter system.
KCl (0.5 M, 200 mL)
Dissolve 7.455 g KCl to a final volume of 200 mL in deionized water.
KOH (1 M)
Dissolve 5.611 g of KOH to a final volume of 100 mL in deionized water.
Lipid stock in chloroform
Lipids are ordered in chloroform at a concentration of 25 mg mL-1, and stored at -20 °C until further use. For longer storage, evaporate the chloroform and store the dried lipid at -20 °C. Before using it, dissolve the 25 mg lipid in 1 mL of chloroform:methanol solution (v:v).
Note: Some lipids may have limited or very poor solubility in chloroform:methanol, and require a mixture of chloroform:methanol:water.
Loading buffer (1 mL)
Take 18.07 µL of 16.6-mM calcein stock (final 300 µM) and top up with buffer A to a final volume of 1 mL. Cover the tube with aluminum foil.
Triton 100-X solution (20% w/v)
Dissolve 200 mg in 1 mL of deionized water.
Acknowledgments
This protocol was adapted from our previous work (Senges et al. 2022). JEB gratefully acknowledges funding from the German Research Foundation (BA 4193/6-1, RTG 2341). HDU is a scholar of the Friedrich Ebert Foundation.
Competing interests
The authors declare that no competing interests exist.
References
- Bae, W., Yoon, T. Y. and Jeong, C. (2021). Direct evaluation of self-quenching behavior of fluorophores at high concentrations using an evanescent field. PLoS One 16(2): e0247326.
- Billesbølle, C. B., Azumaya, C. M., Kretsch, R. C., Powers, A. S., Gonen, S., Schneider, S., Arvedson, T., Dror, R. O., Cheng, Y. and Manglik, A. (2020). Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature 586(7831): 807-811.
- Černiauskas, V., Gruodis, A., Rodaitė-Riševičienė, R., Saulis, G. (2017). Mechanism of Effective Quenching of Calcein Fluorescence by Iron. Ab initio study. In:Innovative Infotechnologies for Science, Business and Education. 2(23): 10-16.
- Dutta, S., Watson, B. G., Mattoo, S. and Rochet, J. C. (2020). Calcein Release Assay to Measure Membrane Permeabilization by Recombinant Alpha-Synuclein. Bio-protocol 10(14): e3690.
- Erdahl, W. L., Chapman, C. J., Taylor, R. W. and Pfeiffer, D. R. (1994). Ca2+ transport properties of ionophores A23187, ionomycin, and 4-BrA23187 in a well defined model system. Biophys J 66(5): 1678-1693.
- Erdahl, W. L., Chapman, C. J., Taylor, R. W. and Pfeiffer, D. R. (1995). Effects of pH conditions on Ca2+ transport catalyzed by ionophores A23187, 4-BrA23187, and ionomycin suggest problems with common applications of these compounds in biological systems. Biophys J 69(6): 2350-2363.
- Erdahl, W. L., Chapman, C. J., Wang, E., Taylor, R. W. and Pfeiffer, D. R. (1996). Ionophore 4-BrA23187 transports Zn2+ and Mn2+ with high selectivity over Ca2+. Biochemistry 35(43): 13817-13825.
- Hamilton, R. T. and Kaler, E. W. (1987). Ionic permeability of synthetic vesicles.J Colloid Interface Sci 116(1): 248-255.
- Hamilton, R. T. and Kaler, E. W. (1990). Alkali metal ion transport through thin bilayers. J Phys Chem 94 (6): 2560-2566.
- Hope, M. J., Bally, M. B., Mayer, L. D. and Cullis, P. R. (1986). Generation of multilamellar and unilamellar phospholipid vesicles. Chem Phys Lipids (40): 89-107.
- Kunding, A. H., Mortensen, M. W., Christensen, S. M. and Stamou, D. (2008). A fluorescence-based technique to construct size distributions from single-object measurements: application to the extrusion of lipid vesicles. Biophys J 95(3): 1176-1188.
- Maherani, B., Arab-Tehrany, E., Kheirolomoom, A., Geny, D. and Linder, M. (2013). Calcein release behavior from liposomal bilayer; influence of physicochemical/mechanical/structural properties of lipids. Biochimie 95(11): 2018-2033.
- Mayer, L. D., Hope, M. J. and Cullis, P. R. (1986). Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta 858(1): 161-168.
- Olson, F., Hunt, C. A., Szoka, F. C., Vail, W. J. and Papahadjopoulos, D. (1979). Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta 557(1): 9-23.
- Picard, V., Govoni, G., Jabado, N. and Gros, P. (2000). Nramp 2 (DCT1/DMT1) expressed at the plasma membrane transports iron and other divalent cations into a calcein-accessible cytoplasmic pool. J Biol Chem 275(46): 35738-35745.
- Senges, C. H. R., Warmuth, H. L., Vazquez-Hernandez, M., Uzun, H. D., Sagurna, L., Dietze, P., Schmidt, C., Mucher, B., Herlitze, S., Kramer, U., et al. (2022). Effects of 4-Br-A23187 on Bacillus subtilis cells and unilamellar vesicles reveal it to be a potent copper ionophore. Proteomics 22(17): e2200061.
- Su, Z. F., Ran, X. Q., Leitch, J. J., Schwan, A. L., Faragher, R. and Lipkowski, J. (2019). How Valinomycin Ionophores Enter and Transport K+ across Model Lipid Bilayer Membranes. Langmuir 35 (51): 16935-16943.
Article Information
Copyright
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
How to cite
Uzun, H. D., Vázquez-Hernández, M., Bandow, J. E. and Pomorski, T. G. (2022). In vitro Assay to Evaluate Cation Transport of Ionophores. Bio-protocol 12(22): e4552. DOI: 10.21769/BioProtoc.4552.
Category
Drug Discovery > Drug Screening
Biochemistry > Other compound > Ion
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link










